The Effect of Different Sludge Pretreatment Methods on Microalgae Performance and the Release of Pollutants in Hydrolysis Acidification Solutions
Abstract
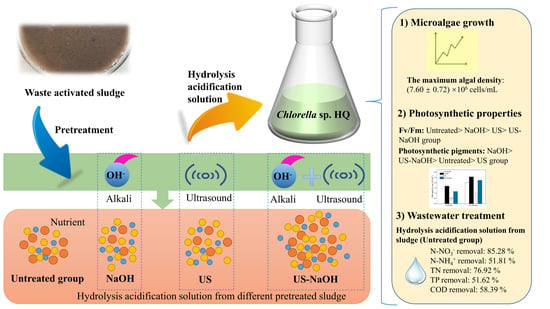
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strain and Wastewater
2.2. Experimental Design
2.3. Analysis Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. Growth Characteristics of Chlorella sp. HQ
3.2. Photosynthetic Characteristics
3.3. Contaminant Removal Using a Hydrolysis-Acidified Solution
3.4. Characteristics of Extracellular Polymer Substances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pang, H.; Jiao, Q.; He, J.; Zhang, Z.; Wang, L.; Yan, Z.; Lu, J. Enhanced short-chain fatty acids production through a short-term anaerobic fermentation of waste activated sludge: Synergistic pretreatment of alkali and alkaline hydrolase blend. J. Clean. Prod. 2022, 342, 130954. [Google Scholar] [CrossRef]
- He, D.; Xiao, J.; Wang, D.; Liu, X.; Li, Y.; Fu, Q.; Li, C.; Yang, Q.; Liu, Y.; Ni, B.J. Understanding and regulating the impact of tetracycline to the anaerobic fermentation of waste activated sludge. J. Clean. Prod. 2021, 313, 127929. [Google Scholar] [CrossRef]
- Zahedi, S.; Icaran, P.; Yuan, Z.; Pijuan, M. Assessment of free nitrous acid pretreatment on a mixture of primary sludge and waste activated sludge: Effect of exposure time and concentration. Bioresour. Technol. 2016, 216, 870–875. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef]
- Duan, N.; Khoshnevisan, B.; Lin, C.; Liu, Z.; Liu, H. Life cycle assessment of anaerobic digestion of pig manure coupled with different digestate treatment technologies. Environ. Int. 2020, 137, 105522. [Google Scholar] [CrossRef]
- Jimenez, R.; Markou, G.; Tayibi, S.; Barakat, A.; Monlau, F. Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes. Appl. Sci. 2020, 10, 3890. [Google Scholar] [CrossRef]
- Chaudry, S. Integrating Microalgae Cultivation with Wastewater Treatment: A Peek into Economics. Appl. Biochem. Biotechnol. 2021, 193, 3395–3406. [Google Scholar] [CrossRef]
- Sharma, R.; Mishra, A.; Pant, D.; Malaviya, P. Recent advances in microalgae-based remediation of industrial and non-industrial wastewaters with simultaneous recovery of value-added products. Bioresour. Technol. 2022, 344, 126129. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Yuan, H.; Zhou, Q.; Gu, G. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007, 41, 683–689. [Google Scholar] [CrossRef]
- Chen, Y.D.; Li, S.; Ho, S.H.; Wang, C.; Lin, Y.C.; Nagarajan, D.; Chang, J.S.; Ren, N.Q. Integration of sludge digestion and microalgae cultivation for enhancing bioenergy and biorefinery. Renew. Sustain. Energy Rev. 2018, 96, 76–90. [Google Scholar] [CrossRef]
- Riau, V.; De la Rubia, M.A.; Pérez, M. Upgrading the temperature-phased anaerobic digestion of waste activated sludge by ultrasonic pretreatment. Chem. Eng. J. 2015, 259, 672–681. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.; Gong, Y.; Yu, Q.; Li, Q.; Sun, J.; Yuan, Z. Technologies for reducing sludge production in wastewater treatment plants: State of the art. Sci. Total Environ. 2017, 587–588, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, V.; Yla-Outinen, A.; Rintala, J. Mesophilic anaerobic digestion of pulp and paper industry biosludge–long-term reactor performance and effects of thermal pretreatment. Water Res. 2015, 87, 105–111. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Martín, M.; Chica, A.F.; Estévez-Pastor, F.; Toro-Baptista, E. Effect of microwave pretreatment on semi-continuous anaerobic digestion of sewage sludge. Renew. Energy 2018, 115, 917–925. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Q.L.; Jiang, G.M. Enhancing methane production from waste activated sludge using a novel indigenous iron activated peroxidation pre-treatment process. Bioresour. Technol. 2015, 182, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, S.; Icaran, P.; Yuan, Z.; Pijuan, M. Exploring alternatives to reduce economical costs associated with FNA pre-treatment of waste activated sludge. Bioresour. Technol. 2017, 243, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.W.; Yang, J.J.; Wu, B.D.; Liu, J.J.; Xu, X.Y.; Wu, W. Enhanced VFAs production from microalgal hydrolytic acidification with ultrasonic-alkali pretreatment. Algal Res. 2023, 71, 103056. [Google Scholar] [CrossRef]
- Jin, B.; Wang, S.; Xing, L.; Li, B.; Peng, Y. Long term effect of alkali types on waste activated sludge hydrolytic acidification and microbial community at low temperature. Bioresour. Technol. 2016, 200, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.L.; Lloréns, M.C.E. Effect of alkaline pretreatment on anaerobic digestion of solid wastes. Waste Manag. 2008, 28, 2229–2234. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hong, Y.; Zhai, Q.Y.; Zhao, G.P.; Zhang, H.K.; Wang, Q. Performance and mechanism of Chlorella in swine wastewater treatment: Roles of nitrogen-phosphorus ratio adjustment and indigenous bacteria. Bioresour. Technol. 2022, 358, 127402. [Google Scholar] [CrossRef]
- Feng, L.Y.; Chen, Y.Z.; Chen, X.T.; Duan, X.; Xie, J.; Chen, Y.G. Anaerobic accumulation of short-chain fatty acids from algae enhanced by damaging cell structure and promoting hydrolase activity. Bioresour. Technol. 2018, 250, 777–783. [Google Scholar] [CrossRef]
- Kabir, S.B.; Khalekuzzaman, M.; Hossain, N.; Jamal, M.; Alam, M.A.; Abomohra, E.F. Progress in biohythane production from microalgae-wastewater sludge co-digestion: An integrated biorefinery approach. Biotechnol. Adv. 2022, 57, 107933. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, H.L.; Li, C.; Peng, Y.Y.; Lu, M.M.; Jin, W.H.; Bao, J.J.; Guo, Y.M. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P. Biogas Production from Algae and Cyanobacteria Through Anaerobic Digestion: A Review, Analysis, and Research Needs. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 873–975. [Google Scholar] [CrossRef]
- Voon, C.P.; Law, Y.S.; Guan, X.Q.; Lim, S.L.; Xu, Z.; Chu, W.T.; Zhang, R.; Sun, F.; Labs, M.; Leister, D.; et al. Modulating the activities of chloroplasts and mitochondria promotes adenosine triphosphate production and plant growth. Energy Res. Manag. 2014, 2, e7. [Google Scholar] [CrossRef]
- Cherepanov, D.A.; Shelaev, I.V.; Gostev, F.E.; Petrova, A.; Semenov, A.Y. Primary charge separation within the structurally symmetric tetrameric Chl2APAPBChl2B chlorophyll exciplex in photosystem I. J. Photochem. Photobiol. B 2021, 217, 112154. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, G.O.; Libby, E.; Pineau, R.; Reinhard, C.T.; Ratcliff, W.C. Oxygen suppression of macroscopic multicellularity. Nat. Commun. 2021, 12, 2838. [Google Scholar] [CrossRef]
- Stablein, M.J.; Baracho, D.H.; Watson, J.T.; Silva, J.C.; Zhang, Y.; Lombardi, A.T. Microalgal photosynthetic inhibition and mixotrophic growth in Post Hydrothermal Liquefaction Wastewater (PHW). Algal Res. 2021, 60, 102548. [Google Scholar] [CrossRef]
- Joun, J.; Sirohi, R.; Sim, S.J. The effects of acetate and glucose on carbon fixation and carbon utilization in mixotrophy of Haematococcus pluvialis. Bioresour. Technol. 2023, 367, 128218. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef]
- Wang, J.H.; Yang, H.Z.; Wang, F. Mixotrophic Cultivation of Microalgae for Biodiesel Production: Status and Prospects. Appl. Biochem. Biotechnol. 2014, 172, 3307–3329. [Google Scholar] [CrossRef]
- Pooi, C.K.; Loka, V.; Ng, H.Y. Treatment and hybrid modeling of domestic reverse osmosis concentrate using biological activated carbon. Desalination 2019, 468, 114047. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ding, S.X.; Song, W.C.; Li, H.W.; Zhang, Y.H.; Ren, W.N.; Li, M.H.; Lu, J.; Ding, J.C. A collaborative effect of algae-bacteria symbiotic and biological activated carbon system on black odorous water pretreated by UV photolysis. Biochem. Eng. J. 2021, 169, 107983. [Google Scholar] [CrossRef]
- Wen, C.; Paul, W.; Leenheer, J.A.; Karl, B. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Qiu, Y.; Frear, C.; Chen, S.; Ndegwa, P.; Harrison, J.; Yao, Y.; Ma, J. Accumulation of long-chain fatty acids from Nannochloropsis salina enhanced by breaking microalgae cell wall under alkaline digestion. Renew. Energy 2020, 149, 691–700. [Google Scholar] [CrossRef]





| Parameter | Untreated | NaOH | US | US-NaOH |
|---|---|---|---|---|
| pH | 7.26 ± 0.03 | 6.96 ± 0.10 | 6.60 ± 0.01 | 6.83 ± 0.02 |
| N-NH4+ (mg/L) | 259.99 | 383.66 (47.57%) | 453.71 (74.51%) | 485.04 (86.56%) |
| TN (mg/L) | 379.50 | 544.15 (43.39%) | 619.35 (63.20%) | 799.65 (110.71%) |
| TP (mg/L) | 12.05 | 22.10 (83.40%) | 21.83 (81.16%) | 33.70 (179.67%) |
| COD (mg/L) | 2462.75 | 1899.38 (−22.88%) | 1963.00 (−20.29%) | 2726.25 (10.70%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hong, Y.; Liu, X. The Effect of Different Sludge Pretreatment Methods on Microalgae Performance and the Release of Pollutants in Hydrolysis Acidification Solutions. Water 2023, 15, 2873. https://doi.org/10.3390/w15162873
Wang X, Hong Y, Liu X. The Effect of Different Sludge Pretreatment Methods on Microalgae Performance and the Release of Pollutants in Hydrolysis Acidification Solutions. Water. 2023; 15(16):2873. https://doi.org/10.3390/w15162873
Chicago/Turabian StyleWang, Xiaoyan, Yu Hong, and Xiaoya Liu. 2023. "The Effect of Different Sludge Pretreatment Methods on Microalgae Performance and the Release of Pollutants in Hydrolysis Acidification Solutions" Water 15, no. 16: 2873. https://doi.org/10.3390/w15162873