High-Performance Catalytic Wet Oxidation of Excess Activated Sludge Derived from Pharmaceutical Wastewater Treatment Process over a Cu/γ-Al2O3 Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reaction System
2.3. Analysis
2.4. Preparation of Catalyst
3. Results and Discussion
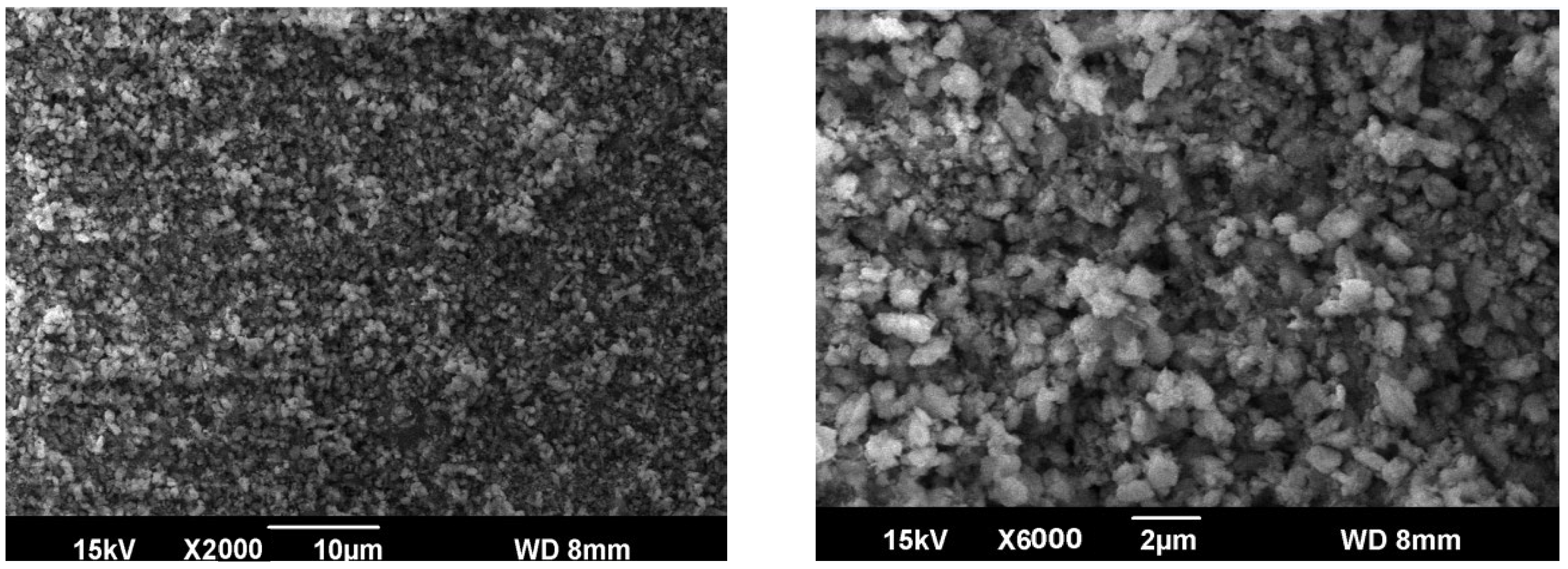
3.1. Characterization of the Catalyst
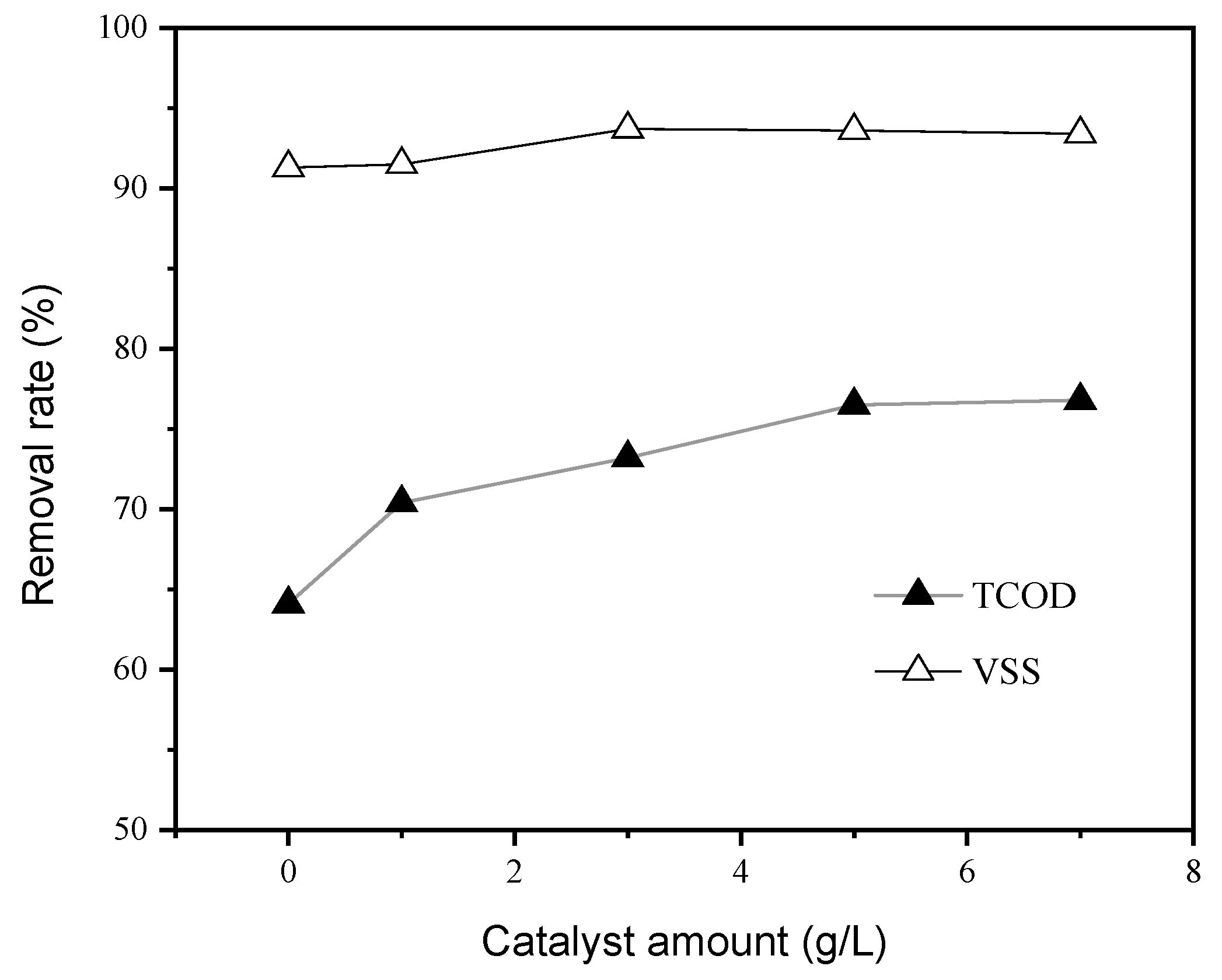
3.2. Effect of Catalyst Amount
3.3. Effect of Reaction Temperature
3.4. Effect of Reaction Time
3.5. Effect of Initial Oxygen Pressure
3.6. Production of VFAs
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arora, P.K.; Srivastava, A.; Singh, V.P. Diversity of 4-chloro-2-nitrophenol-degrading bacteria in a waste water sample. J. Chem. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yu, G.; Cao, Q.M.; Zhang, H.; Lin, Q.; Hong, Y. Occurrence and environmental implications of pharmaceuticals in Chinese municipal sewage sludge. Chemosphere 2013, 93, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J.Y. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Wouters, R.D.; Muraro, P.C.L.; Druzian, D.M.; Viana, A.R.; Pinto, E.d.O.; da Silva, J.K.L.; Vizzotto, B.S.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; et al. Zinc oxide nanoparticlles: Biosynthesis, characterization, biological activity and photocatalytic degradation for tartrazine yellow dye. J. Mol. Liq. 2023, 371, 121090. [Google Scholar] [CrossRef]
- Da Silva, W.L.; Leal, B.C.; Ziulkoski, A.L.; van Leeuwen, P.W.N.M.; dos Santos, J.H.Z.; Schrekker, H.S. Petrochemical residue-derived silica-supported titania-magnesium catalysts for the photocatalytic degradation of imidazolium ionic liquids in water. Sep. Puf. Technol. 2019, 218, 191–199. [Google Scholar] [CrossRef]
- Prince-Pike, A.; Wilson, D.I.; Baroutian, S.; Andrews, J.; Gapes, D.J. A kinetic model of municipal sludge degradation during non-catalytic wet oxidation. Water Res. 2015, 87, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Barbati, S.; Ricq, N.; Ambrosio, M. Intermediates in wet oxidation of cellulose: Identification of hydroxyl radical and characterization of hydrogen peroxide. Water Res. 2002, 36, 4821–4829. [Google Scholar] [CrossRef] [PubMed]
- Saeid, B.; Daniel, J.G.; Ajit, K.S.; Mohammed, M.F.; Brent, R.Y. Formation and degradation of valuable intermediate products during wet oxidation of municipal sludge. Biores. Technol. 2016, 205, 280–285. [Google Scholar]
- Strong, P.J.; McDonald, B.; Gapes, D.J. Combined thermochemical and fermentative destruction of municipal biosolids: A comparison between thermal hydrolysis and wet oxidative pre-treatment. Biores. Technol. 2011, 102, 5520–5526. [Google Scholar] [CrossRef]
- Gomes, H.; Figueiredo, J.; Faria, J.; Serp, P.; Kalck, P. Carbon-supported iridium catalysts in the catalytic wet air oxidation of carboxylic acids: Kinetics and mechanistic interpretation. J. Mol. Catal. A Chem. 2002, 182, 47–60. [Google Scholar] [CrossRef]
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes: A review. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Huang, K.; Xu, Y.; Wang, L.G.; Wu, D.F. Heterogeneous catalytic wet peroxide oxidation of simulated phenol wastewater by copper metal organic frameworks. RSC Adv. 2015, 5, 32795–32803. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Graphene-based materials for the catalytic wet peroxide oxidation of highly concentrated 4-nitrophenol solutions. Catal. Today 2015, 249, 204–212. [Google Scholar] [CrossRef]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Zhou, J.W.; Shangguan, W.F. Catalytic oxidation of low concentration formaldehyde with the assist of ozone over supported cobalt-manganese composite oxides. J. Mol. Catal. 2014, 1, 60–66. [Google Scholar]
- Chou, B.; Tsai, J.L.; Cheng, S. Cu-substituted molecular sieves as liquid phase oxidation catalysts. Microp. Mesop. Mater. 2001, 48, 309–317. [Google Scholar] [CrossRef]
- Taran, O.P.; Zagoruiko, A.N.; Ayusheev, A.B.; Yashnik, S.A.; Prihod’ko, R.V.; Ismagilov, Z.R.; Goncharuk, V.V.; Parmon, V.N. Cu and Fe-containing ZSM-5 zeolites as catalysts for wet peroxide oxidation of organic contaminants: Reaction kinetics. Res. Chem. Intermed. 2015, 41, 9521–9537. [Google Scholar] [CrossRef]
- Barbier, J.; Oliviero, L.; Renard, B.; Duprez, D. Role of ceria-supported noble metal catalysts (Ru, Pd, Pt) in wet air oxidation of nitrogen and oxygen containing compounds. Top. Catal. 2005, 33, 77–86. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhu, W.P.; Yang, S.X.; Wang, W.; Zhou, Y. Catalytic wet air oxidation of phenol with pelletized ruthenium catalysts. Appl. Catal. B Environ. 2008, 78, 30–37. [Google Scholar] [CrossRef]
- Kang, K.; Quitain, A.T.; Daimon, H.; Noda, R.; Goto, N.; Hu, H.Y.; Fujie, K. Optimization of amino acids production from waste fish entrails by hydrolysis in sub- and supercritical water. Can. J. Chem. Eng. 2001, 79, 65–70. [Google Scholar] [CrossRef]
- Gapes, D.J.; Stuthridge, T.R.; Strong, P.J.; Lei, R.J.; Aggrey, A.; James, S.P.; Raymond, S.T. Treating Biomass Comprises Subjecting Biomass to Microbial Digestion, Preferably Anaerobic Microbial Digestion to Produce Volatile Fatty Acids and/or Solvents followed by Wet Oxidation. NZ Patent WO2013128390-A1, 6 September 2013. [Google Scholar]
- Chung, J.; Lee, M.; Ahn, J.; Bae, W.; Lee, Y.W.; Shim, H. Effects of operational conditions on sludge degradation and organic acids formation in low-critical wet air oxidation. J. Hazard. Mater. 2009, 162, 10–16. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hao, O.J.; Olmstead, D.G.; Hsieh, K.P.; Scholze, R.J. Wet air oxidation of anaerobically digested-sludge. J. Water Pollut. Control Feder. 1987, 59, 39–46. [Google Scholar]
- Baroutian, S.; Smit, A.M.; Andrews, J.; Young, B.; Gapes, D. Hydrothermal degradation of organic matter in municipal sludge using non-catalytic wet oxidation. Chem. Eng. J. 2015, 260, 846–854. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, X.; Fang, K. Enhanced Wet Oxidation of Excess Sludge from Pharmaceutical Wastewater Treatment by NaOH. Catalysts 2023, 13, 1070. [Google Scholar] [CrossRef]
- Lipps, W.C.; Baxter, T.E.; Braun-Howland, E.B.; American Public Health Association; American Water Works Association. Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Public Health Association: Washington, DC, USA, 2023. [Google Scholar]
- Yu, C.Y.; Meng, X.; Chen, G.X.; Zhao, P.Q. Catalytic wet air oxidation of high concentration organic pollutants by upflow packed-bed reactor using a Ru-Ce catalyst derived from Ru3(CO)12 precursor. RSC Adv. 2016, 6, 22633–22638. [Google Scholar] [CrossRef]
- Liu, W.M.; Hu, Y.Q.; Tu, S.T. Ruthenium supported on active carbon-ceramic sphere as catalysts for catalytic wet air oxidation (CWAO) of resin effluent. J. Hazard. Mater. 2010, 179, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.M.; Zeng, X.; Jing, Z.Z.; Enomoto, H. A potentially useful technology by mimicking nature by rapid conversion of biomass and CO2 into chemicals and fuels under hydrothermal conditions. Ind. Eng. Chem. Res. 2012, 51, 9921–9937. [Google Scholar] [CrossRef]
- Shanableh, A.; Jomaa, S. Production and transformation of volatile fatty acids from sludge subjected to hydrothermal treatment. Water Sci. Technol. 2001, 44, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, P.; Earnest, F. Generalized kinetic model for wet oxidation of organic compounds. AICHE J. 1991, 37, 1678–1697. [Google Scholar] [CrossRef]
- Urrea, J.; Collado, S.; Oulego, P.; Díaz, M. Wet oxidation of the structural sludge fractions. J. Clean. Prod. 2017, 168, 1163–1170. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, J.; Zhao, J.F. Highly efficient treatment of pharmaceutical sludge by catalytic wet oxidation using CuO-CeO2/gamma-Al2O3 as a catalyst. PLoS ONE 2018, 13, e0199520. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, S.; Zeng, X.; Lin, H.; Zhu, Y. High-Performance Catalytic Wet Oxidation of Excess Activated Sludge Derived from Pharmaceutical Wastewater Treatment Process over a Cu/γ-Al2O3 Catalyst. Water 2023, 15, 3494. https://doi.org/10.3390/w15193494
Chu S, Zeng X, Lin H, Zhu Y. High-Performance Catalytic Wet Oxidation of Excess Activated Sludge Derived from Pharmaceutical Wastewater Treatment Process over a Cu/γ-Al2O3 Catalyst. Water. 2023; 15(19):3494. https://doi.org/10.3390/w15193494
Chicago/Turabian StyleChu, Shangye, Xu Zeng, Hai Lin, and Yuting Zhu. 2023. "High-Performance Catalytic Wet Oxidation of Excess Activated Sludge Derived from Pharmaceutical Wastewater Treatment Process over a Cu/γ-Al2O3 Catalyst" Water 15, no. 19: 3494. https://doi.org/10.3390/w15193494



