Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms
Abstract
:Abbreviations
| COD | Chemical Oxygen Demand |
| SRT | Sludge retention Time |
| MTR | Maximum Tolerable Risk |
| VSS | Volatile suspended solids |
| PAR | Photosynthetic Active Radiation |
| WWTP | Wastewater Treatment Plant |
| PE | Person Equivalent |
1. Introduction
2. Material and Methods
2.1. Scenarios

2.2. Calculations and Parameters
2.2.1. Microalgae
 ) of 0.05 O2/photon. With
) of 0.05 O2/photon. With  the amount of O2 produced per photons received per m2 of ground area is calculated:
the amount of O2 produced per photons received per m2 of ground area is calculated: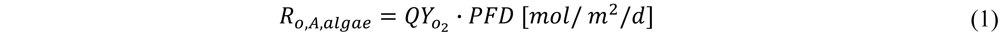
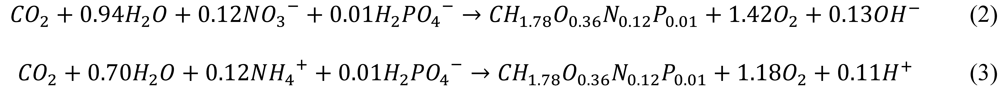

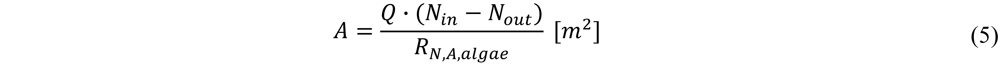
2.2.2. Heterotrophs

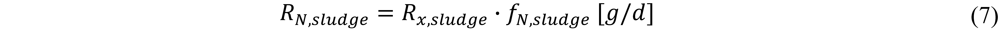
3. Results
| Scenario | Area requirement(m2/PE) | Effluent total N(mg/L) | Effluent total P(mg/L) |
|---|---|---|---|
| Scenario 1 | 0.32 | 5.39 | 0.15 |
| Scenario 2 | 2.10 | 2.20 | 1.40 |
| Scenario 3 | 0.76 | 2.20 | 6.07 |


4. Discussion
4.1. Effluent Concentrations
4.2. Area Requirement
4.3. Seasonal Variation in Temperature and Light Intensity
4.4. Daily Variation in Light Intensity
4.5. Application of Microalgal Biomass
5. Conclusions
Acknowledgements
References
- Brock, T.D.; Madigan, M.T.; Martinko, J.M.; Parker, J. Biology of Microorganisms, 9th ed; Prentice-Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Halterman, S.G.; Toetz, D.W. Kinetics of nitrate uptake by freshwater algae. Hydrobiologia 1984, 114, 209–214. [Google Scholar]
- Collos, Y.; Vaquer, A.; Souchu, P. Acclimation of nitrate uptake by phytoplankton to high substrate levels. J. Phycol. 2005, 41, 466–478. [Google Scholar]
- Eppley, R.W.; Rogers, J.N.; McCarthy, J.J. Half-Saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnol. Oceanogr. 1969, 14, 912–920. [Google Scholar]
- Hwang, S.J.; Havens, K.E.; Steinman, A.D. Phosphorus kinetics of planktonic and benthic assemblages in a shallow subtropical lake. Freshw. Biol. 1998, 40, 729–745. [Google Scholar]
- Stevenson, R.J.; Bothwell, M.L.; Lowe, R.L. Algal Ecology: Freshwater Benthic Ecosystems; Academic Press Elsevier: San diego, CA, USA, 1996. [Google Scholar]
- Di Pippo, F.; Ellwood, N.; Guzzon, A.; Siliato, L.; Micheletti, E.; de Philippis, R.; Albertano, P. Effect of light and temperature on biomass, photosynthesis and capsular polysaccharides in cultured phototrophic biofilms. J. Appl. Phycol. 2011, 24, 1–10. [Google Scholar]
- Sekar, R.; Nair, K.V.K.; Rao, V.N.R.; Venugopalan, V.P. Nutrient dynamics and successional changes in a lentic freshwater biofilm. Freshwater Biol. 2002, 47, 1893–1907. [Google Scholar]
- Roeselers, G.; Loosdrecht, M.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235. [Google Scholar]
- Schumacher, G.; Blume, T.; Sekoulov, I. Bacteria reduction and nutrient removal in small wastewater treatment plants by an algal biofilm. Water Sci. Technol. 2003, 47, 195–202. [Google Scholar]
- Craggs, R.J.; Adey, W.H.; Jenson, K.R.; St. John, M.S.; Green, F.B.; Oswald, W.J. Phosphorous removal from wastewater using algal turf scrubber. Water Sci. Technol. 1996, 33, 191–198. [Google Scholar]
- Shi, J.; Podola, B.; Melkonian, M. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: An experimental study. J. Appl. Phycol. 2007, 19, 417–423. [Google Scholar]
- De Godos, I.; González, C.; Becares, E.; García-Encina, P.; Muñoz, R. Simultaneous nutrients and carbon removal during pretreated swine slurry degradation in a tubular biofilm photobioreactor. Appl. Microbiol. Biotechnol. 2009, 82, 187–194. [Google Scholar]
- González, C.; Marciniak, J.; Villaverde, S.; León, C.; García, P.A.; Munoz, R. Efficient nutrient removal from swine manure in a tubular biofilm photo-bioreactor using algae-bacteria consortia. Water Sci. Technol. 2008, 58, 95–102. [Google Scholar]
- Ley, A.C.; Mauzerall, D.C. Absolute absorption cross-sections for Photosystem II and the minimum quantum requirement for photosynthesis in Chlorella vulgaris. BBA—Bioenergetics 1982, 680, 95–106. [Google Scholar]
- Bjorkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar]
- Qiang, H.; Faiman, D.; Richmond, A. Optimal tilt angles of enclosed reactors for growing photoautotrophic microorganisms outdoors. J. Ferment. Bioeng. 1998, 85, 230–236. [Google Scholar]
- Duboc, P.; Marison, I.; Stockar, U.V. Quantitative calorimetry and biochemical engineering. In Handbook of Thermal Analysis and Calorimetry; Kemp, R.B., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 4. [Google Scholar]
- Healey, F.P. Inorganic nutrient uptake and deficiency in algae. Crit. rev. microbiol. 1973, 3, 69–113. [Google Scholar]
- Ahlgren, G.; Gustafsson, I.B.; Boberg, M. Fatty-acid content and chemical—Composition of fresh-water microalgae. J. Phycol. 1992, 28, 37–50. [Google Scholar]
- Metcalf, I.; Eddy, H. Wastewater Engineering: Treatment and Reuse, 4th ed; McGraw-Hill: Columbus, OH, USA, 2003. [Google Scholar]
- Ho, T.Y.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wyman, K.; Falkowski, P.G.; Morel, F.M.M. The elemental composition of some marine phytoplankton. J. Phycol. 2003, 39, 1145–1159. [Google Scholar]
- Goldman, J.C.; McCarthy, J.J.; Peavey, D.G. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 1979, 279, 210–215. [Google Scholar]
- Elrifi, I.R.; Turpin, D.H. Steady-state luxury consumption and the concept of optimum nutrient ratios: A study with phosphate and nitrate limited Selenastrum minutum (Chlorophyta). J. Phycol. 1985, 21, 592–602. [Google Scholar]
- Klausmeier, C.A.; Litchman, E.; Simon, A.L. Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol. Oceanogr. 2004, 49, 1463–1470. [Google Scholar]
- Powell, N.; Shilton, A.N.; Pratt, S.; Chisti, Y. Factors Influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Envir. Sci. Technol. 2008, 42, 5958–5962. [Google Scholar]
- Christenson, L.B.; Sims, R.C. Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol. Bioeng. 2012. [Google Scholar]
- Liehr, S.K.; Eheart, J.W.; Suidan, M.T. A modeling study of the effect of pH on carbon limited algal biofilms. Water Res. 1988, 22, 1033–1041. [Google Scholar]
- Goldman, J.C.; Carpenter, E.J. A Kinetic approach to the effect of temperature on algal growth. Limnol. Oceanogr. 1974, 19, 756–766. [Google Scholar]
- Vona, V.; Rigano, V.D.M.; Esposito, S.; Carillo, P.; Carfagna, S.; Rigano, C. Growth, photosynthesis, and respiration of Chlorella sorokiniana after N-starvation. Interactions between light, CO2 and NH4+ supply. Physiol. Plantarum 1999, 105, 288–293. [Google Scholar] [CrossRef]
- Hulatt, C.J.; Thomas, D.N. Energy efficiency of an outdoor microalgal photobioreactor sited at mid-temperature latitude. Bioresource technol. 2011, 102, 6687–6695. [Google Scholar]
- Min, M.; Wang, L.; Li, Y.; Mohr, M.J.; Hu, B.; Zhou, W.; Chen, P.; Ruan, R. Cultivating Chlorella sp. in a pilot-scale photobioreactor using centrate wastewater for microalga biomass production and wastewater nutrient removal. Appl. Biochem. Biotech. 2011, 165, 123–137. [Google Scholar] [CrossRef]
- Knuckey, R.M.; Brown, M.R.; Robert, R.; Frampton, D.M.F. Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquacult. Eng. 2006, 35, 300–313. [Google Scholar]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z.; Merzlyak, M. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J. Appl. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar]
- Wetsus Centre of Excellence for Sustainable Water Technology Home Page. Available online: http://www.wetsus.nl (accessed on 16 April 2012).
Correction
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms. Water 2012, 4, 460-473. https://doi.org/10.3390/w4020460
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH. Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms. Water. 2012; 4(2):460-473. https://doi.org/10.3390/w4020460
Chicago/Turabian StyleBoelee, Nadine C., Hardy Temmink, Marcel Janssen, Cees J. N. Buisman, and René H. Wijffels. 2012. "Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms" Water 4, no. 2: 460-473. https://doi.org/10.3390/w4020460
APA StyleBoelee, N. C., Temmink, H., Janssen, M., Buisman, C. J. N., & Wijffels, R. H. (2012). Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms. Water, 4(2), 460-473. https://doi.org/10.3390/w4020460




