Survey on Lombardy Region Wastewater Effluents and Application of Biochar from Biological Sewage Sludge for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. WWTP Effluent Analysis
2.1.1. Monitoring Data
2.1.2. Regulatory Framework for WW Reuse
2.2. Sewage-Sludge-Derived Biochar
2.2.1. Biological Sewage Sludge
2.2.2. Biochar Preparation
2.2.3. Biochar Characterization and Analytical Methods
2.2.4. Adsorption Batch Experiments
Raw Wastewater
Experimental Procedures
- Search for the “optimal thermo-chemical conditions of pyrolysis”: a constant concentration for each biochar (approximately 4.0 gbiochar LWW−1) was used to identify the most effective biochar for the WW adsorption treatment. The different biochar samples were obtained from different thermochemical conditions during the biochar preparation.
- Identification of the “optimal biochar concentration”: different biochar concentrations (0.25–0.4–0.8–1.7–4.0–5.8 gbiochar LWW−1) were used to determine the optimal quantity for the adsorption treatment of the WW, for each of the most promising thermo-chemical treatments.
Analytical Procedures
3. Results and Discussion
3.1. WWTP Effluent Analysis
3.2. Powder Characterization
3.3. Adsorption Batch Experiments
3.3.1. Removal Yields as a Function of the Pyrolysis Operating Conditions
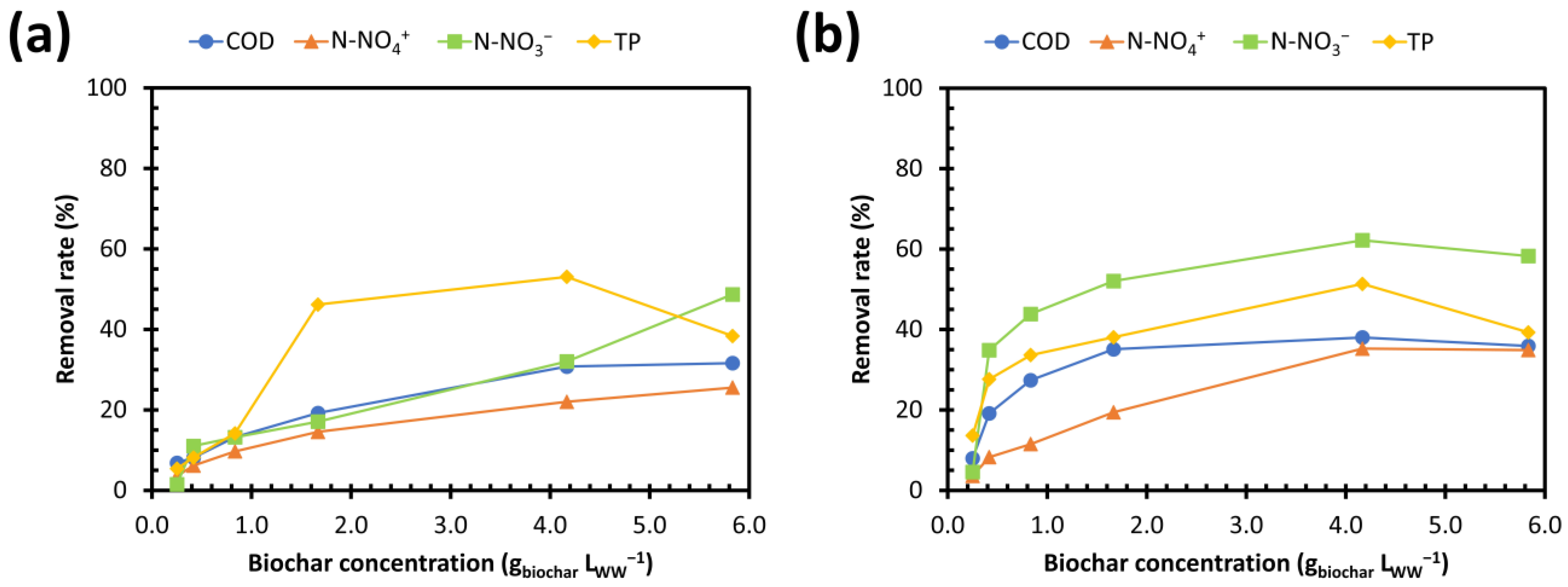
3.3.2. Removal Yields as a Function of Biochar Concentration
3.4. Future Outlooks
4. Conclusions
- (i)
- Pyrolysis at 650 °C + KOH activation for N-NH4+ (32%), P (44%), and N-NO3− (66%);
- (ii)
- Pyrolysis at 950 °C + KOH activation for COD (50%);
- (iii)
- A contact time of 6 h in a batch test for COD, N-NH4+, and P, and 3 h for N-NO3−;
- (iv)
- A concentration range of 4.2–5.8 gbiochar LWW−1 for the highest removal yields.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflectance |
| BOD5 | Biological oxygen demand at 5 days |
| COD | Chemical oxygen demand |
| PE | Population equivalent |
| PXRD | Powder X-ray diffraction |
| TGA | Thermogravimetric analysis |
| TN | Total nitrogen |
| TP | Total phosphorous |
| TSS | Total suspended solids |
| WW | Wastewater |
| WWTP | Wastewater treatment plant |
References
- Zhao, R.; Sun, H.; Xing, L.; Li, R.; Li, M. Effects of anthropogenic climate change on the drought characteristics in China: From frequency, duration, intensity, and affected area. J. Hydrol. 2023, 617, 129008. [Google Scholar] [CrossRef]
- Patra, S.; Shilky; Kumar, A.; Saikia, P. Impact of Land Use Systems and Climate Change on Water Resources: Indian Perspectives. In Advances in Water Resource Planning and Sustainability; Springer Nature: Singapore, 2023; pp. 97–110. [Google Scholar]
- Mahmoud, M.A. Impact of Climate Change on the Agricultural Sector in Egypt. In Conventional Water Resources and Agriculture in Egypt; Springer: Berlin/Heidelberg, Germany, 2017; pp. 213–227. [Google Scholar]
- Suo, X.; Yu, Z.; Cao, S. Utilizing water resources from the Tibetan Plateau: Benefits, risks, and future research requirements. Catena 2023, 230, 107256. [Google Scholar] [CrossRef]
- Marengo, J.A.; Galdos, M.V.; Challinor, A.; Cunha, A.P.; Marin, F.R.; Vianna, M.d.S.; Alvala, R.C.S.; Alves, L.M.; Moraes, O.L.; Bender, F. Drought in Northeast Brazil: A review of agricultural and policy adaptation options for food security. Clim. Resil. Sustain. 2022, 1, e17. [Google Scholar] [CrossRef]
- Karimi, V.; Karami, E.; Keshavarz, M. Climate change and agriculture: Impacts and adaptive responses in Iran. J. Integr. Agric. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Kilinc, E.A.; Tanik, A.; Hanedar, A.; Gorgun, E. Climate change adaptation exertions on the use of alternative water resources in Antalya, Türkiye. Front. Environ. Sci. 2023, 10, 162527. [Google Scholar] [CrossRef]
- Gil-Meseguer, E.; Bernabé-Crespo, M.B.; Gómez-Espín, J.M. Recycled Sewage—A Water Resource for Dry Regions of Southeastern Spain. Water Resour. Manag. 2019, 33, 725–737. [Google Scholar] [CrossRef]
- European Union Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R0741 (accessed on 14 July 2023).
- Vasantha Raman, N.; Dubey, A.; Millar, E.; Nava, V.; Leoni, B.; Gallego, I. Monitoring contaminants of emerging concern in aquatic systems through the lens of citizen science. Sci. Total Environ. 2023, 874, 162527. [Google Scholar] [CrossRef]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- United Nations Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 21 July 2023).
- Tarpani, R.R.Z.; Azapagic, A. Life cycle costs of advanced treatment techniques for wastewater reuse and resource recovery from sewage sludge. J. Clean. Prod. 2018, 204, 832–847. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Canato, M.; Abbà, A.; Carnevale Miino, M. Biosolids: What are the different types of reuse? J. Clean. Prod. 2019, 238, 117844. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef]
- Ifthikar, J.; Jiao, X.; Ngambia, A.; Wang, T.; Khan, A.; Jawad, A.; Xue, Q.; Liu, L.; Chen, Z. Facile One-Pot Synthesis of Sustainable Carboxymethyl Chitosan—Sewage Sludge Biochar for Effective Heavy Metal Chelation and Regeneration. Bioresour. Technol. 2018, 262, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Qian, Z.; Liu, J.; Geng, N.; Hou, J.; Li, D. Investigation on the adsorption of antibiotics from water by metal loaded sewage sludge biochar. Water Sci. Technol. 2021, 83, 739–750. [Google Scholar] [CrossRef]
- Campo, G.; Cerutti, A.; Lastella, C.; Leo, A.; Panepinto, D.; Zanetti, M.; Ruffino, B. Production and Destination of Sewage Sludge in the Piemonte Region (Italy): The Results of a Survey for a Future Sustainable Management. Int. J. Environ. Res. Public Health 2021, 18, 3556. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, L.; Luo, J.; Gong, H.; Zhu, N. A sustainable reuse strategy of converting waste activated sludge into biochar for contaminants removal from water: Modifications, applications and perspectives. J. Hazard. Mater. 2022, 438, 129437. [Google Scholar] [CrossRef]
- ISPRA Special Waste Report. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/rapporti/rapporto-rifiuti-speciali-edizione-2022 (accessed on 17 July 2023). (In Italian)
- Regkouzas, P.; Diamadopoulos, E. Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef]
- Godvin Sharmila, V.; Kumar Tyagi, V.; Varjani, S.; Rajesh Banu, J. A review on the lignocellulosic derived biochar-based catalyst in wastewater remediation: Advanced treatment technologies and machine learning tools. Bioresour. Technol. 2023, 387, 129587. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Norouzi, O.; Mašek, O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef]
- Singh, V.; Srivastava, V.C. Waste-derived biochar/carbon for various environmental and energy applications. In Green Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–363. [Google Scholar]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ambika, S.; Hassani, A.; Nidheesh, P.V. Waste to catalyst: Role of agricultural waste in water and wastewater treatment. Sci. Total Environ. 2023, 858, 159762. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; Boateng, A.A.; Klasson, K.T. Physicochemical and adsorptive properties of fast-pyrolysis bio-chars and their steam activated counterparts. J. Chem. Technol. Biotechnol. 2010, 85, 1515–1521. [Google Scholar] [CrossRef]
- Do Minh, T.; Song, J.; Deb, A.; Cha, L.; Srivastava, V.; Sillanpää, M. Biochar based catalysts for the abatement of emerging pollutants: A review. Chem. Eng. J. 2020, 394, 124856. [Google Scholar] [CrossRef]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment—A sustainability assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, H.; Lv, X.; Zhao, N.; Guo, G.; Zhang, D. Removal of phosphate from aqueous solution by dolomite-modified biochar derived from urban dewatered sewage sludge. Sci. Total Environ. 2019, 687, 460–469. [Google Scholar] [CrossRef]
- Shen, T.; Tang, Y.; Lu, X.Y.; Meng, Z. Mechanisms of copper stabilization by mineral constituents in sewage sludge biochar. J. Clean. Prod. 2018, 193, 185–193. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Liu, L.; Song, K.; Wang, Q. Combined physical and chemical activation of sludge-based adsorbent enhances Cr(Ⅵ) removal from wastewater. J. Clean. Prod. 2019, 238, 117904. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lombardy Region SIRe Acque. Available online: https://www.regione.lombardia.it/wps/portal/istituzionale/HP/DettaglioServizio/servizi-e-informazioni/Enti-e-Operatori/Territorio/governo-delle-acque/servizio-idrico-integrato/sire-acque-controllo-scarichi-impianti-trattamento-acque-reflue-urbane/sire-acq (accessed on 14 July 2023). (In Italian).
- Italian Republic DECREE 12 June 2003, No. 185. Regulation Containing TECHNICAL standards for the Reuse of Wastewater in Implementation of article 26, Paragraph 2, of the Legislative Decree 11 May 1999, n. 152. Available online: https://www.gazzettaufficiale.it/eli/id/2003/07/23/003G0210/sg (accessed on 14 July 2023).
- Lombardy Region Regional Regulation 29 March 2019, n. 6. Available online: https://www.regione.lombardia.it/wps/portal/istituzionale/HP/DettaglioRedazionale/servizi-e-informazioni/Enti-e-Operatori/territorio/governo-delle-acque/scarichi-acque-reflue-impianti-depurazione/scarichi-acque-reflue-impianti-depurazione (accessed on 18 July 2023). (In Italian).
- ASTM D3860-98(2020); Standard Practice for Determination of Adsorptive Capacity of Activated Carbon by Aqueous Phase Isotherm Technique. ASTM International: West Conshohocken, PA, USA, 2020.
- ISPRA. Measurement Procedure for the Determination of the Chemical Oxygen Demand (COD) by Cuvette Test: Method 5135; ISPRA: Rome, Italy, 2014; p. 88. (In Italian) [Google Scholar]
- APAT-IRSA-CNR; Analytical Methods for Water. Section 4000—Non-Metallic Inorganics. 4030—Ammonia Nitrogen. Non-Metallic Inorganic Constituents. Method A1—Spectrophotometric Determination of Indophenol. Italian Agency for the Protection of the Environment and for Technical Services: Rome, Italy, 2003. (In Italian)
- U.S. EPA. Method 300.1; Determination of Inorganic Anions in Drinking Water by Ion Chromatography—Revision 1.0. United States Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- U.S. EPA. Method 3051A (SW-846); Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils—Revision 1.0. United States Environmental Protection Agency: Washington, DC, USA, 2007.
- U.S. EPA. Method 6010D (SW-846); Inductively Coupled Plasma—Optical Emission Spectrometry—Revision 5.0. United States Environmental Protection Agency: Washington, DC, USA, 2018.
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent Organic Matter (EfOM) in Wastewater: Constituents, Effects, and Treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; He, Y.; Li, B.; Dong, Y.; Wang, L. Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour. Technol. 2019, 284, 333–339. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, Q. Preparation of adsorbents made from sewage sludges for adsorption of organic materials from wastewater. J. Hazard. Mater. 2006, 137, 359–366. [Google Scholar] [CrossRef]
- Hadi, P.; Xu, M.; Ning, C.; Sze Ki Lin, C.; McKay, G. A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 2015, 260, 895–906. [Google Scholar] [CrossRef]
- Martin, M.J.; Serra, E.; Ros, A.; Balaguer, M.D.; Rigola, M. Carbonaceous adsorbents from sewage sludge and their application in a combined activated sludge-powdered activated carbon (AS-PAC) treatment. Carbon 2004, 42, 1389–1394. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Elkhlifi, Z.; Sellaoui, L.; Zhao, M.; Ifthikar, J.; Jawad, A.; Shahib, I.I.; Sijilmassi, B.; Lahori, A.H.; Selvasembian, R.; Meili, L.; et al. Lanthanum hydroxide engineered sewage sludge biochar for efficient phosphate elimination: Mechanism interpretation using physical modelling. Sci. Total Environ. 2022, 803, 149888. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-R.; Choi, W.-J.; Kim, T.-J.; Kim, J.-S.; Oh, K.-J. The preparation of an adsorbent from mixtures of sewage sludge and coal-tar pitch using an alkaline hydroxide activation agent. J. Anal. Appl. Pyrolysis 2008, 83, 220–226. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Q.; Gu, W.; Li, S. Application of sludge-based biochar generated by pyrolysis: A mini review. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 00, 1–10. [Google Scholar]
- Rangabhashiyam, S.; Lins, P.V.d.S.; Oliveira, L.M.T.d.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: A critical review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
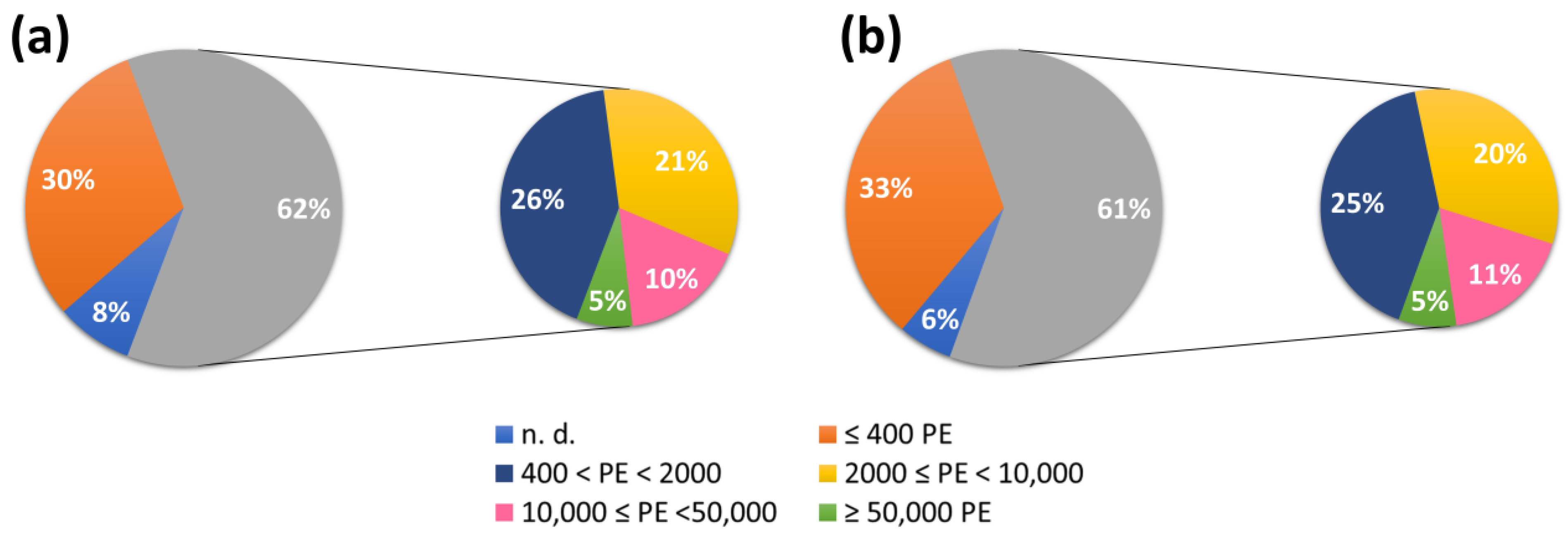
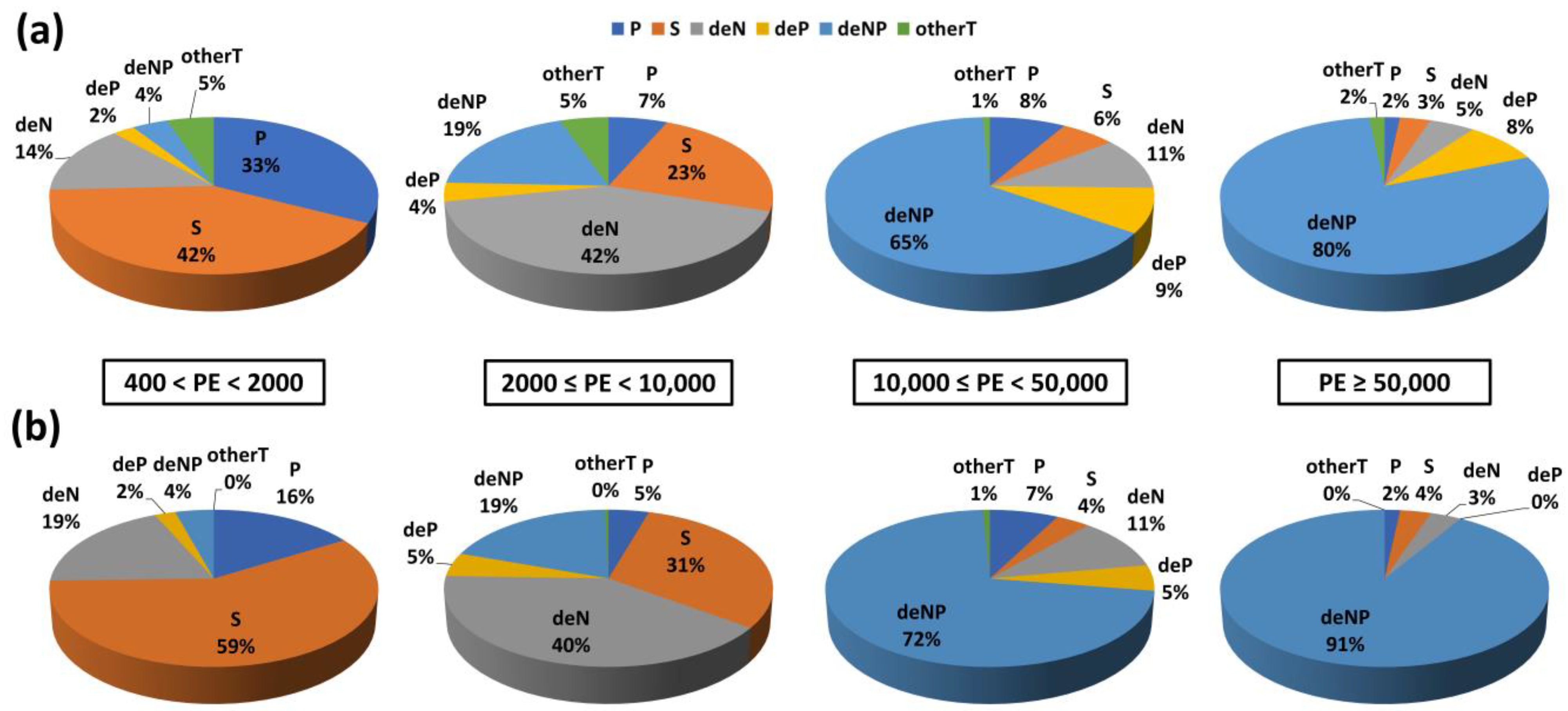




| Parameter | Source of the Legislation and Limit Value (mg L−1) | References |
|---|---|---|
| BOD5 | EU: 10 (*) | [9] |
| COD | Italy: 100 | [41] |
| Lombardy: 60; PE ≥ 50,000 | [42] | |
| TSS | EU, Italy: 10 (*) | [9,41] |
| TP | Italy: 2 | [41] |
| Lombardy: 1 (**) (PE ≥ 50,000) | [42] | |
| TN | Italy: 15 | [41] |
| Lombardy: 10 (PE ≥ 100,000) | [42] | |
| NH4 | Italy: 2 | [41] |
| Parameter | Mean Value | Confidence Interval |
|---|---|---|
| pH | 7.9 (n: 8) | ±0.2 |
| COD (mg L−1) | 362 (n: 8) | ±190 |
| N-NH4+ (mg L−1) | 24.4 (n: 8) | ±10.2 |
| N-NO3- (mg L−1) | 0.6 (n: 8) | ±0.1 |
| TP (mg L-1) | 4.3 (n: 8) | ±1.0 |
| Sample | Weight % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Na | Mg | Al | Si | P | S | Cl | K | Ca | Ti | Fe | |
| Dried sludge | 40.63 | 43.53 | 0.29 | 0.3 | 2.55 | 2.21 | 2.99 | 0.96 | - | 0.2 | 3.54 | - | 2.81 |
| Pyrolyzed at 350 °C | 34.86 | 37.36 | 0.28 | 0.65 | 5.5 | 4.58 | 5.94 | 0.8 | - | 0.49 | 5.09 | - | 4.28 |
| Pyrolyzed at 650 °C | 26.2 | 36.77 | 0.29 | 0.9 | 6.33 | 7.02 | 6.79 | 0.85 | 0.27 | 0.82 | 7.32 | 0.27 | 6.18 |
| Pyrolyzed at 950 °C | 24.51 | 34.53 | 0.43 | 0.89 | 7.15 | 7.14 | 7.97 | 0.84 | 0.38 | 0.96 | 9.97 | 0.4 | 4.85 |
| Pyrolyzed at 350 °C + activation | 8.71 | 47.97 | - | 0.22 | 9.27 | 12.81 | 1.5 | - | - | 14.48 | 2.34 | - | 2.69 |
| Pyrolyzed at 650 °C + activation | 18.4 | 46.42 | - | 0.27 | 13.65 | 10.38 | 0.33 | 0.33 | - | 7.06 | 1.57 | - | 1.6 |
| Pyrolyzed at 950 °C + activation | 18.6 | 43.43 | - | 0.28 | 13.4 | 6.24 | 5.09 | 0.26 | - | 8.05 | 1.33 | 0.23 | 3.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collivignarelli, M.C.; Caccamo, F.M.; Bellazzi, S.; Llamas, M.M.; Sorlini, S.; Milanese, C. Survey on Lombardy Region Wastewater Effluents and Application of Biochar from Biological Sewage Sludge for Wastewater Treatment. Water 2023, 15, 3636. https://doi.org/10.3390/w15203636
Collivignarelli MC, Caccamo FM, Bellazzi S, Llamas MM, Sorlini S, Milanese C. Survey on Lombardy Region Wastewater Effluents and Application of Biochar from Biological Sewage Sludge for Wastewater Treatment. Water. 2023; 15(20):3636. https://doi.org/10.3390/w15203636
Chicago/Turabian StyleCollivignarelli, Maria Cristina, Francesca Maria Caccamo, Stefano Bellazzi, Maria Medina Llamas, Sabrina Sorlini, and Chiara Milanese. 2023. "Survey on Lombardy Region Wastewater Effluents and Application of Biochar from Biological Sewage Sludge for Wastewater Treatment" Water 15, no. 20: 3636. https://doi.org/10.3390/w15203636






