River Habitat Survey: Does This Help to Explain the Nature of Water Mite (Acari and Hydrachnidia) Assemblages?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Sites, and Field Work
2.2. Laboratory Work
2.3. Statistical Analyses
3. Results
3.1. Hydromorphological Assessment
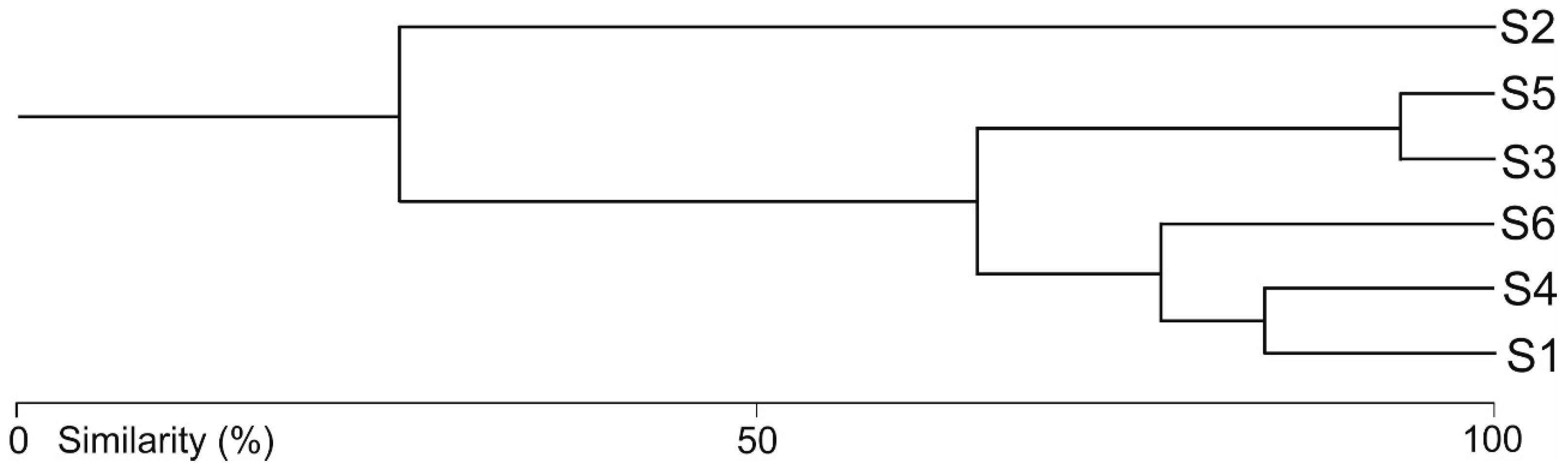
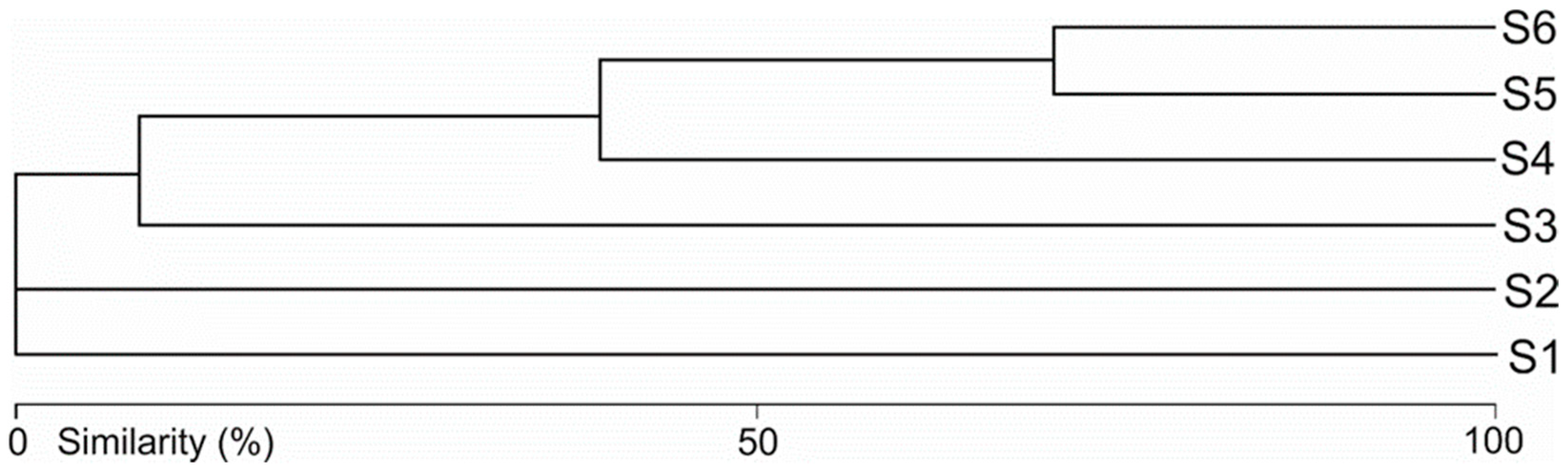
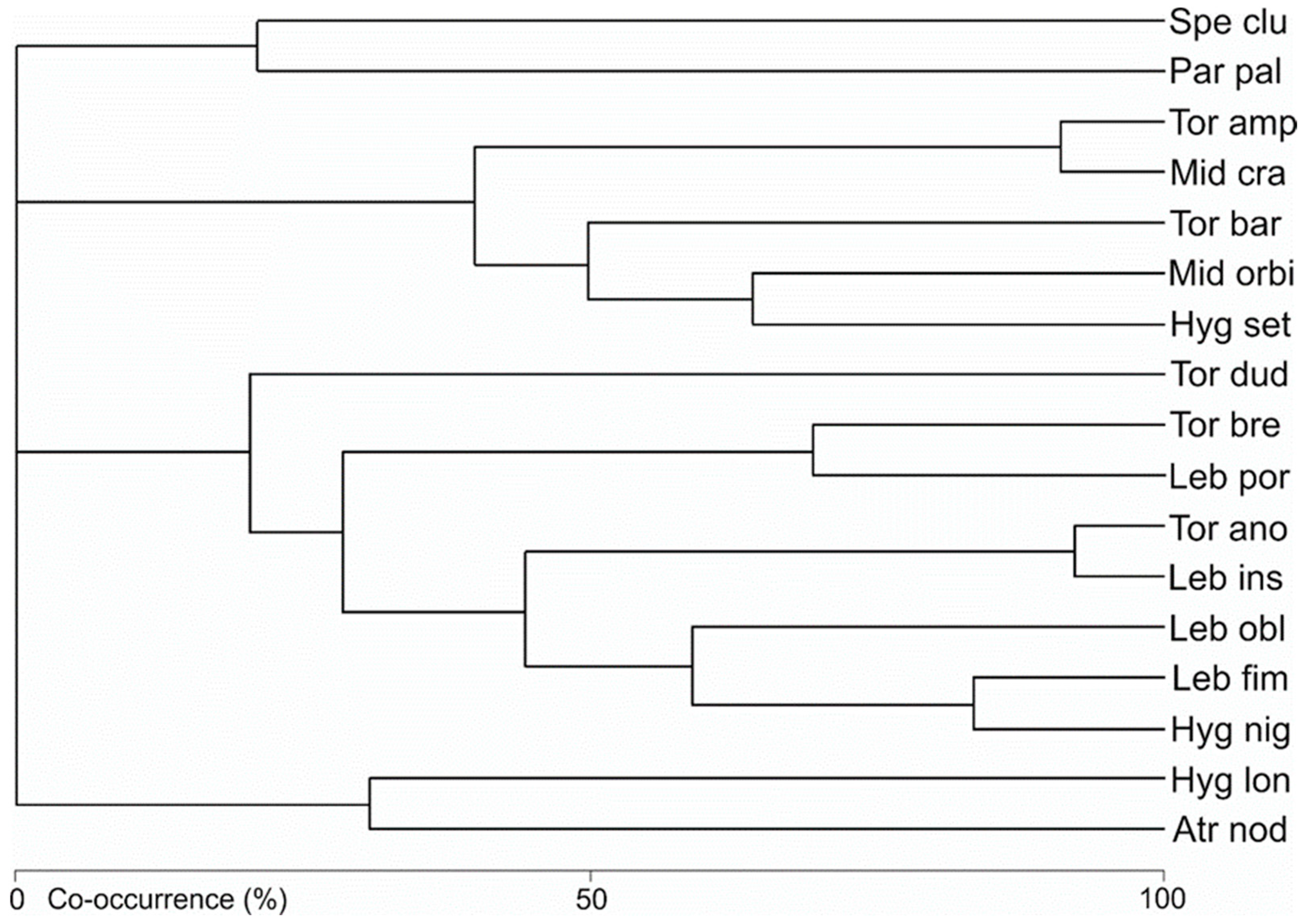
3.2. Water Mite Communities
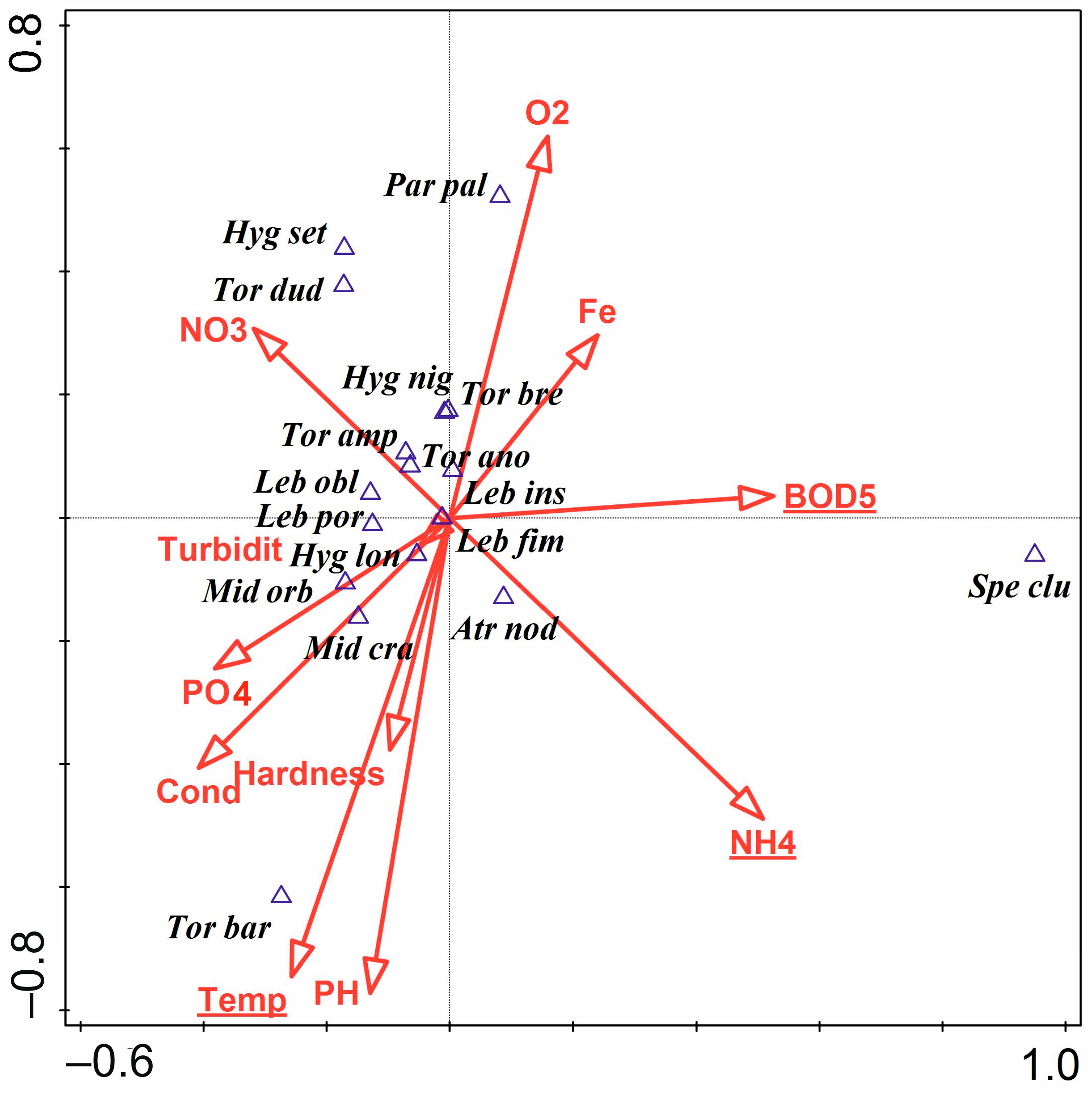
3.3. Water Mites and Environmental Factors
4. Discussion
5. Conclusions
- The biology and ecology of water mites make them no less suitable bioindicators of the environment—including hydromorphological modifications—than aquatic insects, commonly used for this purpose.
- The hydromorphological characteristics of the habitats, expressed as hydromorphological indices, explain the nature of water mite communities in the river at the level of general population indices (the number of individuals and species), whereas at the species level, especially in parts of the river that are not highly modified, general regularities in water mite fauna distribution in river ecosystems, the continuity of the river ecosystem, and characteristics at a smaller spatial scale (habitat scale) better explain water mite community structure than the hydromorphological indices determined for a given site or section of the river (without distinguishing specific habitats). However, these two components complement one another, and ultimately both of them together explain the nature of the water mite fauna in the river (Figure 10).
- More studies should be carried out to provide a better understanding of the connections and dependencies between water mite fauna, which is a very important element of multi-taxa invertebrate communities in lotic ecosystems, and the hydromorphological characteristics of the environment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moreyra, A.K.; Padovesi-Fonseca, C. Environmental effects and urban impacts on aquatic macroinvertebrates in a stream of central Brazilian Cerrado. Sustain. Water Resour. Manag. 2015, 1, 125–136. [Google Scholar] [CrossRef]
- Tampo, L.; Kaboré, I.; Alhassan, E.H.; Ouéda, A.; Bawa, L.M.; Djaneye-Boundjou, G. Benthic Macroinvertebrates as Ecological Indicators: Their Sensitivity to the Water Quality and Human Disturbances in a Tropical River. Front. Water 2021, 3, 662765. [Google Scholar] [CrossRef]
- Goertzen, D.; Schneider, A.-K.; Eggers, T.O.; Suhling, F. Temporal changes of biodiversity in urban running waters—Results of a twelve-year monitoring study. Basic Appl. Ecol. 2022, 58, 74–87. [Google Scholar] [CrossRef]
- Orozco-González, C.E.; Ocasio-Torres, M.E. Aquatic Macroinvertebrates as Bioindicators of Water Quality: A Study of an Ecosystem Regulation Service in a Tropical River. Ecologies 2023, 4, 209–228. [Google Scholar] [CrossRef]
- EU. Directive 2000/60/EC of the European parliament and of the council of 23 establishing a framework for community action in the field of water policy. Off. J. Commun. 2000, L327, 1–72. [Google Scholar]
- Raven, P.; Holmes, N.; Charrier, P.; Dawson, F.; Naura, M.; Boon, P. Towards a harmonized approach for hydromorphological assessment of rivers in Europe: A qualitative comparison of three survey methods. Aquat. Conserv. Mar. Freshw. Ecosyst. 2002, 12, 405–424. [Google Scholar] [CrossRef]
- Feld, C.K. Identification and measure of hydromorphological degradation in Central European lowland streams. Hydrobiologia 2004, 516, 69–90. [Google Scholar] [CrossRef]
- Lorenz, A.; Hering, D.; Feld, C.K.; Rolauffs, P. A new method for assessing the impact of hydromorphological degradation on the macroinvertebrate fauna of five German stream types. Hydrobiologia 2004, 516, 107–127. [Google Scholar] [CrossRef]
- Raven, P.J.; Fox, P.; Everard, M.; Holmes, H.T.H.; Dawson, F.H. River Habitat Survey: A new system for classifying rivers according to their habitat quality. In Freshwater Quality: Defining the Indefinable? Boon, P.J., Howell, D.L., Eds.; The Stationary Office: Edinburgh, UK, 1997; pp. 215–234. [Google Scholar]
- Raven, P.J.; Holmes, N.T.H.; Dawson, F.H.; Fox, P.J.A.; Everard, M.; Fozzard, I.R.; Rauen, K.J. River Habitat Quality, The Physical Character of Rivers and Streams in the UK and Isle of Man; River Habitat Survey No. 2; Environmental Agency: Bristol, UK, 1998; pp. 1–84.
- Tavzes, B.; Urbanič, G. New indices for assessment of hydromorphological alteration of rivers and their evaluation with benthic invertebrate communities; Alpine case study. Rev. Hydrobiol. 2009, 2, 133–161. [Google Scholar]
- Szoszkiewicz, K.; Gebler, D. Ocena warunków hydromorfologicznych rzek w Polsce metodą River Habitat Survey. Ochrona Środowiska i Zasobów Naturalnych 2011, 47, 70–81. [Google Scholar]
- Logan, P.; Furse, M. Preparing for the European Water Framework Directive? making the links between habitat and aquatic biota. Aquat. Conserv. Mar. Freshw. Ecosyst. 2002, 12, 425–437. [Google Scholar] [CrossRef]
- Cortes, R.M.V.; Varandas, S.; Hughes, S.J.; Ferreira, M.T. Combining habitat and biological characterization: Ecological validation of the river habitat survey. Limnetica 2008, 27, 39–56. [Google Scholar] [CrossRef]
- Lorenz, A.; Feld, C.K.; Hering, D. Typology of streams in Germany based on benthic invertebrates: Ecoregions, zonation, geology and substrate. Limnologica 2004, 34, 379–389. [Google Scholar] [CrossRef]
- Dahm, V.; Hering, D.; Nemitz, D.; Graf, W.; Schmidt-Kloiber, A.; Seebacher, A.; Leitner, P.; Melcher, A.; Feld, C.K. Effects of physico-chemistry, land use and hydromorphologyon three riverine organism groups: A comparative analysis with monitoring data from Germany and Austria. Hydrobiologia 2013, 704, 389–415. [Google Scholar] [CrossRef]
- Corneil, D.; Villeneuve, B.; Piffady, J.; Chandesris, A.; Usseglio-Polatera, P.; Souchon, Y. Introducing nested spatial scales in multi-stress models: Towards better assessment of human impacts on river ecosystems. Hydrobiologia 2018, 806, 347–361. [Google Scholar] [CrossRef]
- Knehtl, M.; Podgornik, S.; Urbanič, G. Scale-depended effects of hydromorphology and riparian land-use on benthic invertebrates and fish: Implications for large river management. Hydrobiologia 2021, 848, 3447–3467. [Google Scholar] [CrossRef]
- Meyer, A.; Grac, C.; Combroux, I.; Schmitt, L.; Trémolières, M. Biological feedback of unprecedented hydromorphological side channel restoration along the Upper Rhine (France). Hydrobiologia 2021, 848, 1593–1609. [Google Scholar] [CrossRef]
- Omoniyi, G.E.; Piscart, C.; Pellan, L.; Bergerot, B. Responses of Macroinvertebrate Communities to Hydromorphological Restoration of Headwater Streams in Brittany. Water 2022, 14, 553. [Google Scholar] [CrossRef]
- Erba, S.; Cazzola, M.; Belfiore, C.; Buffagni, A. Macroinvertebrate metrics responses to morphological alteration in Italian rivers. Hydrobiologia 2020, 847, 2169–2191. [Google Scholar] [CrossRef]
- Erba, S.; Buffagni, A.; Holmes, N.; O’hare, M.; Scarlett, P.; Stenico, A. Preliminary testing of River Habitat Survey features for the aims of the WFD hydro-morphological assessment: An overview from the STAR Project. Hydrobiologia 2006, 566, 281–296. [Google Scholar] [CrossRef]
- Johnson, R.K.; Hering, D.; Furse, M.T.; Clarke, R.T. Detection of ecological change using multiple organism groups: Metrics and uncertainty. Hydrobiologia 2006, 566, 115–137. [Google Scholar] [CrossRef]
- Tavzes, B.; Urbanič, G.; Toman, M.J. Biological and hydromorphological integrity of the small urban stream. Phys. Chem. Earth 2006, 31, 1062–1074. [Google Scholar] [CrossRef]
- Raven, P.J.; Holmes, N.T.; Vaughan, I.P.; Dawson, F.H.; Scarlett, P. Benchmarking habitat quality: Observations using River Habitat Survey on near-natural streams and rivers in northern and western Europe. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, S13–S30. [Google Scholar] [CrossRef]
- Kokeš, J. River channel habitat diversity (RCHD) and macroinvertebrate community. Biologia 2011, 66, 328–334. [Google Scholar] [CrossRef]
- Kalaninová, D.; Bulánková, E.; Šporka, F. Caddisfly assemblages of high mountain streams (The High Tatra Mts, Slovakia) influenced by a major windstorm event. Biologia 2013, 68, 501–509. [Google Scholar] [CrossRef]
- Urbanič, G. Hydromorphological degradation impact on benthic invertebrates in large rivers in Slovenia. Hydrobiologia 2014, 729, 191–207. [Google Scholar] [CrossRef]
- Lewin, I.; Czerniawska-Kusza, I.; Szoszkiewicz, K.; Ławniczak, A.E.; Jusik, S. Biological indices applied to benthic macroinvertebrates at reference conditions of mountain streams in two ecoregions (Poland, the Slovak Republic). Hydrobiologia 2013, 709, 183–200. [Google Scholar] [CrossRef]
- Lewin, I.; Jusik, S.; Szoszkiewicz, K.; Czerniawska-Kusza, I.; Ławniczak, A.E. Application of the new multimetric MMI_PL index for biological water quality assessment in reference and human-impacted streams (Poland, the Slovak Republic). Limnologica 2014, 49, 42–51. [Google Scholar] [CrossRef]
- Petkovska, V.; Urbanič, G. The links between river morphological variables and benthic invertebrate assemblages: Comparison among three European ecoregions. Aquat. Ecol. 2015, 49, 159–173. [Google Scholar] [CrossRef]
- Sabatino, A.; Gerecke, R.; Martin, P. The biology and ecology of lotic water mites (Hydrachnidia). Freshw. Biol. 2000, 44, 47–62. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Smit, H.; Gerecke, R.; Goldschmidt, T.; Matsumoto, N.; Cicolani, B. Global diversity of water mites (Acari, Hydrachnidia; Arachnida) in freshwater. Hydrobiologia 2008, 595, 303–315. [Google Scholar] [CrossRef]
- Smith, I.M.; Cook, D.R.; Smith, B.P. Chapter 15. Water mites (Hydrachnidiae) and other Arachnids. In Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Thorp, J.H., Covich, A.P., Eds.; Academic Press: London, UK, 2010; pp. 485–586. [Google Scholar]
- Stryjecki, R.; Zawal, A.; Krepski, T.; Stępień, E.; Buczyńska, E.; Buczyński, P.; Czachorowski, S.; Jankowiak, Ł.; Pakulnicka, J.; Sulikowska-Drozd, A.; et al. Anthropogenic transformations of river ecosystems are not always bad for the environment: Multi-taxa analyses of changes in aquatic and terrestrial environments after dredging of a small lowland river. PeerJ 2011, 9, e122242021. [Google Scholar] [CrossRef]
- Davids, C.; Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H.; van der Hammen, H. Acari: Hydrachnidia, I. In Freshwater Fauna of Central Europe; Gerecke, R., Ed.; Spektrum Akademischer Verlag: München, Germany, 2007; Volume 7/2-1, pp. 241–388. [Google Scholar]
- Martin, P. Water mites (Hydrachnidia, Acari) as predators in lotic environments. Phytophaga 2005, 14, 307–321. [Google Scholar]
- Matveev, V.T.; Martinez, C.C. Can water mites control populations of planktonic Cladocera? Hydrobiologia 1990, 198, 227–231. [Google Scholar] [CrossRef]
- Proctor, H.C.; Garga, N. Red, distasteful water mites: Did fish make them that way? Exp. Appl. Acarol. 2004, 34, 127–147. [Google Scholar] [CrossRef]
- Goldschmidt, T. Water mites (Acari, Hydrachnidia): Powerful but widely neglected bioindicators—A review. Neotrop. Biodivers. 2016, 2, 12–25. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I.; Szoszkiewicz, K. Biologiczna i Hydromorfologiczna Ocena wód Płynących na Przykładzie Rzeki Mała Panew; Katedra Ochrony Powierzchni Ziemi, Uniwersytet Opolski: Opole, Poland, 2007. [Google Scholar]
- Zawal, A.; Stryjecki, R.; Stępień, E.; Buczyńska, E.; Buczyński, P.; Czachorowski, S.; Pakulnicka, J.; Śmietana, P. The influence of environmental factors on water mite assemblages (Acari, Hydrachnidia) in a small lowland river—An analysis at different levels of organization of the environment. Limnology 2017, 18, 333–343. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Gerecke, R.; Gledhill, T.; Smit, H. Chelicerata: Acari II. In Freshwater Fauna of Central Europe; Gerecke, R., Ed.; Spektrum Akademischer Verlag: München, Germany, 2010; Volume 7/2–2, pp. 1–234. [Google Scholar]
- Gerecke, R.; Gledhill, T.; Pešić, V.; Smit, H. Chelicerata: Acari III. In Freshwater Fauna of Central Europe; Gerecke, R., Ed.; Spektrum Akademischer Verlag: München, Germany, 2010; Volume 7, pp. 1–429. [Google Scholar]
- Smit, H.; van der Hammen, H. Atlas of the Dutch water mites (Acari: Hydrachnidia). Ned. Faunist. Mededel. 2000, 13, 1–266. (In Dutch) [Google Scholar]
- Biesiadka, E. Wodopójki (Hydrachnidia). In Fauna of Poland—Characteristic and Checklist of Species; Bogdanowicz, W., Chudzicka, E., Pilipiuk, J., Skibińska, E., Eds.; Fauna Polski—Charakterystyka i Wykaz Gatunków; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 2008; Volume 3, pp. 149–219. [Google Scholar]
- Gerecke, R.; Martin, P.; Gledhill, T. Water mites (Acari: Parasitengona: Hydrachnidia) as inhabitants of groundwater-influenced habitats—Considerations following an update of Limnofauna Europaea. Limnologica 2018, 69, 81–93. [Google Scholar] [CrossRef]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software; Jointly Developed by the Scottish Association for Marine Science and the Natural History Museum London. 1997. Available online: https://www.sams.ac.uk/science/outputs/ (accessed on 24 October 2023).
- O’Hara, R.; Kotze, J. Do not log-transform count data. Nat. Précéd. 2010, 9, e12224. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination; Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Buffagni, A.; Crosa, G.A.; Harper, D.M.; Kemp, J.L. Using macroinvertebrate species assemblages to identify river channel habitat units: An application of the functional habitats concept to a large, unpolluted Italian river (River Ticino, Northern Italy). Hydrobiologia 2000, 435, 213–225. [Google Scholar] [CrossRef]
- Beavan, L.; Sadler, J.; Pinder, C. The invertebrate fauna of a physically modified urban river. Hydrobiologia 2001, 445, 97–108. [Google Scholar] [CrossRef]
- Kowalik, W.; Biesiadka, E. Occurence of water mites (Hydracarina) in the river Wieprz polluted with domesticindustry sewage. Acta Hydrobiol. 1981, 23, 331–348. [Google Scholar]
- Cicolani, B.; Di Sabatino, A. Sensitivity of water mites to water pollution. In Modern Acarology; Dusbábek, F., Bukva, V., Eds.; Academia: Prague, Czech Republic; SPB Academic Publishing bv: The Hague, The Netherlands, 1991; Volume 1, pp. 465–474. [Google Scholar]
- Gerecke, R.; Schwoerbel, J. Water quality and water mites (Acari, Actinedida) in the upper Danube region, 1959–1984. In Modern Acarology; Dusbábek, F., Bukva, V., Eds.; Academia: Prague, Czech Republic; SPB Academic Publishing bv: The Hague, The Netherlands, 1991; Volume 1, pp. 483–491. [Google Scholar]
- Hammen, H.; Smit, H. The water mites (Acari: Hydrachnidia) of streams in The Netherlands: Distribution and ecological aspects on a regional scale. Aquat. Ecol. 1996, 30, 175–185. [Google Scholar] [CrossRef]
- Zawal, A.; Stępień, E.; Szlauer-Łukaszewska, A.; Michoński, G.; Kłosowska, M.; Bańkowska, A.; Myśliwy, M.; Stryjecki, R.; Buczyńska, E.; Buczyński, P. The influence of dredging of a lowland river (the Krąpiel in NW Poland) on water mite fauna (Acari: Hydrachnidia). Fund. Appl. Limnol. 2015, 186, 217–232. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedel, J.R.; Cushing, C.E. River continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Schwoerbel, J. Die Wassermilben (Hydrachnellae und Limnohalacaridae) als Indikatoren einer biozönotischen Gliederung von Breg und Brigach sowie der obersten Donau. Arch. Hydrobiol, Suppl. 1964, 27, 386–417. [Google Scholar]
- Biesiadka, E. Hydracarina of the river Raba and some of its tributaries. Acta Hydrobiol. 1974, 16, 31–50. [Google Scholar]
- Angelier, E.; Angelier, M.-L.; Lauga, J. Recherches sur l’écologie des Hydracariens (Hydrachnellae, Acari) dans les eaux courantes. Ann. Limnol. 1985, 21, 25–64. [Google Scholar] [CrossRef]
- Cichocka, M. Wodopójki (Hydracarina) rzeki Pasłęki. Fragm. Faun. 1996, 39, 179–205. [Google Scholar] [CrossRef]
- Cichocka, M. Water mites (Hydrachnidia, Acari) in the running waters of the Masurian Landscape Park. Suppl. ad Acta Hydrobiol. 2006, 8, 33–53. [Google Scholar]
- Stryjecki, R.; Bańkowska, A. A faunistic and ecological characterization of the water mites (Acari: Hydrachnidia) of the Bukowa River (central-eastern Poland). Acta Biol. 2018, 25, 77–94. [Google Scholar] [CrossRef]
- Vidoša, T.; Pozojević, I.; Maoduš, I.V.; Mihaljević, Z. Longitudinal Changes in Diverse Assemblages of Water Mites (Hydrachnidia) along a Lowland River in Croatia. Diversity 2023, 15, 139. [Google Scholar] [CrossRef]
- Martin, P. Diel and seasonal drift of water mites (Acari, Hydrachnidia) in two streams of the North German Lowland. In Ecology and Evolution of the Acari; Series Entomologica; Bruin, J., van der Geest, L.P.S., Sabelis, M.W., Eds.; Springer: Dordrecht, The Netherlands, 1999; Volume 55, pp. 451–457. [Google Scholar]
- Biesiadka, E. Wodopójki (Hydracarina) dolnego biegu rzeki Wełny. Fragm. Faun. 1970, 5, 43–55. [Google Scholar] [CrossRef]
- Biesiadka, E. Wodopójki (Hydracarina) Pienin. Fragm. Faun. 1979, 24, 97–173. [Google Scholar] [CrossRef]
- Martin, P. Faunistisch-ökologische Benthosstudien an den Wassermilben (Hydrachnidia, Acari) zweier Bäche des Norddeutschen Tieflandes (Ostholsteinisches Hügelland, Schleswig-Holstein). Faun.-Ökol. Mitteil. 1996, 7, 153–167. [Google Scholar]
- Stryjecki, R. A faunistic and ecological characterization of the water mites (Acari: Hydrachnidia) of the Branew River (central-eastern Poland). Acta Biol. 2019, 26, 99–115. [Google Scholar] [CrossRef]
- Brookes, A.; Gregory, K.J. Channelisation, river engineering and geomorphology. In Geomorphology in Environmental Planning; Hooke, J.M., Ed.; John Wiley & Sons: Chichester, UK, 1998; pp. 68–145. [Google Scholar]
- Pedersen, E.R.; Perkins, M.A. The use of benthic invertebrate data for evaluating impacts of urban runoff. Hydrobiologia 1986, 139, 13–22. [Google Scholar] [CrossRef]
- Giller, P.S.; Malmqvist, B. The Biology of Streams and Rivers; Oxford University Press: Oxford, UK, 1998; pp. 1–296. [Google Scholar]
- Bis, B.; Higler, L.W.G. Riparian vegetation of streams and the macroinvertebrate community structure. Int. J. Ecohydrol. Hydrobiol. 2001, 1, 253–260. [Google Scholar]
- Sponseller, A.R.; Benfield, F.E.; Valett, H.M. Relationship between land use, spatial scale and stream macroinvertebrate communities. Freshw. Biol. 2001, 46, 1409–1424. [Google Scholar] [CrossRef]
- Sandin, L.; Johnson, R.K. Local, landscape and regional factors structuring benthic macroinvertebrate assemblages in Swedish streams. Landsc. Ecol. 2004, 19, 501–515. [Google Scholar] [CrossRef]
- Bottger, K. Types of parasitism by larvae of water mites (Acari: Hydrachnellae). Freshw. Biol. 1976, 6, 497–500. [Google Scholar] [CrossRef]
- Schwoerbel, J. Die Bedeutung der Wassermilben für die biozönotische Gliederung. Verh. Int. Ver. Limnol. 1961, 14, 355–361. [Google Scholar] [CrossRef]
- Statzner, B.; Gore, J.A.; Resh, V.H. Hydraulic Stream Ecology: Observed Patterns and Potential Applications. J. N. Am. Benthol. Soc. 1988, 7, 307–360. [Google Scholar] [CrossRef]
- Brooks, N.; Adger, W.N.; Kelly, P.M. The determinants of vulnerability and adaptive capacity at the national level and the implications for adaptation. Glob. Environ. Chang. 2005, 15, 151–163. [Google Scholar] [CrossRef]
- Gerecke, R. The water mites (Acari, Hydrachnidia) of a little disturbed forest stream in southwest Germany—A study on seasonality and habitat preference, with remarks on diversity patterns in different geographical areas. In Acarid Phylogeny and Evolution; Adaptations in Mites and Ticks; Bernini, F., Nannelli, R., Nuzzaci, G., de Lillo, E., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 69–89. [Google Scholar]
- Tickner, D.; Armitage, P.D.; Bickerton, M.A.; Hall, K.A. Assessing stream quality using information on mesohabitat distribution and character. Aquat. Conserv. 2000, 10, 179–196. [Google Scholar] [CrossRef]
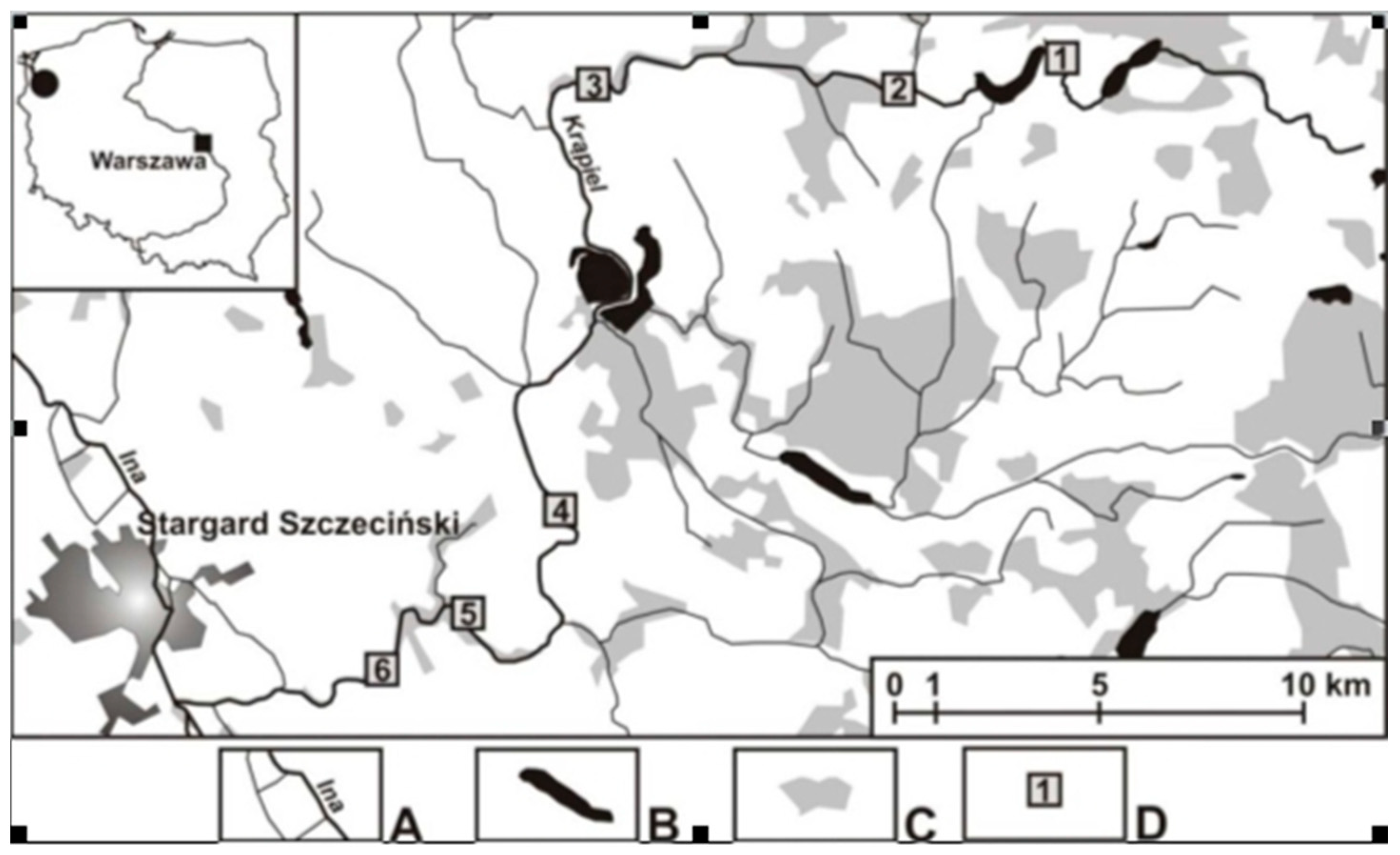





| S/h | Type | Depth | Flow [m/s] | River Width | Organic Matter | Mineral Matter | Surroundings | Bottom | Vegetation/Remarks |
|---|---|---|---|---|---|---|---|---|---|
| [m] | [m] | ||||||||
| S1/1 | R | 0.2 | 0.16–0.18 | 1.0 | 0.55–0.58 | 99.42–99.45 | alder carr | sand | |
| S1/2 | R | 0.2 | 0.38–0.47 | 0.11–0.46 | 99.54–99.89 | stones | |||
| S1/3 | P | 0.5 | 0.001–0.003 | 2.49–2.78 | 97.22–97.51 | sand, silt, mud | |||
| S1/4 | R | 0.2 | 0.48–0.62 | 0.01–0.10 | 99.90–99.99 | rocks | mosses, algae | ||
| S1/5 | R | 0.2 | 0.24–0.31 | 0.35–0.43 | 99.57–99.65 | gravel, sand | |||
| S1/6 | P | 0.4 | 0.005–0.01 | 0.87–1.23 | 98.77–99.13 | sand, silt | absence of water mites | ||
| S2/1 | R | 1.2 | 0.35–0.42 | 4.0 | 0.45–0.58 | 99.42–99.55 | alder carr, willow thickets | gravel, sand | absence of water mites |
| S2/2 | P | 0.5 | 0.02–0.04 | 32.70–36.30 | 63.70–67.30 | silt, mud | Phragmites australis (Cav.) Trin. ex Steud. | ||
| S2/3 | P | 0.5 | 0.05–0.07 | 1.41–1.84 | 98.16–98.59 | silt, mud | |||
| S3/1 | R | 0.2 | 0.2–0.36 | 7.0 | 1.21–2.49 | 97.51–98.79 | alder carr, willow thickets | gravel, sand, mud, leaves | |
| S3/2 | R | 0.5 | 0.41–0.57 | 0.15–0.19 | 99.81–99.85 | gravel | Sparganium emersum Rehmann absence of water mites | ||
| S3/3 | R | 0.7 | 0.31–0.36 | 0.01 | 99.99 | rocks, stones | Fontinalis antipyretica Hedw. | ||
| S3/4 | P | 0.2 | 0.05–0.06 | 1.58–2.23 | 97.77–98.42 | sand, mud | |||
| S3/5 | P | 0.1 | 0.01–0.02 | 1.23–1.31 | 98.69–98.77 | sand, mud | Mentha aquatica L., Carex acutiformis Ehrh. | ||
| S4/1 | R | 0.4 | 0.28–0.38 | 5.0 | 1.78–2.89 | 97.11–98.22 | alluvial forests with Alnus glutinosa and Fraxinus excelsior | sand, leaves | grasses |
| S4/2 | P | 0.2 | 0.1–0.12 | 32.20–35.30 | 64.70–67.80 | mud | Glyceria maxima (Hartm.) Holmb. | ||
| S4/3 | R | 0.5 | 0.12–0.18 | 0.82–0.88 | 99.12–99.18 | sand, gravel | |||
| S4/4 | P | 0.2 | 0.01–0.02 | 1.13–1.59 | 98.41–98.87 | sand, mud | Sagittaria sagittifolia L. | ||
| S4/5 | R | 0.4 | 0.2–0.22 | 0.59–0.78 | 99.22–99.41 | sand, mud | Sagittaria sagittifolia L. | ||
| S4/6 | R | 0.7 | 0.27–0.31 | 4.50–4.84 | 95.16–95.50 | sand, mud | |||
| S5/1 | R | 0.7 | 0.2–0.4 | 10.0 | 0.98–9.39 | 90.61–99.02 | oak–hornbeam stands | sand, leaves | |
| S5/2 | P | 0.2 | 0.01 | 21.00–23.40 | 76.60–79.00 | sand, mud | |||
| S5/3 | R | 0.5 | 0.43–0.57 | 0.73–0.76 | 99.24–99.27 | gravel, stones | |||
| S6/1 | R | 0.7 | 0.18–0.35 | 10.0 | 0.29–0.35 | 99.65–99.71 | alder carr | sand | |
| S6/2 | P | 0.2 | 0.07–0.12 | 0.88–1.10 | 98.90–99.12 | sand, mud | sedges | ||
| S6/3 | R | 0.7 | 0.34–0.54 | 1.35–3.63 | 96.37–98.65 | rocks | |||
| S6/4 | P | 0.4 | 0.05–0.16 | 0.28–1.25 | 98.75–99.72 | sand, mud |
| Study Site | HQA | HMS | RHQ | RHM | RHS Class |
|---|---|---|---|---|---|
| S1 | 53 | 24 | 135,50 | 14,5 | 4 |
| S2 | 22 | 34 | 86,31 | 35,2 | 5 |
| S3 | 55 | 0 | 131,23 | 0 | 2 |
| S4 | 47 | 13 | 137,85 | 5,8 | 3 |
| S5 | 57 | 0 | 125,35 | 0 | 1 |
| S6 | 56 | 15 | 143,94 | 25,4 | 3 |
| Species | SG | S1 | S2 | S3 | S4 | S5 | S6 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | R | P | R | P | ||||
| 1. | Eylais degenerata Koenike, 1897 | lb | 1 | 1 | |||||||||||
| 2. | Parathyas palustris (Koenike, 1912) | cr | 9 | 9 | |||||||||||
| 3. | Hydryphantes hellichi Thon, 1899 | lb | 1 | 1 | |||||||||||
| 4. | Lebertia fimbriata Thor, 1899 | rb | 1 | 6 | 7 | ||||||||||
| 5. | Lebertia inaequalis (Koch, 1837) | rf | 1 | 2 | 3 | ||||||||||
| 6. | Lebertia insignis Neuman, 1880 | rf | 2 | 1 | 4 | 7 | |||||||||
| 7. | Lebertia oblonga Koenike, 1911 | rb | 4 | 2 | 6 | 12 | |||||||||
| 8. | Lebertia porosa Thor, 1900 | rf | 3 | 4 | 3 | 1 | 5 | 11 | 27 | ||||||
| 9. | Lebertia pusilla Koenike, 1911 | rb | 2 | 2 | |||||||||||
| - | Lebertia sp. Neuman, 1880 | - | 1 | 2 | 1 | 4 | 8 | ||||||||
| 10. | Sperchon clupeifer Piersig, 1896 | rb | 62 | 4 | 3 | 3 | 4 | 76 | |||||||
| 11. | Sperchon setiger Thor, 1898 | rb | 2 | 2 | |||||||||||
| 12. | Sperchon thienemanni Koenike, 1907 | cr | 2 | 2 | |||||||||||
| - | Sperchon sp. Kramer, 1877 | - | 1 | 1 | 2 | ||||||||||
| 13. | Sperchonopsis verrucosa (Protz, 1896) | rb | 1 | 1 | |||||||||||
| 14. | Torrenticola amplexa (Koenike, 1908) | rb | 1 | 35 | 1 | 58 | 8 | 31 | 30 | 164 | |||||
| 15. | Torrenticola anomala (Koch, 1837) | rb | 2 | 1 | 3 | 6 | |||||||||
| 16. | Torrenticola barsica (Szalay, 1933) | rb | 43 | 43 | |||||||||||
| 17. | Torrenticola brevirostris (Halbert, 1911) | rb | 1 | 14 | 7 | 22 | |||||||||
| 18. | Torrenticola dudichi (Szalay, 1933) | rb | 14 | 3 | 17 | ||||||||||
| 19. | Torrenticola similis (K. Viets, 1939) | rb | 1 | 1 | |||||||||||
| - | Torrenticola sp. Piersig, 1896 | - | 2 | 3 | 3 | 8 | |||||||||
| 20. | Albia stationis Thon, 1899 | rb | 1 | 1 | 2 | ||||||||||
| 21. | Aturus scaber Kramer, 1875 | rb | 2 | 2 | |||||||||||
| 22. | Parabrachypoda montii (Maglio, 1924) | lb | 2 | 2 | |||||||||||
| 23. | Atractides nodipalpis Thor, 1899 | rb | 18 | 1 | 19 | ||||||||||
| 24. | Atractides neumani (Lundblad, 1962) | rb | 1 | 1 | |||||||||||
| 25. | Hygrobates fluviatilis (Ström, 1768) | rf | 1 | 1 | 2 | ||||||||||
| 26. | Hygrobates longipalpis (Hermann, 1804) | rf | 1 | 4 | 2 | 7 | |||||||||
| 27. | Hygrobates longiporus Thor, 1898 | rf | 1 | 1 | |||||||||||
| 28. | Hygrobates nigromaculatus Lebert, 1879 | lb | 5 | 5 | |||||||||||
| 29. | Hygrobates setosus Besseling, 1942 | rf | 2 | 12 | 1 | 32 | 1 | 20 | 68 | ||||||
| - | Hygrobates sp. Koch, 1837 | - | 5 | 5 | |||||||||||
| 30. | Limnesia maculata (Müller, 1776) | lb | 1 | 1 | |||||||||||
| 31. | Forelia variegator (Koch, 1837) | lb | 1 | 1 | |||||||||||
| - | Piona sp. Koch, 1842 | - | 1 | 1 | 2 | ||||||||||
| 32. | Neumania callosa (Koenike, 1895) | lb | 1 | 1 | |||||||||||
| 33. | Neumania papillosa (Soar, 1902) | rf | 2 | 2 | |||||||||||
| 34. | Mideopsis crassipes Soar, 1904 | rf | 8 | 12 | 4 | 71 | 6 | 58 | 159 | ||||||
| 35. | Mideopsis orbicularis (Müller, 1776) | lb | 1 | 1 | 5 | 21 | 3 | 10 | 41 | ||||||
| In-habitat specimens | 73 | 4 | 0 | 3 | 7 | 17 | 83 | 26 | 141 | 138 | 84 | 166 | 742 | ||
| species | 3 | 1 | 0 | 2 | 4 | 5 | 10 | 6 | 12 | 9 | 14 | 13 | |||
| At-site specimens | 77 | 3 | 24 | 109 | 279 | 250 | 742 | ||||||||
| species | 3 | 2 | 6 | 12 | 15 | 20 | |||||||||
| Water Mite Zone | Site | Distance from the Source (km) |
|---|---|---|
| Sperchon | 1 | 11.2 |
| - | 2 | 19.7 |
| (Hygrobates) | 3 | 25.8 |
| Torrenticola–Mideopsis | 4 | 46.2 |
| Torrenticola–Mideopsis | 5 | 53.2 |
| Torrenticola–Mideopsis | 6 | 56.4 |
| HQA | HMS | RHQ | RHM | RHS Class | |
|---|---|---|---|---|---|
| Number of individuals | 0.77 * | −0.45 | 0.48 | −0.21 | −0.69 |
| Number of species | 0.77 | −0.61 | 0.53 | −0.26 | −0.69 |
| Shannon–Wiener index | 0.43 | −0.62 | 0.44 | −0.31 | −0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stryjecki, R.; Pešić, V.; Szlauer-Łukaszewska, A.; Michoński, G.; Bańkowska, A.; Pakulnicka, J.; Filip, E.; Lewin, I.; Chatterjee, T.; Zawal, A. River Habitat Survey: Does This Help to Explain the Nature of Water Mite (Acari and Hydrachnidia) Assemblages? Water 2023, 15, 3751. https://doi.org/10.3390/w15213751
Stryjecki R, Pešić V, Szlauer-Łukaszewska A, Michoński G, Bańkowska A, Pakulnicka J, Filip E, Lewin I, Chatterjee T, Zawal A. River Habitat Survey: Does This Help to Explain the Nature of Water Mite (Acari and Hydrachnidia) Assemblages? Water. 2023; 15(21):3751. https://doi.org/10.3390/w15213751
Chicago/Turabian StyleStryjecki, Robert, Vladimir Pešić, Agnieszka Szlauer-Łukaszewska, Grzegorz Michoński, Aleksandra Bańkowska, Joanna Pakulnicka, Ewa Filip, Iga Lewin, Tapas Chatterjee, and Andrzej Zawal. 2023. "River Habitat Survey: Does This Help to Explain the Nature of Water Mite (Acari and Hydrachnidia) Assemblages?" Water 15, no. 21: 3751. https://doi.org/10.3390/w15213751






