Photochlorination of Anthracene in Saline Ice under Simulated Solar Light
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Preparation of ANT Reaction Ice
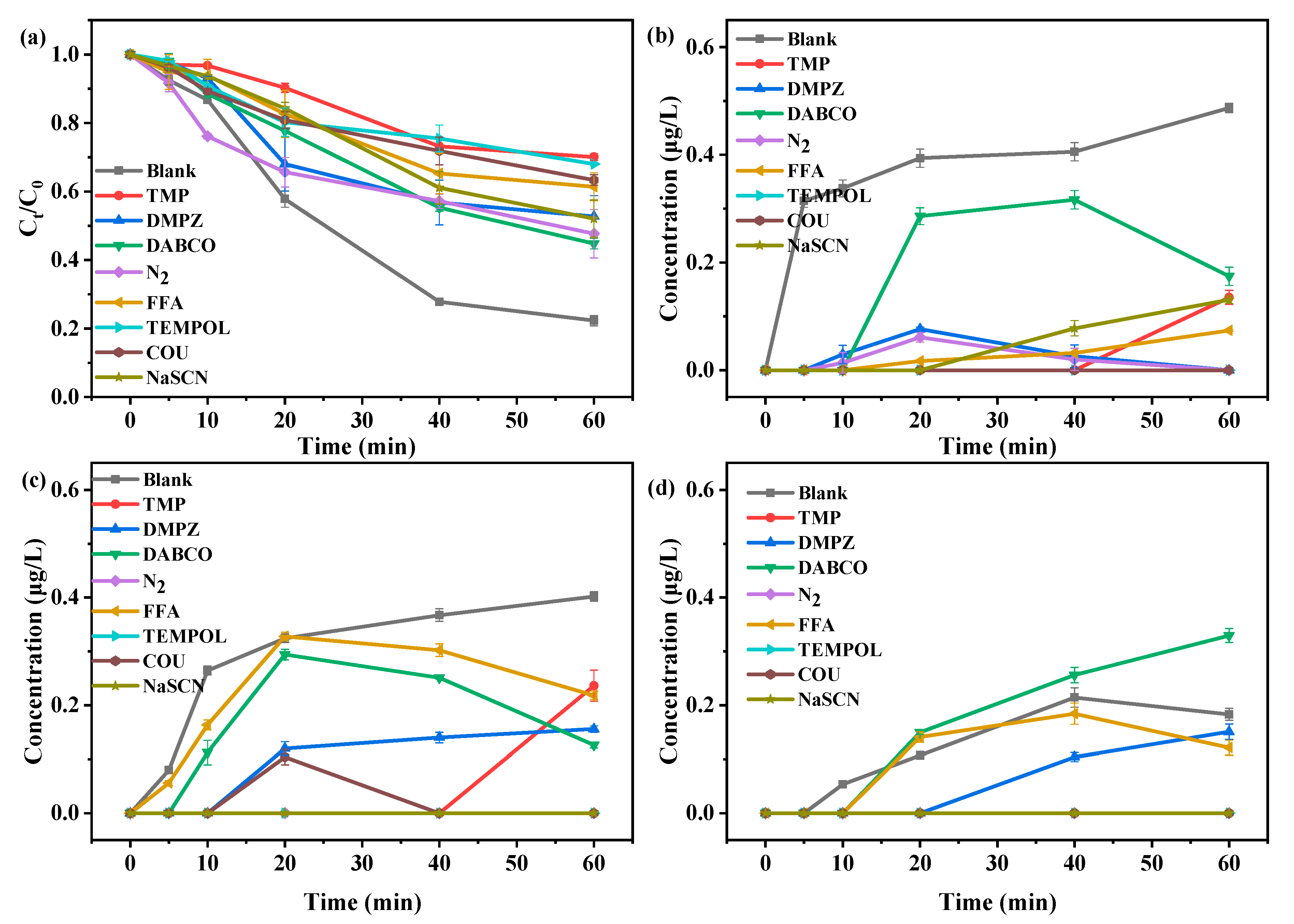
2.3. Trapping Experiments
2.4. Irradiation Experiments
2.5. Sample Preparation
2.6. Methods of Analysis
3. Results and Discussion
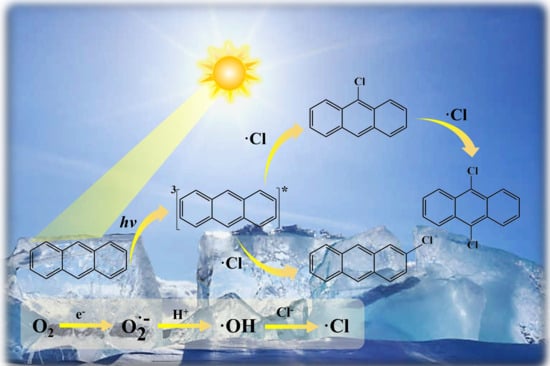
3.1. Phototransformation of ANT and Formation of Its Chlorinated Products in Saline Ice
3.2. Reactive Oxygen Species
3.3. Laser Flash Photolysis Experiment
3.4. The Reaction Pathways of ANT
3.5. The Influence of Temperature
3.6. The Influence of pH
3.7. The Influence of Fulvic Acid
3.8. ANT Phototransformation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Li, M.; Tan, J.; He, M.; Wu, H.; Kang, Y.; Hu, Z.; Zhang, J.; Guo, Z. Distribution, sources, ecological risk and microbial response of polycyclic aromatic hydrocarbons in Qingdao bays, China. Environ. Pollut. 2023, 338, 122687. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, Q.; Liang, H.; Gao, B.; Alam, E. Distribution characteristics and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in underground coal mining environment of Xuzhou. Hum. Ecol. Risk Assess. 2019, 25, 1564–1578. [Google Scholar] [CrossRef]
- Ma, B.; Chen, H.; He, Y.; Wang, H.; Xu, J. Evaluation of toxicity risk of polycyclic aromatic hydrocarbons (PAHs) in crops rhizosphere of contaminated field with sequential extraction. J. Soils Sediments 2010, 10, 955–963. [Google Scholar] [CrossRef]
- Yao, Y.; Meng, X.; Wu, C.; Bao, L.; Wang, F.; Wu, F.-C.; Zeng, E.Y. Tracking human footprints in Antarctica through passive sampling of polycyclic aromatic hydrocarbons in inland lakes. Environ. Pollut. 2016, 213, 412–419. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, A.R.; Jayaraman, S.; Lenka, S.; Gurjar, S.; Sarkar, A.; Saha, J.K.; Patra, A.K. Polycyclic aromatic hydrocarbons in diverse agricultural soils of central India: Occurrence, sources, and potential risks. Int. J. Environ. Anal. Chem. 2022, 102, 1–15. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, J.; Su, G.; Liu, Z.; Shi, B.; Wang, T. Source apportionment and risk assessment for polycyclic aromatic hydrocarbons in soils at a typical coking plant. Ecotoxicol. Environ. Saf. 2021, 222, 112509. [Google Scholar] [CrossRef]
- Qi, A.; Wang, P.; Lv, J.; Zhao, T.; Huang, Q.; Wang, Y.; Zhang, X.; Wang, M.; Xiao, Y.; Yang, L.; et al. Distributions of PAHs, NPAHs, OPAHs, BrPAHs, and ClPAHs in air, bulk deposition, soil, and water in the Shandong Peninsula, China: Urban-rural gradient, interface exchange, and long-range transport. Ecotoxicol. Environ. Saf. 2023, 265, 115494. [Google Scholar] [CrossRef]
- Chongtai, C.; Tian, L.; Xu, S.; Zilan, W.; Jianhui, T. Spatiotemporal distribution and particle-water partitioning of polycyclic aromatic hydrocarbons in Bohai Sea, China. Water Res. 2023, 244, 120440. [Google Scholar]
- Casal, P.; Cabrerizo, A.; Vila-Costa, M.; Pizarro, M.; Jiménez, B.; Dachs, J. Pivotal role of snow deposition and melting driving fluxes of polycyclic aromatic hydrocarbons at Coastal Livingston Island (Antarctica). Environ. Sci. Technol. 2018, 52, 12327–12337. [Google Scholar] [CrossRef]
- Liu, M.; Cai, M.; Duan, M.; Chen, M.; Lohmann, R.; Lin, Y.; Liang, J.; Ke, H.; Zhang, K. PAHs in the North Atlantic Ocean and the Arctic Ocean: Spatial distribution and water mass transport. J. Geophys. Res. Oceans 2022, 127, e2021JC018389. [Google Scholar] [CrossRef]
- Iriarte, J.; Dachs, J.; Casas, G.; Martínez-Varela, A.; Berrojalbiz, N.; Vila-Costa, M. Snow-dependent biogeochemical cycling of polycyclic aromatic hydrocarbons at coastal Antarctica. Environ. Sci. Technol. 2023, 57, 1625–1636. [Google Scholar] [CrossRef]
- Sanches, S.; Leitão, C.; Penetra, A.; Cardoso, V.V.; Ferreira, E.; Benoliel, M.J.; Crespo, M.T.B.; Pereira, V.J. Direct photolysis of polycyclic aromatic hydrocarbons in drinking water sources. J. Hazard. Mater. 2011, 192, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Sankoda, K.; Kuribayashi, T.; Nomiyama, K.; Shinohara, R. Occurrence and source of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) in tidal flats of the Ariake Bay, Japan. Environ. Sci. Technol. 2013, 47, 7037–7044. [Google Scholar] [CrossRef] [PubMed]
- Sankoda, K.; Nomiyama, K.; Kuribayashi, T.; Shinohara, R. Halogenation of polycyclic aromatic hydrocarbons by photochemical reaction under simulated tidal flat conditions. Polycycl. Aromat. Compd. 2013, 33, 236–253. [Google Scholar] [CrossRef]
- Huang, C.; Xu, X.; Wang, D.; Ma, M.; Rao, K.; Wang, Z. The aryl hydrocarbon receptor (AhR) activity and DNA-damaging effects of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs). Chemosphere 2018, 211, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, C.; Horii, Y.; Tanaka, S.; Asante, K.A.; Ballesteros, F.; Viet, P.H.; Itai, T.; Takigami, H.; Tanabe, S.; Fujimori, T. Occurrence, profiles, and toxic equivalents of chlorinated and brominated polycyclic aromatic hydrocarbons in E-waste open burning soils. Environ. Pollut. 2017, 225, 252–260. [Google Scholar] [CrossRef]
- Xinyan, L.; Mei, M.; Bilin, Z.; Na, L.; Ling, F.; Donghong, W.; Tiangang, L. Chlorinated polycyclic aromatic hydrocarbons induce immunosuppression in THP-1 macrophages characterized by disrupted amino acid metabolism. Environ. Sci. Technol. 2022, 56, 16012–16023. [Google Scholar]
- Xu, X.; Xiao, R.; Dionysiou, D.D.; Spinney, R.; Fu, T.; Li, Q.; Wang, Z.; Wang, D.; Wei, Z. Kinetics and mechanisms of the formation of chlorinated and oxygenated polycyclic aromatic hydrocarbons during chlorination. Chem. Eng. J. 2018, 351, 248–257. [Google Scholar] [CrossRef]
- Wang, Y.; Su, P.; Ge, X.; Ren, H.; Ma, S.; Shen, G.; Chen, Q.; Yu, Y.; An, T. Identification of specific halogenated polycyclic aromatic hydrocarbons in surface soils of petrochemical, flame retardant, and electronic waste dismantling industrial parks. J. Hazard. Mater. 2022, 436, 129160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, G.; Zheng, M.; Liu, S.; Yang, Q.; Liu, X.; Wang, M.; Yang, L. Discovery of significant atmospheric emission of halogenated polycyclic aromatic hydrocarbons from secondary zinc smelting. Ecotoxicol. Environ. Saf. 2022, 238, 113594. [Google Scholar] [CrossRef]
- Jin, R.; Bu, D.; Liu, G.; Zheng, M.; Lammel, G.; Fu, J.; Yang, L.; Li, C.; Habib, A.; Yang, Y.; et al. New classes of organic pollutants in the remote continental environment—Chlorinated and brominated polycyclic aromatic hydrocarbons on the Tibetan Plateau. Environ. Int. 2020, 137, 105574. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.T.; Thang, P.Q.; Ohura, T.; Choi, S.-D. Chlorinated and brominated polycyclic aromatic hydrocarbons in ambient air: Seasonal variation, profiles, potential sources, and size distribution. Rev. Environ. Sci. Biotechnol. 2020, 19, 259–273. [Google Scholar] [CrossRef]
- Grossman, J.N.; Stern, A.P.; Kirich, M.L.; Kahan, T.F. Anthracene and pyrene photolysis kinetics in aqueous, organic, and mixed aqueous-organic phases. Atmos. Environ. 2016, 128, 158–164. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, H.; Chang, F.; Hu, X. Self-sensitized photochlorination of benzo[a]pyrene in saline water under simulated solar light irradiation. J. Hazard. Mater. 2020, 408, 124445. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Xiang, W.; Zhu, F.; Huo, Z.; Wang, Z.; Qu, R. Oxidative degradation and possible interactions of coexisting decabromodiphenyl ether (BDE-209) on polystyrene microplastics in UV/chlorine process. Water Res. 2023, 245, 120560. [Google Scholar] [CrossRef]
- Pouch, A.; Zaborska, A.; Mazurkiewicz, M.; Winogradow, A.; Pazdro, K. PCBs, HCB and PAHs in the seawater of Arctic fjords—Distribution, sources and risk assessment. Mar. Pollut. Bull. 2021, 164, 111980. [Google Scholar] [CrossRef] [PubMed]
- Malley, P.P.A.; Kahan, T.F. Nonchromophoric organic matter suppresses polycyclic aromatic hydrocarbon photolysis in ice and at ice surfaces. J. Phys. Chem. A 2014, 118, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Dubowski, Y.; Hoffmann, M.R. Photochemical transformations in ice: Implications for the fate of chemical species. Geophys. Res. Lett. 2000, 27, 3321–3324. [Google Scholar] [CrossRef]
- Ram, K.; Anastasio, C. Photochemistry of phenanthrene, pyrene, and fluoranthene in ice and snow. Atmos. Environ. 2009, 43, 2252–2259. [Google Scholar] [CrossRef]
- Vetráková, Ľ.; Neděla, V.; Runštuk, J.; Heger, D. The morphology of ice and liquid brine in an environmental scanning electron microscope: A study of the freezing methods. Cryosphere 2019, 13, 2385–2405. [Google Scholar] [CrossRef]
- Noble, J.A.; Michoulier, E.; Aupetit, C.; Mascetti, J. Influence of ice structure on the soft UV photochemistry of PAHs embedded in solid water. Astron. Astrophys. 2020, 644, A22. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kahan, T.F. Physical characterization of frozen aqueous solutions containing sodium chloride and humic acid at environmentally relevant temperatures. ACS Earth Space Chem. 2020, 4, 305–310. [Google Scholar] [CrossRef]
- Zhengnan, T.; Yumeng, Q.; Xiaosheng, T.; Zunyao, W.; Ruijuan, Q. Photochemical transformation of anthracene (ANT) in surface soil: Chlorination and hydroxylation. J. Hazard. Mater. 2023, 452, 131252. [Google Scholar]
- Kahan, T.F.; Donaldson, D.J. Photolysis of polycyclic aromatic hydrocarbons on water and ice surfaces. J. Phys. Chem. A 2007, 111, 1277–1285. [Google Scholar] [CrossRef]
- Xue, S.; Sun, J.; Liu, Y.; Zhang, Z.; Lin, Y.; Liu, Q. Effect of dissolved organic matter fractions on photodegradation of phenanthrene in ice. J. Hazard. Mater. 2019, 361, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Qu, R.; Ge, J.; Wang, Z.; Gu, C. Enhanced Removal of Chlorophene and 17β-estradiol by Mn(III) in a Mixture Solution with Humic Acid: Investigation of Reaction Kinetics and Formation of Co-oligomerization Products. Environ. Sci. Technol. 2018, 52, 13222–13230. [Google Scholar] [CrossRef]
- Blaha, K. Effects of Sodium Chloride on Anthracene Photolysis Kinetics in Aqueous-Organic Solutions. Ph.D. Thesis, Syracuse University, Syracuse, NY, USA, 2017. [Google Scholar]
- Zhao, S.; Xue, S.; Zhang, J.; Zhang, Z.; Sun, J. Dissolved organic matter-mediated photodegradation of anthracene and pyrene in water. Sci. Rep. 2020, 10, 3413. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Clemente, A.; Chica, E.; Peñuela, G.A. Photolysis of a mixture of anthracene and benzo[a]pyrene at ultra-trace levels in natural water with disinfection purposes. JEnvS 2020, 92, 79–94. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, C.; Fang, G.; Chu, L.; Gu, C.; Gao, J. Persistent free radicals from low-molecular-weight organic compounds enhance cross-coupling reactions and toxicity of anthracene on amorphous silica surfaces under light. Environ. Sci. Technol. 2021, 55, 3716–3726. [Google Scholar] [CrossRef]
- Zijin, L.; Xianbo, S.; Jie, F.; Wen, L.; Zhengqing, C. Elevated nitrate promoted photodegradation of PAHs in aqueous phase: Implications for the increased nutrient discharge. J. Hazard. Mater. 2023, 443, 130143. [Google Scholar]
- Jialu, F.; Xianbo, S.; Yongdi, L.; Dongye, Z.; Xiaodi, H.; Wen, L.; Zhengqing, C. New insight into environmental photochemistry of PAHs induced by dissolved organic matters: A model of naphthalene in seawater. Process. Saf. Environ. Prot. 2022, 161, 325–333. [Google Scholar]
- Grossman, J.N.; Kowal, S.F.; Stubbs, A.D.; Cawley, C.N.; Kahan, T.F. Anthracene and pyrene photooxidation kinetics in saltwater environments. ACS Earth Space Chem. 2019, 3, 2695–2703. [Google Scholar] [CrossRef]
- Sobron, P.; Wang, A. Low-temperature raman spectroscopy of materials relevant for planetary. Proc. Lunar. Planet. Sci. 2011, 42, 1580. [Google Scholar]
- Jin, R.; Zheng, M.; Lammel, G.; Bandowe, B.A.M.; Liu, G. Chlorinated and brominated polycyclic aromatic hydrocarbons: Sources, formation mechanisms, and occurrence in the environment. Prog. Energy Combust. Sci. 2020, 76, 100803. [Google Scholar] [CrossRef]
- Glover, C.M.; Rosario-Ortiz, F.L. Impact of halides on the photoproduction of reactive intermediates from organic matter. Environ. Sci. Technol. 2013, 47, 13949–13956. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, T.; Nunomura, M.; Katoh, T. Detection of superoxide anion radical in phospholipid liposomal. Biochim. Biophys. Acta. Biomembr. 1999, 1421, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.J.; Connery, K.A.; DiPippo, M.M.; Feng, J.; Chateauneuf, J.E.; Brennecke, J.F. Laser flash photolysis investigation of the triplet-triplet annihilation of anthracene in supercritical water. J. Phys. Chem. A 1999, 103, 6591–6598. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, Y.; Lei, X.; Liang, X.; Cheng, S.; Ouyang, G.; Yang, X. Assessing the use of probes and quenchers for understanding the reactive species in advanced oxidation processes. Environ. Sci. Technol. 2023, 57, 5433–5444. [Google Scholar] [CrossRef] [PubMed]
- Hullar, T.; Magadia, D.; Anastasio, C. Photodegradation rate constants for anthracene and pyrene are similar in/on ice and in aqueous solution. Environ. Sci. Technol. 2018, 52, 12225–12234. [Google Scholar] [CrossRef]
- Bünau, G.V.; Weber, S.; Wolff, T. Time and temperature dependence of anthracene triplet yields in frozen aqueous micellar solutions: An electron paramagnetic resonance study. J. Photochem. Photobiol. A Chem. 1994, 80, 363–368. [Google Scholar] [CrossRef]
- Greiner, G. The unusual temperature dependence of the fluorescence intensity and lifetime of anthracene in ethanol. J. Photochem. Photobiol. A Chem. 2000, 137, 1–7. [Google Scholar] [CrossRef]
- Matson, P.G.; Martz, T.R.; Hofmann, G.E. High-frequency observations of pH under Antarctic sea ice in the southern Ross Sea. Antarct. Sci. 2011, 23, 607–613. [Google Scholar] [CrossRef]
- Cummings, V.J.; Barr, N.G.; Budd, R.G.; Marriott, P.M.; Safi, K.A.; Lohrer, A.M. In situ response of Antarctic under-ice primary producers to experimentally altered pH. Sci. Rep. 2019, 9, 6069. [Google Scholar] [CrossRef] [PubMed]
- Shaban, Y.A. Solar light-induced photodegradation of chrysene in seawater in the presence of carbon-modified n-TiO2 nanoparticles. Arab. J. Chem. 2019, 12, 652–663. [Google Scholar] [CrossRef]
- Pawar, R.M. The Effect of Soil pH on Degradation of Polycyclic Aromatic Hydrocarbons (PAHs). Ph.D. Thesis, University of Hertfordshire, Hatfield, UK, 2012. [Google Scholar]
- Ohura, T.; Miwa, M. Photochlorination of polycyclic aromatic hydrocarbons in acidic brine solution. Bull. Environ. Contam. Toxicol. 2016, 96, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xue, S.; Zhao, S.; Sun, J.; Zhang, Z. Indirect photodegradation of anthracene and pyrene induced by dissolved organic matter derived reactive species in ice. J. Environ. Chem. Eng. 2023, 11, 111086. [Google Scholar] [CrossRef]
- Kong, Q.; Ye, L.; Pan, Y.; Zhou, Y.; Lei, Y.; Zeng, Z.; Chen, S.; Yao, L.; Zhang, X.; Westerhoff, P.; et al. Photochemical transformation of free chlorine induced by triplet state dissolved organic matter. Environ. Sci. Technol. 2023, 57, 10849–10859. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Cao, S.; Halsall, C.; Niu, J.; Bai, D.; He, G.; Zhang, P.; Ma, H. Photodegradation of hydroxyfluorenes in ice and water: A comparison of kinetics, effects of water constituents, and phototransformation by-products. JEnvS 2023, 124, 139–145. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Quan, X.; Zhang, Y.; Chen, S. Formation of chlorinated intermediate from bisphenol a in surface saline water under simulated solar light irradiation. Environ. Sci. Technol. 2009, 43, 7712–7717. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, X.; Xie, H.; Cai, B.; Bai, Y. Photochlorination of Anthracene in Saline Ice under Simulated Solar Light. Water 2024, 16, 1237. https://doi.org/10.3390/w16091237
Li Y, Hu X, Xie H, Cai B, Bai Y. Photochlorination of Anthracene in Saline Ice under Simulated Solar Light. Water. 2024; 16(9):1237. https://doi.org/10.3390/w16091237
Chicago/Turabian StyleLi, Yujie, Xuefeng Hu, Hao Xie, Beichuan Cai, and Yaxing Bai. 2024. "Photochlorination of Anthracene in Saline Ice under Simulated Solar Light" Water 16, no. 9: 1237. https://doi.org/10.3390/w16091237