Mechanistic Insight into Degradation of Cetirizine under UV/Chlorine Treatment: Experimental and Quantum Chemical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. UV/Chlorine Degradation Experiments
2.3. Analytical Methods
2.4. Quantum Chemical Calculation Methods
3. Results and Discussion
3.1. Degradation of CTZ and Influencing Factors during UV/Chlorine Treatment
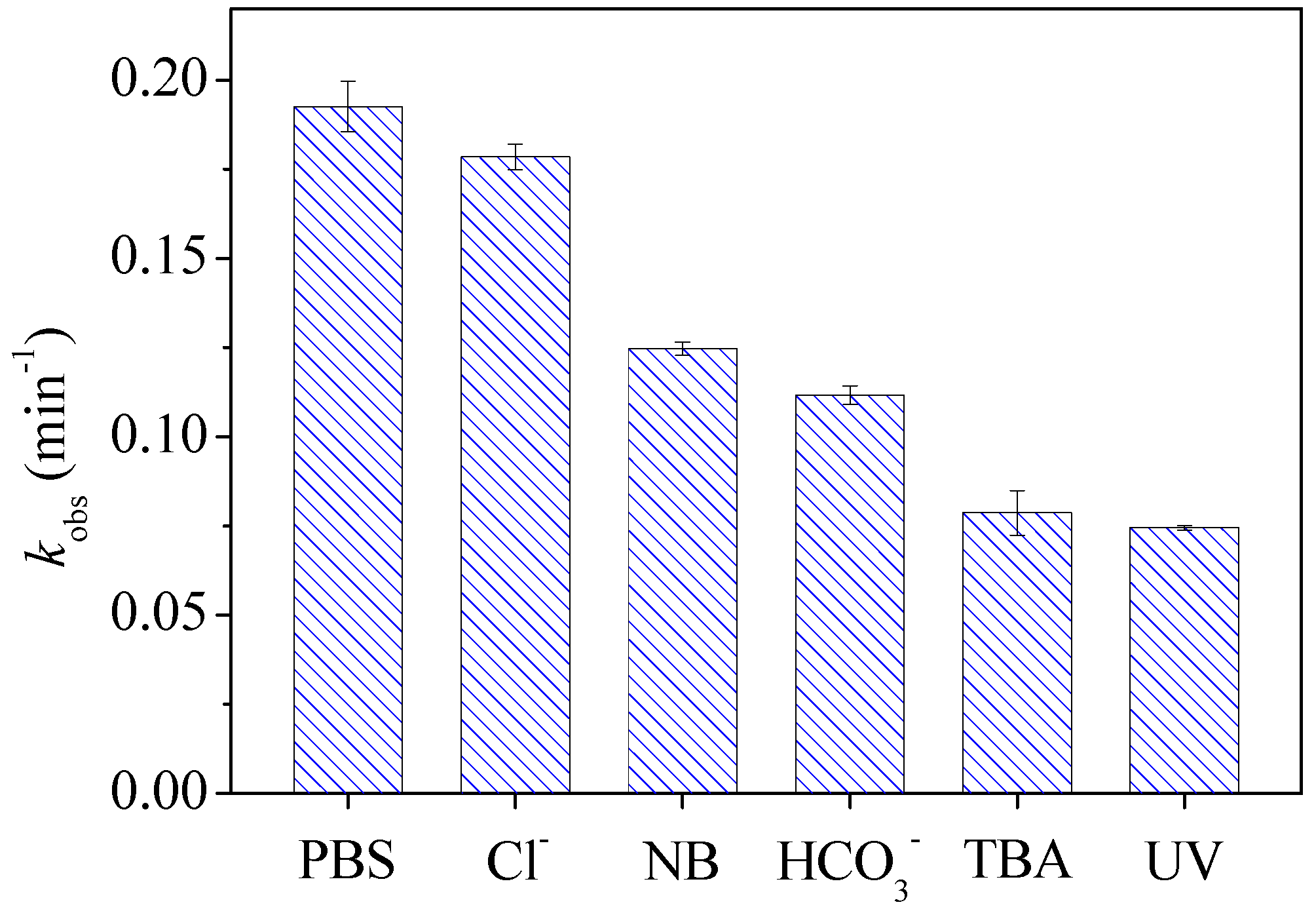
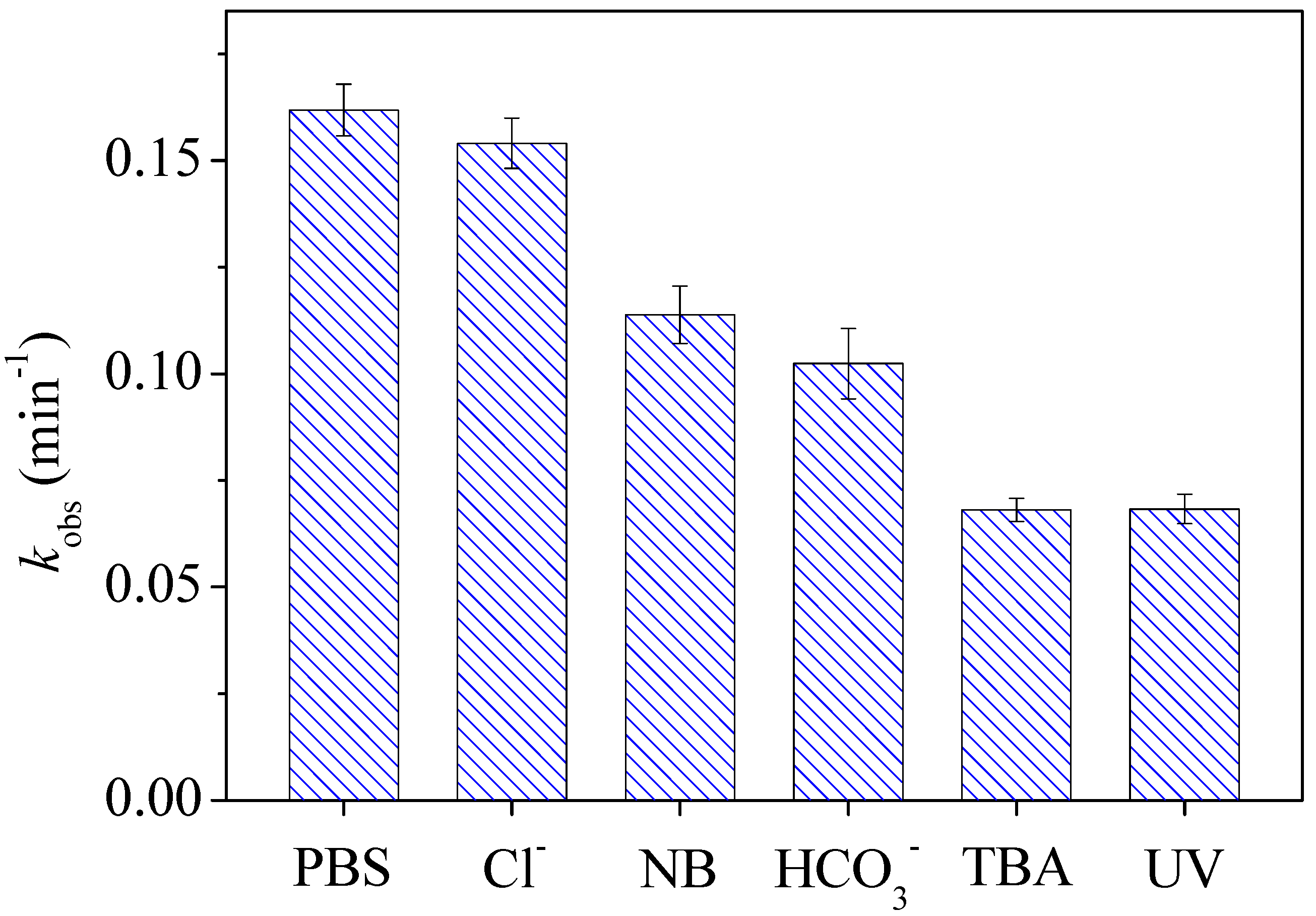
3.2. Roles of Reactive Species in Degradation of CTZ
3.3. Degradation Pathways of CTZ in UV/Chlorine System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.-X.; Chen, J.; Wu, D.; Liu, Y.-S.; Yang, Y.-Q.; He, L.-X.; Ye, P.; Zhao, J.-L.; Liu, S.-S.; Yang, B.; et al. Highly enhanced biodegradation of pharmaceutical and personal care products in a novel tidal flow constructed wetland with baffle and plants. Water Res. 2021, 193, 116870. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Shi, X.; Jin, X.; Wang, X.C.; Jin, P. Comprehensive evaluation of pharmaceuticals and personal care products (PPCPs) in urban sewers: Degradation, intermediate products and environmental risk. Chem. Eng. J. 2021, 404, 127024. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, T.; Huang, C.-H.; Ben, W.; Yao, H.; Liu, R.; Sun, P. PPCP degradation by chlorine–UV processes in ammoniacal water: New reaction insights, kinetic modeling, and DBP formation. Environ. Sci. Technol. 2018, 52, 7833–7841. [Google Scholar] [CrossRef]
- Jonsson, M.; Fick, J.; Klaminder, J.; Brodin, T. Antihistamines and aquatic insects: Bioconcentration and impacts on behavior in damselfly larvae (Zygoptera). Sci. Total Environ. 2014, 472, 108–111. [Google Scholar] [CrossRef] [PubMed]
- He, X.; O’Shea, K.E. Rapid transformation of H1-antihistamines cetirizine (CET) and diphenhydramine (DPH) by direct peroxymonosulfate (PMS) oxidation. J. Hazard. Mater. 2020, 398, 123219. [Google Scholar] [CrossRef]
- Jonsson, M.; Andersson, M.; Fick, J.; Brodin, T.; Klaminder, J.; Piovano, S. High-speed imaging reveals how antihistamine exposure affects escape behaviours in aquatic insect prey. Sci. Total Environ. 2019, 648, 1257–1262. [Google Scholar] [CrossRef]
- Kristofco, L.A.; Brooks, B.W. Global scanning of antihistamines in the environment: Analyses of occurrence and hazards in aquatic systems. Sci. Total Environ. 2017, 592, 477–487. [Google Scholar] [CrossRef]
- Bittner, L.; Teixidó, E.; Keddi, I.; Escher, B.I.; Klüvera, N. pH-Dependent uptake and sublethal effects of antihistamines in Zebrafish (Danio rerio) embryos. Environ. Toxicol. Chem. 2019, 38, 1012–1022. [Google Scholar] [CrossRef]
- Paakkari, I. Cardiotoxicity of new antihistamines and cisapride. Toxicol. Lett. 2002, 127, 279–284. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Montanaro, D.; Lavecchia, R.; Petrucci, E.; Zuorro, A. UV-assisted electrochemical degradation of coumarin on boron-doped diamond electrodes. Chem. Eng. J. 2017, 323, 512–519. [Google Scholar] [CrossRef]
- Mar-Ortiz, A.F.; Salazar-Rábago, J.J.; Sánchez-Polo, M.; Rozalen, M.; Cerino-Córdova, F.J.; Loredo-Cancino, M. Photodegradation of antihistamine chlorpheniramine using a novel iron-incorporated carbon material and solar radiation. Environ. Sci. Water Res. Technol. 2020, 6, 2607. [Google Scholar] [CrossRef]
- Ennouri, R.; Lavecchia, R.; Zuorro, A.; Elaoud, S.C.; Petrucci, E. Degradation of chloramphenicol in water by oxidation on a boron-doped diamond electrode under UV irradiation. J. Water Process Eng. 2021, 41, 101995. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, J.-R.; Lin, C.-H.; Huang, C.-P. Catalytic degradation of chlorpheniramine over GO-Fe3O4 in the presence of H2O2 in water: The synergistic effect of adsorption. Sci. Total Environ. 2020, 736, 139468. [Google Scholar] [CrossRef]
- Sun, P.; Meng, T.; Wang, Z.; Zhang, R.; Yao, H.; Yang, Y.; Zhao, L. Degradation of organic micropollutants in UV/NH2Cl advanced oxidation process. Environ. Sci. Technol. 2019, 53, 9024–9033. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, J.; Fu, W.; Shang, C.; Li, Y.; Chen, Y.; Gan, W.; Fang, J. PPCP degradation by UV/chlorine treatment and its impact on DBP formation potential in real waters. Water Res. 2016, 98, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-K.; Ha, H.-H.; Yu, Y.H.; Kim, B.-J.; Bang, H.-J.; Lee, H.; Jung, S.-C. The photocatalytic destruction of cimetidine using microwave-assisted TiO2 photocatalysts hybrid system. J. Hazard. Mater. 2020, 391, 122568. [Google Scholar] [CrossRef]
- Borowska, E.; Bourgin, M.; Hollender, J.; Kienle, C.; McArdell, C.S.; von Gunten, U.D. Oxidation of cetirizine, fexofenadine and hydrochlorothiazide during ozonation: Kinetics and formation of transformation products. Water Res. 2019, 94, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Hu, C.; Hu, X.; Qu, J.; Yang, M. Degradation of selected pharmaceuticals in aqueous solution with UV and UV/H2O2. Water Res. 2009, 43, 1766–1774. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Yan, S.; Yao, B.; Song, W.; Hua, Z.; Zhang, X.; Kong, X.; Li, X.; Fang, J. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water Res. 2018, 147, 184–194. [Google Scholar] [CrossRef]
- Minakata, D.; Kamath, D.; Maetzold, S. Mechanistic insight into the reactivity of chlorine-derived radicals in the aqueous-phase UV−chlorine advanced oxidation process: Quantum mechanical calculations. Environ. Sci. Technol. 2017, 51, 6918–6926. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Li, T.; Zhang, T.; Zhang, Y.; Yang, C.; Luo, S. Degradation of organic filter 2-Phenylbenzidazole-5-Sulfonic acid by light-driven free chlorine process: Reactive species and mechanisms. Chem. Eng. J. 2022, 430, 132684. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.; Xiang, Y.; Shang, C.; Li, X.; Meng, F.; Yang, X. Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways. Water Res. 2016, 104, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Cheng, S.; Luo, N.; Yang, X.; An, T. Rate constants and mechanisms of the reactions of Cl• and Cl2•− with trace organic contaminants. Environ. Sci. Technol. 2019, 53, 11170–11182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hou, Z.; Li, Y.; Lei, Y.; Xu, Z.; Gu, J.; Tian, S. Modeling degradation kinetics of gemfibrozil and naproxen in the UV/chlorine system: Roles of reactive species and effects of water matrix. Water Res. 2021, 202, 117445. [Google Scholar] [CrossRef] [PubMed]
- Remucal, C.K.; Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 565–579. [Google Scholar] [CrossRef]
- Wang, W.L.; Wu, Q.Y.; Huang, N.; Wang, T.; Hu, H.Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Shang, C.; Yao, B.; Hou, S.; Yang, X.; Song, W.; Fang, J. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, S.; Yang, X.; Ren, J.; Fang, J.; Shang, C.; Song, W.; Lian, L.; Zhang, X. UV/chlorine treatment of carbamazepine: Transformation products and their formation kinetics. Water Res. 2017, 116, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Mei, Q.; Han, D.; Wei, B.; An, Z.; Cao, H.; Xie, J.; He, M. The roles of HO•, ClO• and BrO• radicals in caffeine degradation: A theoretical study. Sci. Total Environ. 2021, 768, 144733. [Google Scholar] [CrossRef]
- Ren, X.; Sun, Y.; Fu, X.; Zhu, L.; Cui, Z. DFT comparison of the OH-initiated degradation mechanisms for five chlorophenoxy herbicides. J. Mol. Modeling 2013, 19, 2249–2263. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Chen, J.; Zhou, C.; Xie, Q. Phototransformation of 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE) in natural waters: Important roles of dissolved organic matter and chloride ion. Environ. Sci. Technol. 2018, 52, 10490–10499. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, H.-B.; Chen, J.; Yang, X.; Zhang, Y.; Qiao, X. Predicting gaseous reaction rates of short chain chlorinated paraffins with •OH: Overcoming the difficulty in experimental determination. Environ. Sci. Technol. 2014, 48, 13808–13816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, F.; He, D.; Zhang, Y.; Qu, J.; Yang, X.; Chen, J.; Peijnenburg, W.J.G.M. Effect of UV/chlorine treatment on photophysical and photochemical properties of dissolved organic matter. Water Res. 2021, 192, 116857. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Guo, Y.; Ye, Z.; Guo, Z.; Lei, Y.; Yang, X.; Zhang, L.; Niu, J. Dichlorine radicals (Cl2•−) promote the photodegradation of propranolol in estuarine and coastal waters. J. Hazard. Mater. 2021, 414, 125536. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Mai, J.; Wu, S.; Liu, C.; Tang, L.; Mo, Z.; Zhang, M.; Guo, L.; Liu, M.; Ma, J. UV/chlorine process for degradation of benzothiazole and benzotriazole in water: Efficiency, mechanism and toxicity evaluation. Sci. Total Environ. 2021, 760, 144304. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Gadipelly, C.R.; Rathod, V.K.; Marathe, K.V. Persulfate assisted photo-catalytic abatement of cetirizine hydrochloride from aqueous waste: Biodegradability and toxicityanalysis. J. Mol. Catal. A Chem. 2016, 414, 116–121. [Google Scholar] [CrossRef]
- Wang, Y.; dos Santos, M.M.; Ding, X.; Labanowski, J.; Gombert, B.; Snyder, S.A.; Croué, J.-P. Impact of EfOM in the elimination of PPCPs by UV/chlorine: Radical chemistry and toxicity bioassays. Water Res. 2021, 204, 117634. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.C.; Huang, C. Aqueous chlorination of the antibacterial agent trimethoprim: Reaction kinetics and pathways. Water Res. 2007, 41, 647–655. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Xiang, Y.; Fang, J.; Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water Res. 2016, 90, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; DeCaprio, A.; Tarifa, A.; O’Shea, K. Ultrasound-induced remediation of the second-generation antihistamine, Cetirizine. J. Environ. Chem. Eng. 2020, 8, 103680. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Cheng, F.; Zhong, W.; Qu, J.; Zhang, Y.-n.; Yu, H. Mechanistic Insight into Degradation of Cetirizine under UV/Chlorine Treatment: Experimental and Quantum Chemical Studies. Water 2022, 14, 1323. https://doi.org/10.3390/w14091323
Zhu B, Cheng F, Zhong W, Qu J, Zhang Y-n, Yu H. Mechanistic Insight into Degradation of Cetirizine under UV/Chlorine Treatment: Experimental and Quantum Chemical Studies. Water. 2022; 14(9):1323. https://doi.org/10.3390/w14091323
Chicago/Turabian StyleZhu, Boyi, Fangyuan Cheng, Wenjing Zhong, Jiao Qu, Ya-nan Zhang, and Hongbin Yu. 2022. "Mechanistic Insight into Degradation of Cetirizine under UV/Chlorine Treatment: Experimental and Quantum Chemical Studies" Water 14, no. 9: 1323. https://doi.org/10.3390/w14091323





