Phytohormone Supplementation for Nutrient Removal from Mariculture Wastewater by Oocystis borgei in Sequential Batch Operation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae, Phytohormones, and Synthetic Mariculture Wastewater
2.2. Determination of Optimal Concentration of Phytohormone
2.3. Nitrogen and Phosphorus Determination
2.4. Determination of Photosynthetic Pigments and Chlorophyll Fluorescence Parameter
2.5. Determination of Nitrogen-Metabolism-Related Enzymes and Antioxidant Enzymes
2.6. Economic Feasibility Analysis of Phytohormones for MW Treatment
2.7. Statistical Analyses
3. Results and Discussion
3.1. Evaluating the Effect of Phytohormone on Growth of O. borgei
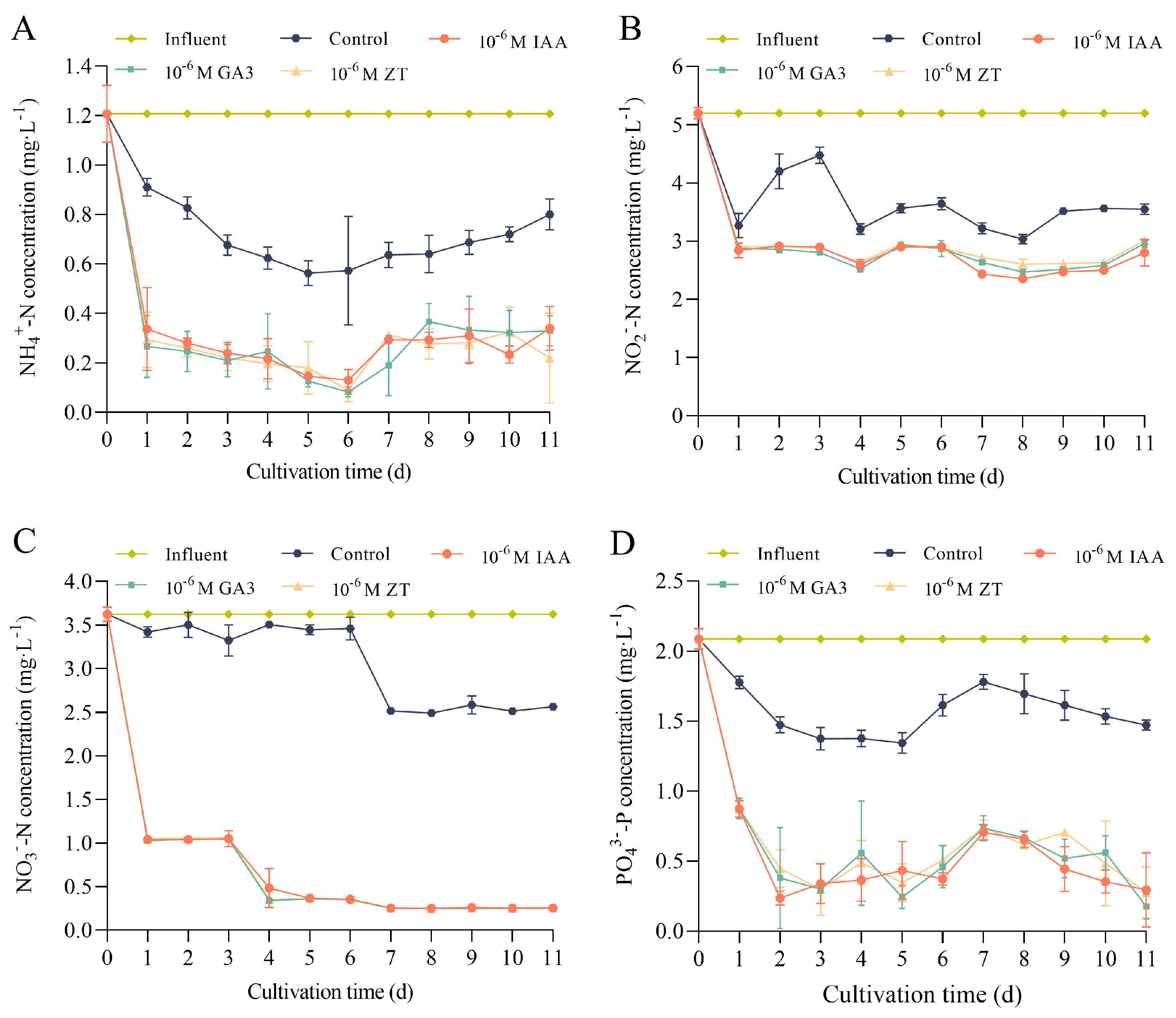
3.2. Phytohormones Facilitate the Removal of N and P by O. borgei
3.3. Mechanism of Phytohormones to Promote N and P Removal by O. borgei
3.3.1. Promotion of Photosynthetic Metabolism of O. borgei
3.3.2. Improving the Nitrogen Metabolism of O. borgei
3.3.3. Mitigation of Oxidative Stress Damage Caused by Abiotic Stresses
3.4. Economic Evaluation of Phytohormones for MW Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Ding, Y.; Liu, F.; You, H.; Qi, P.; Wang, F.; Li, Y.; Jin, C. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Xie, B.; Li, Z.; Si, D.; Yang, X.; Qu, X.; Liang, H.; Yan, Z.; You, H. Enhancement of the mariculture wastewater treatment based on the bacterial-microalgal consortium. Mater. Sci Energy Technol. 2022, 5, 110–115. [Google Scholar] [CrossRef]
- Poste, A.E.; Muir, D.C.G.; Guildford, S.J.; Hecky, R.E. Bioaccumulation and Biomagnification of mercury in African lakes: The importance of trophic status. Sci. Total Environ. 2015, 506–507, 126–136. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rahman, A.; Behrens, J.; Brennan, C.; Ham, B.; Kim, H.S.; Nho, C.W.; Yun, S.; Azam, H.; Kwon, M.J. Nutrient removal from hydroponic wastewater by a microbial consortium and a culture of Paracercomonas Saepenatans. New Biotechnol. 2018, 41, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, N.; Wang, B.; Zhang, T.; Ma, P.; Zhagn, W.; Nusrat, Z.S.; Ma, Z.; Zhang, Y.; Ying, L. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J. Environ. Manag. 2022, 320, 115673. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Mehariya, S.; Kumar, P.; Vinayak, V.; Saratale, G.D.; Raj, T.; Kim, S.; Yang, Y. An overview on microalgal-bacterial granular consortia for resource recovery and wastewater treatment. Bioresour. Technol. 2022, 351, 127028. [Google Scholar] [CrossRef]
- Chu, G.; Wang, Q.; Song, C.; Liu, J.; Zhao, Y.; Lu, S.; Zhang, Z.; Jin, C.; Gao, M. Platymonas helgolandica-driven nitrogen removal from mariculture wastewater under different photoperiods: Performance evaluation, enzyme activity and transcriptional response. Bioresour. Technol. 2023, 372, 128700. [Google Scholar] [CrossRef]
- Santos, F.M.; Pires, J.C.M. Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Bioresour. Technol. 2018, 267, 725–731. [Google Scholar] [CrossRef]
- De Morais, E.G.; Sampaio, I.C.F.; Gonzalez-Flo, E.; Ferrer, I.; Uggetti, E.; García, J. Microalgae harvesting for wastewater treatment and resources recovery: A review. New Biotechnol. 2023, 78, 84–94. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef]
- Torres-Franco, A.; Passos, F.; Figueredo, C.; Mota, C.; Muñoz, R. Current advances in microalgae-based treatment of high-strength wastewaters: Challenges and opportunities to enhance wastewater treatment performance. Rev. Environ. Sci. Biotechnol. 2021, 20, 209–235. [Google Scholar] [CrossRef]
- Liu, T.; Liu, F.; Wang, C.; Wang, Z.; Li, Y. The boosted biomass and lipid accumulation in chlorella vulgaris by supplementation of synthetic phytohormone analogs. Bioresour. Technol. 2017, 232, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Staden, J. van Potential of Phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef]
- Yu, Z.; Song, M.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. The effects of combined agricultural phytohormones on the growth, carbon partitioning and cell morphology of two screened algae. Bioresour. Technol. 2017, 239, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Hashtroudi, M.S.; Ghassempour, A.; Riahi, H.; Shariatmadari, Z.; Khanjir, M. Endogenous auxins in plant growth-promoting Cyanobacteria—Anabaena Vaginicola and Nostoc Calcicola. J. Appl. Phycol. 2013, 25, 379–386. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella Vulgaris (Trebouxiophyceae). Plant Physiol. Biochem. 2013, 71, 290–297. [Google Scholar] [CrossRef]
- Dao, G.; Wu, G.; Wang, X.; Zhuang, L.; Zhang, T.; Hu, H. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus Sp. LX1 by two types of auxin. Bioresour.Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef]
- Seemashree, M.H.; Chauhan, V.S.; Sarada, R. Phytohormone supplementation mediated enhanced biomass production, lipid accumulation, and modulation of fatty acid profile in Porphyridium Purpureum and Dunaliella Salina cultures. Biocatal. Agric. Biotechnol. 2022, 39, 102253. [Google Scholar] [CrossRef]
- Babu, A.G.; Wu, X.; Kabra, A.N.; Kim, D. Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res. 2017, 23, 178–185. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Aziz, A.; Cha, T.S. Gibberellin promotes cell growth and induces changes in fatty acid biosynthesis and upregulates fatty acid biosynthetic genes in Chlorella Vulgaris UMT-M1. Appl. Biochem. Biotech. 2019, 188, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Islam, S.; Mohammad, F.; Siddiqui, M.H. Gibberellic acid: A versatile regulator of plant growth, development and stress responses. J. Plant Growth Regul. 2023, 42, 7352–7373. [Google Scholar] [CrossRef]
- Du, K.; Tao, H.; Wen, X.; Geng, Y.; Li, Y. Enhanced growth and lipid production of Chlorella Pyrenoidosa by plant growth regulator GA3. Fresenius Environ. Bull. 2015, 24, 3414–3419. [Google Scholar]
- Du, H.; Ahmed, F.; Lin, B.; Li, Z.; Huang, Y.; Sun, G.; Ding, H.; Wang, C.; Meng, C.; Gao, Z. The effects of plant growth regulators on cell growth, protein, carotenoid, PUFAs and lipid production of Chlorella Pyrenoidosa ZF strain. Energies 2017, 10, 1696. [Google Scholar] [CrossRef]
- Mousavi, P.; Morowvat, M.H.; Montazeri-Najafabady, N.; Abolhassanzadeh, Z.; Mohagheghzadeh, A.; Hamidi, M.; Niazi, A.; Ghasemi, Y. Investigating the effects of phytohormones on growth and β-carotene production in a naturally isolates stain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 6, 164–171. [Google Scholar] [CrossRef]
- Madani, N.S.H.; Shamsaie Mehrgan, M.; Hosseini Shekarabi, S.P.; Pourang, N. Regulatory effect of gibberellic acid (GA3) on the biomass productivity and some metabolites of a marine microalga, Isochrysis galbana. J. Appl. Phycol. 2021, 33, 255–262. [Google Scholar] [CrossRef]
- Arumugam, M.; Udayan, A.; Sabapathy, H.; Abraham, B. Plant growth regulator triggered metabolomic profile leading to increased lipid accumulation in an edible marine microalga. J. Appl. Phycol. 2021, 33, 1353–1365. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae Biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers. Manag. 2017, 140, 14–23. [Google Scholar] [CrossRef]
- Park, W.; Yoo, G.; Moon, M.; Kim, C.W.; Choi, Y.E.; Yang, J. Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl. Biochem. 2013, 171, 1128–1142. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.; Kabra, A.N.; Ji, M.; Kim, J.R.; Min, B.; Jeon, B. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 2014, 172, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.J.; TurpinJelfs, T.; Nicholes, M.J.; Yallop, M.L.; Anesio, A.M.; Tranter, M. Macro-nutrient stoichiometry of glacier algae from the southwestern margin of the greenland ice sheet. Front. Plant Sci. 2021, 12, 673614. [Google Scholar] [CrossRef]
- Huang, H.; Zhong, S.; Wen, S.; Luo, C.; Long, T. Improving the efficiency of wastewater treatment and microalgae production for biofuels. Resour. Conserv. Recy. 2022, 178, 106094. [Google Scholar] [CrossRef]
- Zhao, P.; Lin, Z.; Wang, Y.; Chai, H.; Li, Y.; He, L.; Zhou, J. Facilitating effects of plant hormones on biomass production and nutrients removal by Tetraselmis cordiformis for advanced sewage treatment and its mechanism. Sci. Total Environ. 2019, 693, 133650. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Li, Y.; Yang, Z.; Xie, Z.; Hou, Q.; Nie, C. Inclined algal biofilm photobioreactor (IABPBR) for cost-effective cultivation of lipid-rich microalgae and treatment of seawater-diluted anaerobically digested effluent from kitchen waste with the aid of phytohormones. Bioresour. Technol. 2020, 315, 123761. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Jin, W.; Zhang, C.; Liang, Y.; He, Z.; Chen, Y.; Han, W.; Jiang, G. Enhancing cultivation of biodiesel-promising microalgae Chlorella pyrenoidosa using plant hormones in municipal wastewater. Biomass Convers. Bior. 2021, 13, 9753–9763. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Wang, Y.; Zhou, M. Effects of environmental factors on the uptake rates of dissolved nitrogen by a salt-water green alga (Oocystis borgei Snow). Bull. Environ. Contam. Toxicol. 2012, 89, 905–909. [Google Scholar] [CrossRef]
- Michels, M.H.A.; van der Goot, A.J.; Vermuë, M.H.; Wijffels, R.H. Cultivation of shear stress sensitive and tolerant microalgal species in a tubular photobioreactor equipped with a centrifugal pump. J. Appl. Phycol. 2016, 28, 53–62. [Google Scholar] [CrossRef]
- Ren, J.; Hong, T.; Zhang, N.; Huang, X.; Li, C. Effects of salinity on chlorophyll fluorescence parameters and cloning and characterization of PsbA gene in Oocystis borgei. J. Guangdong Ocean Univ. 2020, 10, 30–39. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Li, S. Effects of Oocystis borgei on Growth of Vibrios. J. Guangdong Ocean Univ. 2010, 30, 33–38. (In Chinese) [Google Scholar] [CrossRef]
- Xie, L.; Zhang, N.; Li, C.; Huang, X. Effects of sodium nitrate on growth, biochemical components and sedimentation of Oocystis borgei. J. Guangdong Ocean Univ. 2020, 8, 48–55. (In Chinese) [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Liu, C.; Wang, Z. Study on the N and P nutrient demand of Oocystis borgei. Mar. Sci. Bull. 2002, 21, 32–38. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Li, C.; Qian, X.; Li, J. Uptake rate of ammonium by Oocystis borgei under different conditions. J. Guangdong Ocean Univ. 2012, 32, 29–34. (In Chinese) [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Zhang, R.; Li, C.; Gu, B. Uptake of urea nitrogen by Oocystis borgei in Prawn (Litopenaeus vannamei) aquaculture ponds. B. Environ. Contam. Tox. 2018, 101, 586–591. (In Chinese) [Google Scholar] [CrossRef]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Preparation of artificial seawater. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Yang, X. Optimizing Conditions and Applying Microalgae for Efficient Utilization of Ammonia Nitrogen and Inorganic Carbon Sources; Fuzhou University: Fuzhou, China, 2018. [Google Scholar]
- Han, Q.; Liu, J.; Huang, X.; Dong, M.; Li, M. Optimization of polysaccharide extraction conditions of Oocystis borgei by response surface methodology. J. Guangdong Ocean Univ. 2018, 38, 23–29. (In Chinese) [Google Scholar] [CrossRef]
- Sözgen Başkan, K.; Tütem, E.; Özer, N.; Apak, R. Spectrophotometric and chromatographic assessment of contributions of carotenoids and chlorophylls to the total antioxidant capacities of plant foods. J. Agric. Food Chem. 2013, 61, 11371–11381. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise estimation of chlorophyll a, b and carotenoid content by deconvolution of the Absorption spectrum and new simultaneous equations for chl determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Winter, A. Studies on the hormonal relationships of algae in pure culture: I. The effect of indole-3-acetic acid on the growth of blue-green and green algae. Planta 1968, 78, 277–286. [Google Scholar] [CrossRef]
- Chung, T.; Kuo, C.; Lin, W.; Wang, W.; Chou, J. Indole-3-acetic-acid-induced phenotypic plasticity in Desmodesmus algae. Sci. Rep. 2018, 8, 10270. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Gruz, J.; Strnad, M.; Ördög, V.; van Staden, J. Effect of gibberellins on growth and biochemical constituents in Chlorella minutissima (Trebouxiophyceae). S. Afr. J. Bot. 2019, 126, 92–98. [Google Scholar] [CrossRef]
- Na, H.; Jo, S.W.; Do, J.M.; Kim, I.S.; Yoon, H.S. Production of algal biomass and high-value compounds mediated by interaction of microalgal Oocystis Sp. KNUA044 and Bacterium Sphingomonas KNU100. J. Microbiol. Biotechnol. 2021, 31, 387–397. [Google Scholar] [CrossRef]
- Fan, H.; Wang, K.; Wang, C.; Yu, F.; He, X.; Ma, J.; Li, X. A comparative study on growth characters and nutrients removal from wastewater by two microalgae under optimized light regimes. Environ. Technol. Innov. 2020, 19, 100849. [Google Scholar] [CrossRef]
- Furnish, B.J.; Keller, T.A. Carbon limitation in hypereutrophic, periphytic algal wastewater treatment systems. PLoS ONE 2020, 15, e0240525. [Google Scholar] [CrossRef]
- Fu, J.; Lin, Z.; Zhao, P.; Wang, Y.; He, L.; Zhou, J. Establishment and efficiency analysis of a single-stage denitrifying phosphorus removal system treating secondary effluent. Bioresour. Technol. 2019, 288, 121520. [Google Scholar] [CrossRef]
- Liu, T.; Luo, F.; Wang, Z.; Li, Y. The enhanced biomass and lipid accumulation in Coccomyxa Subellipsoidea with an integrated treatment strategy initiated by brewery effluent and phytohormones. World J. Microb. Biot. 2018, 34, 25. [Google Scholar] [CrossRef]
- Yu, J.; You, X.; Gao, Y.; Guo, L.; Yang, X.; Gao, M.; Zhao, Y.; Jin, C.; Ji, J.; She, Z. The impact of auxin analogues on microalgal intracellular component accumulation and nutrient removal for mariculture wastewater treatment basing on bacterial-algal coupling technology. Process Saf. Environ. 2022, 164, 660–668. [Google Scholar] [CrossRef]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater treatment by seven species of microalgae and an algal bloom: Biomass production, N and P removal kinetics and harvestability. Water Res. 2015, 83, 42–51. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, H.; Yang, Z.; Wang, X.; Chen, S.; Li, Y.; Xie, Z. Microalgae nourished by mariculture wastewater aids aquaculture self-reliance with desirable biochemical composition. Bioresour. Technol. 2019, 278, 205–213. [Google Scholar] [CrossRef]
- Fierli, D.; Aranyos, A.; Barone, M.E.; Parkes, R.; Touzet, N. Influence of exogenous phytohormone supplementation on the pigment and fatty acid content of three marine diatoms. Appl. Microbiol. Biotechnol. 2022, 106, 6195–6207. [Google Scholar] [CrossRef]
- Dawiec-Liśniewska, A.; Podstawczyk, D.; Bastrzyk, A.; Czuba, K.; Pacyna-Iwanicka, K.; Okoro, O.V.; Shavandi, A. New trends in biotechnological applications of photosynthetic microorganisms. Biotechnol. Adv. 2022, 59, 107988. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Vasilieva, S.; Lobakova, E. Chlorophyll fluorescence as a valuable multitool for microalgal biotechnology. Biophys. Rev. 2022, 14, 973–983. [Google Scholar] [CrossRef]
- Best, R.J.; Lyczakowski, J.J.; Abalde-Cela, S.; Yu, Z.; Abell, C.; Smith, A.G. Label-free analysis and sorting of microalgae and Cyanobacteria in microdroplets by intrinsic chlorophyll fluorescence for the identification of fast growing strains. Anal. Chem. 2016, 88, 10445–10451. [Google Scholar] [CrossRef]
- Dao, G.; Wang, S.; Wang, X.; Chen, Z.; Wu, Y.; Wu, G.; Lu, Y.; Liu, S.; Hu, H. Enhanced Scenedesmus Sp. growth in response to gibberellin secretion by symbiotic bacteria. Sci. Total Environ. 2020, 740, 140099. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef]
- Paes, C.R.; Faria, G.R.; Tinoco, N.A.; Castro, D.J.; Barbarino, E.; Lourenço, S.O. Growth, Nutrient uptake and chemical composition of Chlorella Sp. and Nannochloropsis Oculata under nitrogen starvation. Lat. Am. J. Aquat. Res. 2016, 44, 275–292. [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Li, C.; Gu, B. Study on the uptake of dissolved nitrogen by Oocystis borgei in Prawn (Litopenaeus vannamei) aquaculture ponds and establishment of uptake model. Aquacult. Int. 2020, 28, 1445–1458. [Google Scholar] [CrossRef]
- Calatrava, V.; ChamizoAmpudia, A.; SanzLuque, E.; OcañaCalahorro, F.; Llamas, A.; Fernandez, E.; Galvan, A. How Chlamydomonas handles nitrate and the nitric oxide cycle. J. Exp. Bot. 2017, 68, 2593–2602. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Jeong, E.; Kim, S.; Seo, J.S.; Um, T.; Shim, J.S. Signaling pathways underlying nitrogen transport and metabolism in plants. BMB Rep. 2023, 56, 56–64. [Google Scholar] [CrossRef]
- Scarcelli, P.G.; Ruas, G.; Lopez-Serna, R.; Serejo, M.L.; Blanco, S.; Boncz, M.Á.; Muñoz, R. Integration of algae-based sewage treatment with anaerobic digestion of the bacterial-algal biomass and biogas upgrading. Bioresour. Technol. 2021, 340, 125552. [Google Scholar] [CrossRef]
- Wang, M.; Keeley, R.; Zalivina, N.; Halfhide, T.; Scott, K.; Zhang, Q.; van der Steen, P.; Ergas, S.J. Advances in algal-prokaryotic wastewater treatment: A Review of nitrogen transformations, reactor configurations and molecular tools. J. Environ. Manag. 2018, 217, 845–857. [Google Scholar] [CrossRef]
- Tian, Y.S.; Wang, R.T.; Zhao, W.; Xing, X.J.; Fu, X.Y.; Peng, R.H.; Yao, Q.H. Distinct properties of two glutamine synthetase isoforms in soybean root nodules. Appl. Biochem. Microbiol. 2016, 52, 643–649. [Google Scholar] [CrossRef]
- Guan, M.; de Bang, T.C.; Pedersen, C.; Schjoerring, J.K. Cytosolic glutamine synthetase Gln1;2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity. Plant Physiol. 2016, 171, 1921–1933. [Google Scholar] [CrossRef]
- Konishi, N.; Ishiyama, K.; Beier, M.P.; Inoue, E.; Kanno, K.; Yamaya, T.; Takahashi, H.; Kojima, S. Contributions of two cytosolic glutamine synthetase isozymes to ammonium assimilation in Arabidopsis roots. J. Exp. Bot. 2017, 68, 613–625. [Google Scholar] [CrossRef]
- Moreira, E.; Coimbra, S.; Melo, P. Glutamine synthetase: An unlikely case of functional redundancy in Arabidopsis thaliana. Plant Biol. 2022, 24, 713–720. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Le, T.V.A.; Show, P.L.; Nguyen, T.T.; Tran, M.H.; Tran, T.N.T.; Lee, S.Y. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar] [CrossRef]
- Xu, Y.; Milledge, J.J.; Abubakar, A.; Swamy, R.A.R.; Bailey, D.; Harvey, P.J. Effects of centrifugal stress on cell disruption and glycerol leakage from Dunaliella salina. Microalgae Biotechnol. 2015, 1, 20–27. [Google Scholar] [CrossRef]
- Gomes, T.; Xie, L.; Brede, D.; Lind, O.; Solhaug, K.A.; Salbu, B.; Tollefsen, K.E. Sensitivity of the green algae Chlamydomonas reinhardtii to gamma radiation: Photosynthetic performance and ROS formation. Aquat. Toxicol. 2017, 183, 1–10. [Google Scholar] [CrossRef]
- Motone, K.; Takagi, T.; Aburaya, S.; Miura, N.; Aoki, W.; Ueda, M. A zeaxanthin-producing bacterium isolated from the algal phycosphere protects coral endosymbionts from environmental stress. mBio 2020, 11, e01019-19. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Tsiplakou, E.; Zerva, A.; Pantiora, P.D.; Georgakis, N.D.; Tsintzou, G.P.; Madesis, P.; Labrou, N.E. Microalgae as a sustainable source of antioxidants in animal nutrition, health and livestock development. Antioxidants 2023, 12, 1882. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E.; Bralska, M. Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green alga Acutodesmus obliquus. Plant Physiol. Biochem. 2018, 132, 535–546. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, Y.; Wakisaka, M.; Yang, Z.; Yin, Y.; Fang, W.; Xu, Y.; Omura, T.; Yu, R.; Zheng, A.L.T. Mitigation of oxidative stress damage caused by abiotic stress to improve biomass yield of microalgae: A review. Sci. Total Environ. 2023, 896, 165200. [Google Scholar] [CrossRef]



| Bulk | Amount of Phytohormone (g·m−3) | Price | Value for Cultivation Day | Cell Density (1012 Cells·m−3) | Cost (CNY·109 Cells−1) | |
|---|---|---|---|---|---|---|
| Phytohormone (CNY·g−1) | SMW (CNY·m−3) | |||||
| Control | 0 | 0 | 1970 | 4 | 1.48 | 5.32 |
| IAA | 0.18 | 3.01 | 1970 | 1.82 | 4.33 | |
| GA3 | 0.35 | 5.04 | 1970 | 1.73 | 4.56 | |
| ZT | 0.22 | 2170 | 1970 | 1.75 | 5.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Deng, C.; Song, X.; Hu, Z.; Li, F.; Zhang, Y.; Li, C.; Huang, X.; Zhang, N. Phytohormone Supplementation for Nutrient Removal from Mariculture Wastewater by Oocystis borgei in Sequential Batch Operation. Water 2024, 16, 552. https://doi.org/10.3390/w16040552
Liu Y, Deng C, Song X, Hu Z, Li F, Zhang Y, Li C, Huang X, Zhang N. Phytohormone Supplementation for Nutrient Removal from Mariculture Wastewater by Oocystis borgei in Sequential Batch Operation. Water. 2024; 16(4):552. https://doi.org/10.3390/w16040552
Chicago/Turabian StyleLiu, Yang, Chengcheng Deng, Xinyue Song, Zhangxi Hu, Feng Li, Yulei Zhang, Changling Li, Xianghu Huang, and Ning Zhang. 2024. "Phytohormone Supplementation for Nutrient Removal from Mariculture Wastewater by Oocystis borgei in Sequential Batch Operation" Water 16, no. 4: 552. https://doi.org/10.3390/w16040552







