Enhanced Nitrate Nitrogen Removal from Constructed Wetland via Fe3O4/Granular Activated Carbon Anode Microbial Electrolysis Cell under Low C/N Ratio
Abstract
:1. Introduction
2. Materials and Methods
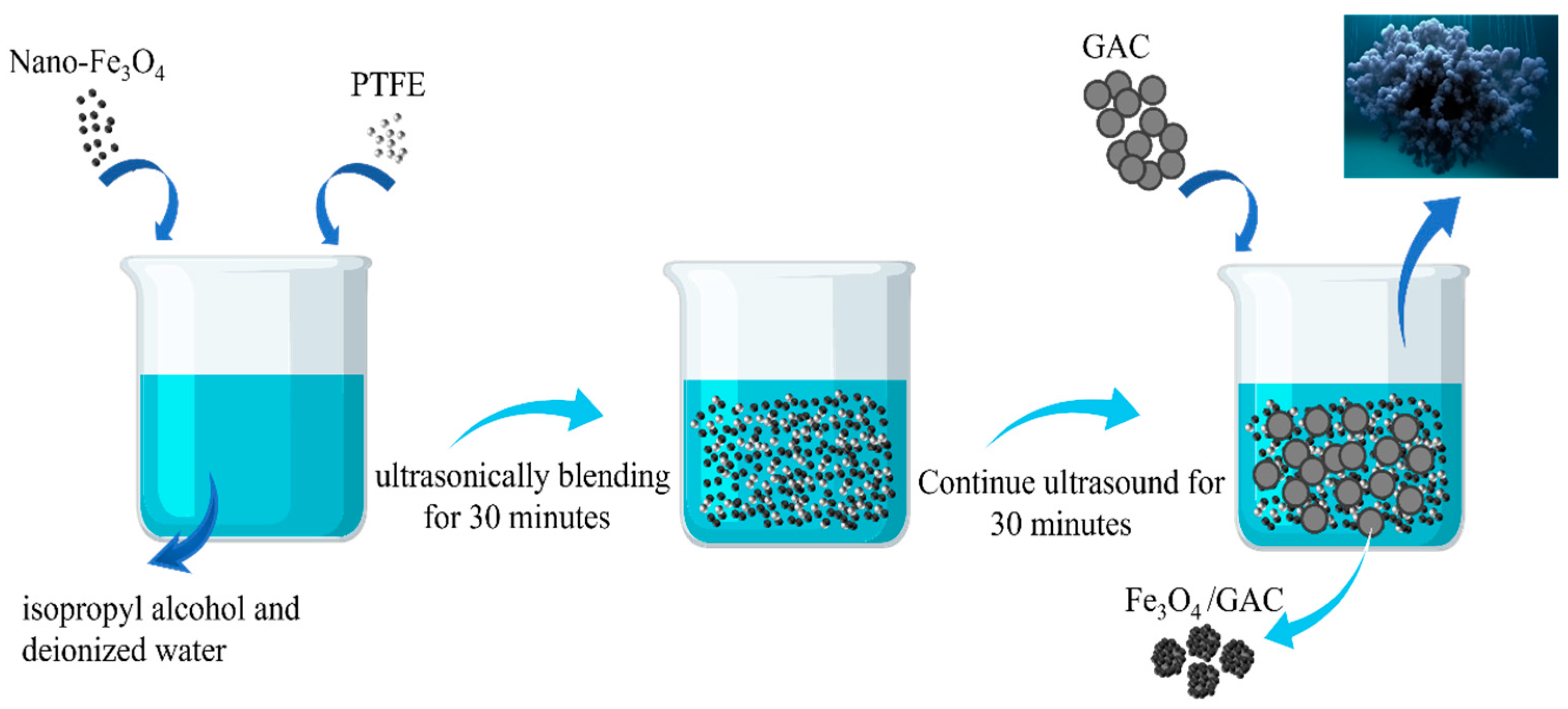
2.1. Synthesis of Fe3O4/GAC Composite Particles
2.2. Experimental Start-Up
2.3. The Experimental Water Characteristics and Water Quality Determination
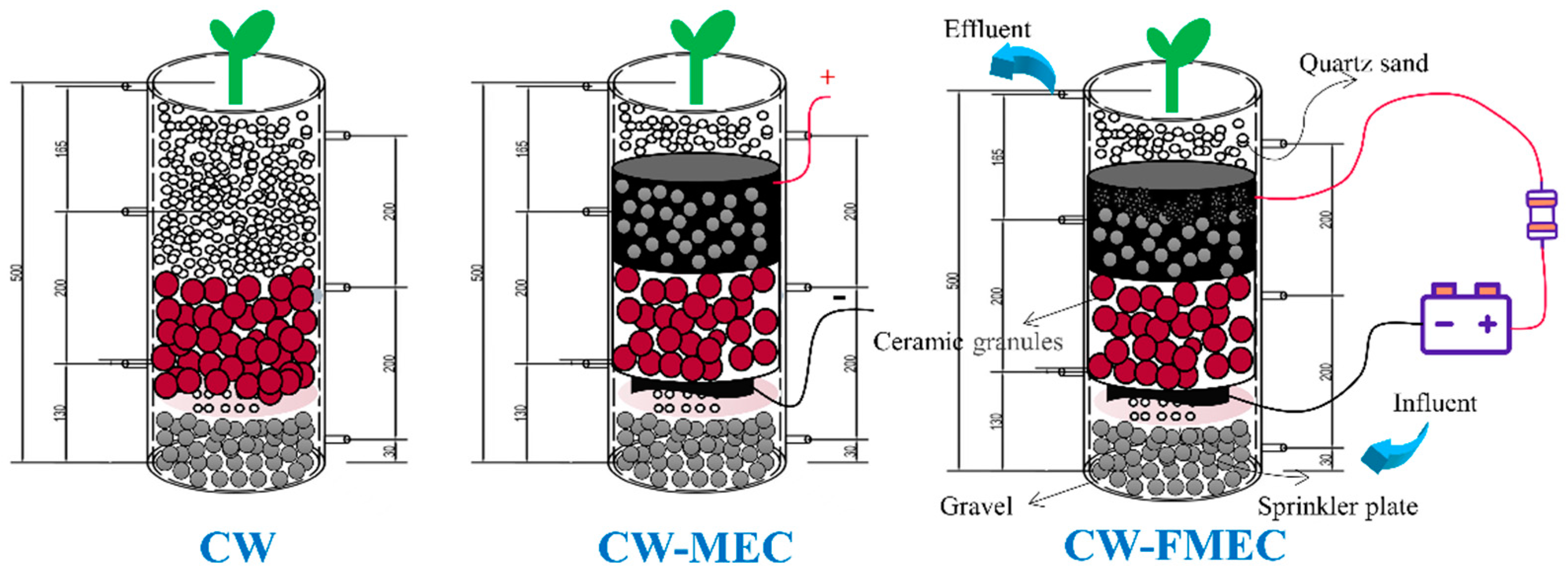
2.4. Wetlands Start-Up and Operation
2.5. Analytical Methods
3. Results and Discussion
3.1. Characterization of Electrode Material
3.2. Treatment Performance
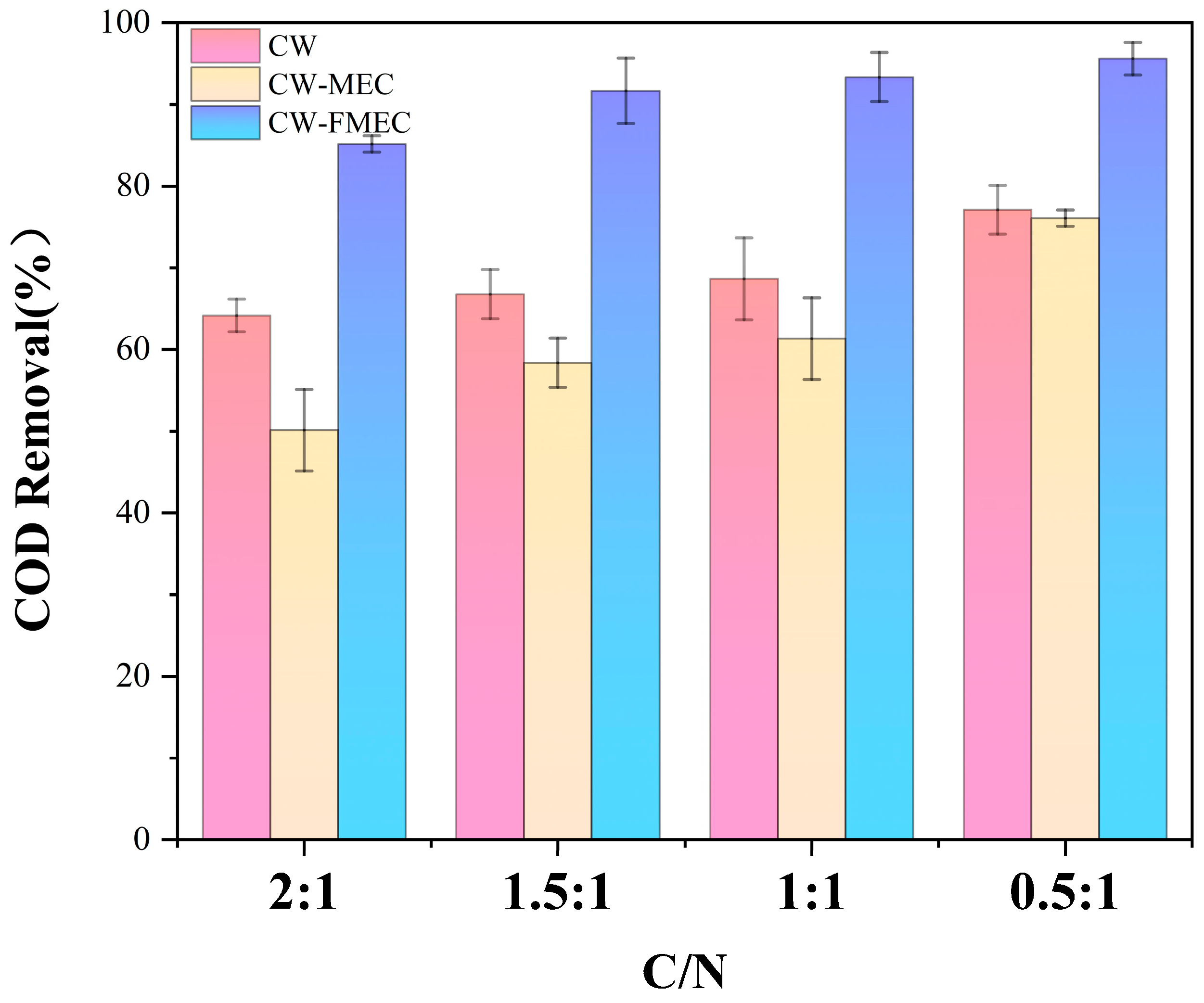
3.3. COD Removal
3.4. Functional Genes of Different Reactors under Optimal Conditions
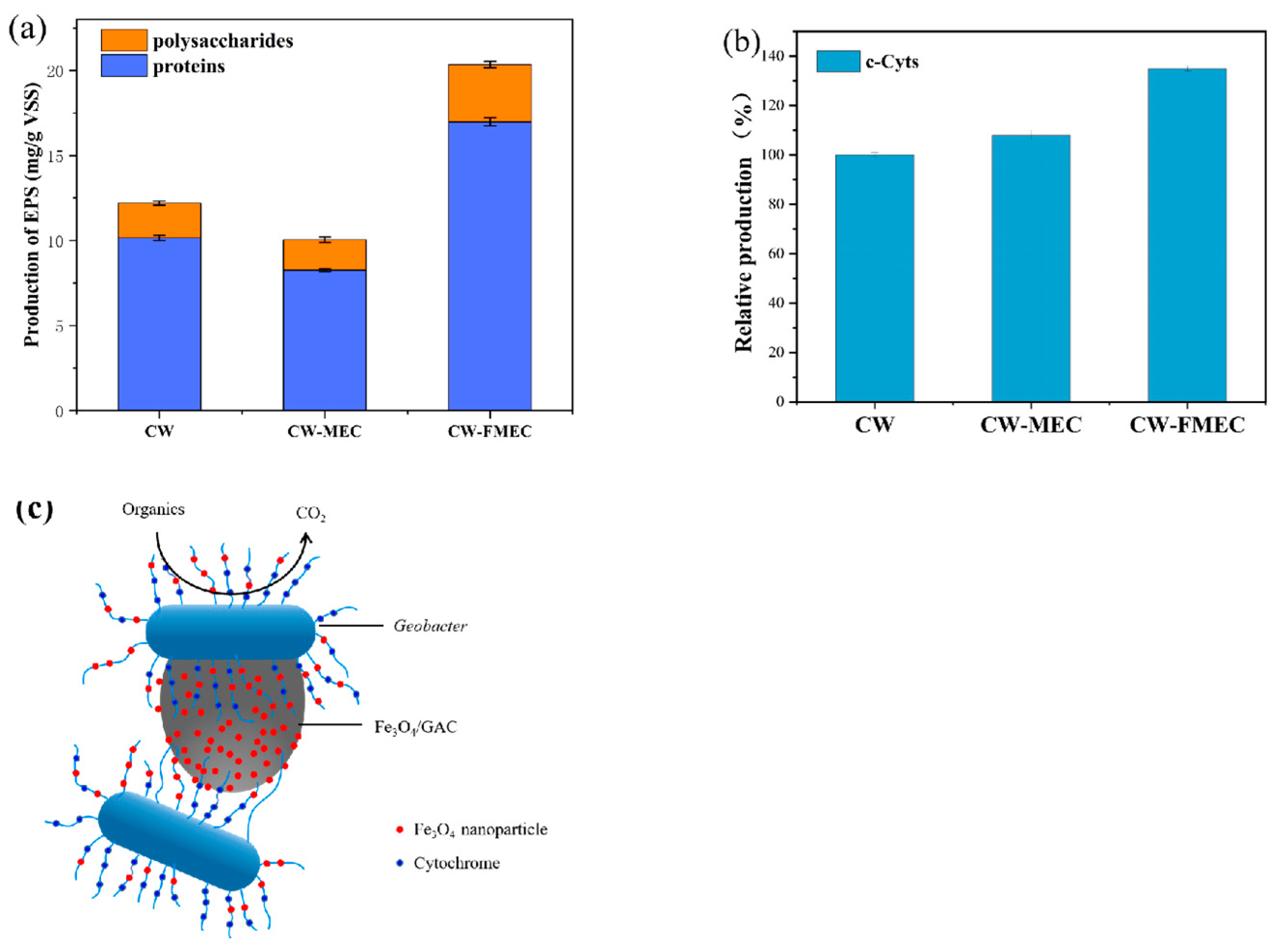
3.5. Relative Content of EPS and c-Cyts under Optimal Conditions
3.6. Microbial Communities and Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Juntakut, P.; Snow, D.D.; Haacker, E.M.K.; Ray, C. The long term effect of agricultural, vadose zone and climatic factors on nitrate contamination in the Nebraska’s groundwater system. J. Contam. Hydrol. 2019, 220, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Sajedi-Hosseini, F.; Malekian, A.; Choubin, B.; Rahmati, O.; Cipullo, S.; Coulon, F.; Pradhan, B. A novel machine learning-based approach for the risk assessment of nitrate groundwater contamination. Sci. Total Environ. 2018, 644, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.H.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Wan, D.J.; Wang, J.K.; Shi, Y.H.; Qu, D.; Zhang, J.H. Construction of continuous-flow electrodialysis ion-exchange membrane bioreactor for effective removal of nitrate and perchlorate: Modelling and impact analysis of environmental variables. Chem. Eng. J. 2023, 462, 142144. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Tang, Z.B.; Yao, Y.; Wang, X.L.; Park, B. Interaction between particulate fouling and precipitation fouling: Sticking probability and deposit bond strength. Int. J. Heat Mass Transf. 2019, 144, 118700. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef] [PubMed]
- Zekker, I.; Mandel, A.; Rikmann, E.; Jaagura, M.; Salmar, S.; Ghangrekar, M.M.; Tenno, T. Ameliorating effect of nitrate on nitrite inhibition for denitrifying P-accumulating organisms. Sci. Total Environ. 2021, 797, 149133. [Google Scholar] [CrossRef]
- Wu, H.M.; Zhang, J.; Ngo, H.H.; Guo, W.S.; Hu, Z.; Liang, S.; Fan, J.L.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Kill, K.; Grinberga, L.; Koskiaho, J.; Mander, U.; Wahlroos, O.; Lauva, D.; Parn, J.; Kasak, K. Phosphorus removal efficiency by in-stream constructed wetlands treating agricultural runoff: Influence of vegetation and design. Ecol. Eng. 2022, 180, 106664. [Google Scholar] [CrossRef]
- Zhang, N.; Li, C.Y.; Xie, H.J.; Yang, Y.X.; Hu, Z.; Gao, M.M.; Liang, S.; Feng, K.S. Mn oxides changed nitrogen removal process in constructed wetlands with a microbial electrolysis cell. Sci. Total Environ. 2021, 770, 144761. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Gao, B.; Jia, W.; Miao, A.J.; Xiao, L.; Yang, L.Y. Highly efficient removal of nitrogen and phosphorus in an electrolysis integrated horizontal subsurface-flow constructed wetland amended with biochar. Water Res. 2018, 139, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Zhao, C.E.; Gai, P.P.; Guo, D.; Jiang, L.P.; Zhang, Q.; Zhang, J.R.; Zhu, J.J. Graphene/Fe3O4 Nanocomposites as Efficient Anodes to Boost the Lifetime and Current Output of Microbial Fuel Cells. Chem.-Asian J. 2017, 12, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, J.; Shuang, C.; Zhou, Q.; Li, A. Effects of Fe3O4 on the denitrification performance of Pseudomonas stutzeri. Sheng Wu Gong Cheng Xue Bao 2021, 37, 3685–3695. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 4644–4652. [Google Scholar] [CrossRef]
- Keithley, S.E.; Kirisits, M.J. An improved protocol for extracting extracellular polymeric substances from granular filter media. Water Res. 2018, 129, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in Anthrone Method for Determination of Carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Li, N.Y.; Quan, X.C.; Zhuo, M.H.; Zhang, X.F.; Quan, Y.P.; Liang, P. Enhancing methanogenesis of anaerobic granular sludge by incorporating Fe/Fe oxides nanoparticles aided with biofilm disassembly agents and mediating redox activity of extracellular polymer substances. Water Res. 2022, 216, 118293. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhong, H.; Li, P.; Zhang, C.J. Microbial communities responded to tetracyclines and Cu(II) in constructed wetlands microcosms with Myriophyllum aquaticum. Ecotoxicol. Environ. Saf. 2020, 205, 111362. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Khiew, P.S.; Isa, D.; Tan, T.K.; Chiu, W.S.; Chia, C.H.; Hamid, M.A.A.; Shamsudin, R. Nano Fe3O4-Activated Carbon Composites for Aqueous Supercapacitors. Sains Malays. 2014, 43, 885–894. [Google Scholar]
- Vrtovsek, J.; Ros, M. Denitrification of groundwater in the biofilm reactor with a specific biomass support material. Acta Chim. Slov. 2006, 53, 396–400. [Google Scholar]
- Chiu, Y.C.; Chung, M.S. Determination of optimal COD/nitrate ratio for biological denitrification. Int. Biodeterior. Biodegrad. 2003, 51, 43–49. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Abbassi, R.; Garaniya, V.; Lewis, T. Denitrification in a low carbon environment of a constructed wetland incorporating a microbial electrolysis cell. J. Environ. Chem. Eng. 2018, 6, 5602–5607. [Google Scholar] [CrossRef]
- Zhu, M.H.; Fan, J.K.; Zhang, M.L.; Li, Z.Y.; Yang, J.D.; Liu, X.T.; Wang, X.H. Current intensities altered the performance and microbial community structure of a bio-electrochemical system. Chemosphere 2021, 265, 129069. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.R.; Lin, X.Y.; Zhao, F.; Pu, Y.; Chen, Y.N.; Tang, X.H. Nano-α-Fe2O3 for enhanced denitrification in a heterotrophic/biofilm-electrode autotrophic denitrification reactor. Water Environ. Res. 2022, 94, e10814. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Yoon, S.; Cruz-García, C.; Sanford, R.; Ritalahti, K.M.; Löffler, F.E. Denitrification versus respiratory ammonification: Environmental controls of two competing dissimilatory NO3−/NO2− reduction pathways in Shewanella loihica strain PV-4. Isme J. 2015, 9, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, X.M.; Fan, W.H.; Wang, J.L. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. ND7 isolated from municipal activated sludge. Bioresour. Technol. 2020, 301, 122749. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.H.; Li, D.S.; Yang, X.; Zhu, S.B.; Li, J.L. Process of nitrogen transformation and microbial community structure in the Fe(0)-carbon-based bio-carrier filled in biological aerated filter. Environ. Sci. Pollut. Res. 2016, 23, 6621–6630. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.G.; Zhang, S.Q.; Oh, Y.; Zhou, Z.B.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Liu, S.Q.; Wang, C.; Hou, J.; Wang, P.F.; Miao, L.Z. Effects of Ag NPs on denitrification in suspended sediments via inhibiting microbial electron behaviors. Water Res. 2020, 171, 115436. [Google Scholar] [CrossRef]
- Berks, B.C.; Ferguson, S.J.; Moir, J.W.; Richardson, D.J. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1995, 1232, 97–173. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Dai, X.H.; Dai, L.L. Principles and advancements in improving anaerobic digestion of organic waste via direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 148, 111367. [Google Scholar] [CrossRef]
- Liu, T.X.; Luo, X.B.; Wu, Y.D.; Reinfelder, J.R.; Yuan, X.; Li, X.M.; Chen, D.D.; Li, F.B. Extracellular Electron Shuttling Mediated by Soluble c-Type Cytochromes Produced by Shewanella oneidensi s MR-1. Environ. Sci. Technol. 2020, 54, 10577–10587. [Google Scholar] [CrossRef]
- He, Y.; Guo, J.B.; Song, Y.Y.; Chen, Z.; Lu, C.C.; Han, Y.; Li, H.B.; Hou, Y.A.; Zhao, R. Acceleration mechanism of bioavailable Fe(III) on Te(IV) bioreduction of Shewanella oneidensis MR-1: Promotion of electron generation, electron transfer and energy level. J. Hazard. Mater. 2021, 403, 123728. [Google Scholar] [CrossRef]
- Wan, R.; Chen, Y.G.; Zheng, X.; Su, Y.L.; Huang, H.N. Effect of CO2 on NADH production of denitrifying microbes via inhibiting carbon source transport and its metabolism. Sci. Total Environ. 2018, 627, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 2015, 17, 648–655. [Google Scholar] [CrossRef]
- Peng, T.; Feng, C.P.; Hu, W.W.; Chen, N.; He, Q.C.; Dong, S.S.; Xu, Y.X.; Gao, Y.; Li, M. Treatment of nitrate-contaminated groundwater by heterotrophic denitrification coupled with electro-autotrophic denitrifying packed bed reactor. Biochem. Eng. J. 2018, 134, 12–21. [Google Scholar] [CrossRef]
- Tian, Z.J.; Li, G.W.; Xiong, Y.; Cao, X.X.; Pang, H.T.; Tang, W.Z.; Liu, Y.L.; Bai, M.X.; Zhu, Q.H.; Du, C.L.; et al. Step-feeding food waste fermentation liquid as supplementary carbon source for low C/N municipal wastewater treatment: Bench scale performance and response of microbial community. J. Environ. Manag. 2023, 345, 118434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Wei, D.Y.; Morrison, L.; Ge, Z.B.; Zhan, X.M.; Li, R.H. Nutrient removal through pyrrhotite autotrophic denitrification: Implications for eutrophication control. Sci. Total Environ. 2019, 662, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, X.W.; Wu, Z.Q.; Li, J.Y.; Cui, Y.; Liu, J.; Li, B.A. Insights on nitrogen and phosphorus removal mechanism in a single-stage Membrane Aeration Biofilm Reactor (MABR) dominated by denitrifying phosphorus removal coupled with anaerobic/aerobic denitrification. J. Water Process Eng. 2023, 52, 103583. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Y.T.; Hu, S.Y.; Li, Q.X.; Chen, S.W. Assembled denitrifying consortia for efficient nitrate removal under low-COD/N conditions. Chem. Eng. J. 2023, 460, 141655. [Google Scholar] [CrossRef]
- Liessens, J.; Vanbrabant, J.; De Vos, P.; Kersters, K.; Verstraete, W. Mixed culture hydrogenotrophic nitrate reduction in drinking water. Microb. Ecol. 1992, 24, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.T.; Yang, J.Q.; Yu, F.F.; Cai, L.; Li, J.; Ganigu, R. Understanding the role of polyurethane sponges on rapid formation of aerobic granular sludge and enhanced nitrogen removal. Chem. Eng. J. 2023, 460, 141670. [Google Scholar] [CrossRef]
- Duffner, C.; Holzapfel, S.; Wunderlich, A.; Einsiedl, F.; Schloter, M.; Schulz, S. Dechloromonas and close relatives prevail during hydrogenotrophic denitrification in stimulated microcosms with oxic aquifer material. Fems Microbiol. Ecol. 2021, 97, fiab004. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Han, Y.X.; Xu, C.Y.; Han, H.J.; Zhong, D.; Zheng, M.Q.; Ma, W.W. Effect of low-intensity direct current electric field on microbial nitrate removal in coal pyrolysis wastewater with low COD to nitrogen ratio. Bioresour. Technol. 2019, 287, 121465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Y.; Feng, Q.; Chen, Y.; Shi, X.S.; Qin, K.; Lu, M.Y.; Qin, F.; Fu, S.F.; Guo, R.B. Enhancement of biological nitrogen removal performance from low C/N municipal wastewater using novel carriers based on the nano-Fe3O4 br. Bioresour. Technol. 2022, 363, 127914. [Google Scholar] [CrossRef] [PubMed]




| Reactor Type | Influent Nitrate (mg/L) | Current Intensity (mA) | Removal Efficiencies (%) | Nitrogen Coulomb Reduction Rate (mg/C) | |
|---|---|---|---|---|---|
| Pratiksha et al. [27] | CW-MEC | 50 | 0.233 | 69.1 | 0.17 |
| Zhu et al. [28] | Bielectrochemical system | 35 | 400 | 94.2 | 0.031 |
| Yin et al. [29] | Heterotrophic/biofilm–electrode autotrophic denitrification reactor | 60 | 60 | 95.83 | 0.015 |
| Current study | CW-FMEC | 40 | 3 | 88.9 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Tan, S.; Huang, Y.; Tang, X. Enhanced Nitrate Nitrogen Removal from Constructed Wetland via Fe3O4/Granular Activated Carbon Anode Microbial Electrolysis Cell under Low C/N Ratio. Water 2024, 16, 1377. https://doi.org/10.3390/w16101377
Yang H, Tan S, Huang Y, Tang X. Enhanced Nitrate Nitrogen Removal from Constructed Wetland via Fe3O4/Granular Activated Carbon Anode Microbial Electrolysis Cell under Low C/N Ratio. Water. 2024; 16(10):1377. https://doi.org/10.3390/w16101377
Chicago/Turabian StyleYang, Heng, Shenyu Tan, Yu Huang, and Xinhua Tang. 2024. "Enhanced Nitrate Nitrogen Removal from Constructed Wetland via Fe3O4/Granular Activated Carbon Anode Microbial Electrolysis Cell under Low C/N Ratio" Water 16, no. 10: 1377. https://doi.org/10.3390/w16101377





