Upgrading of Wastewater Treatment Plants Through the Use of Unconventional Treatment Technologies: Removal of Lidocaine, Tramadol, Venlafaxine and Their Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization of ACs and Lab-Scale Adsorption Experiments
2.3. Projects at the WWTPs and Sample Collection
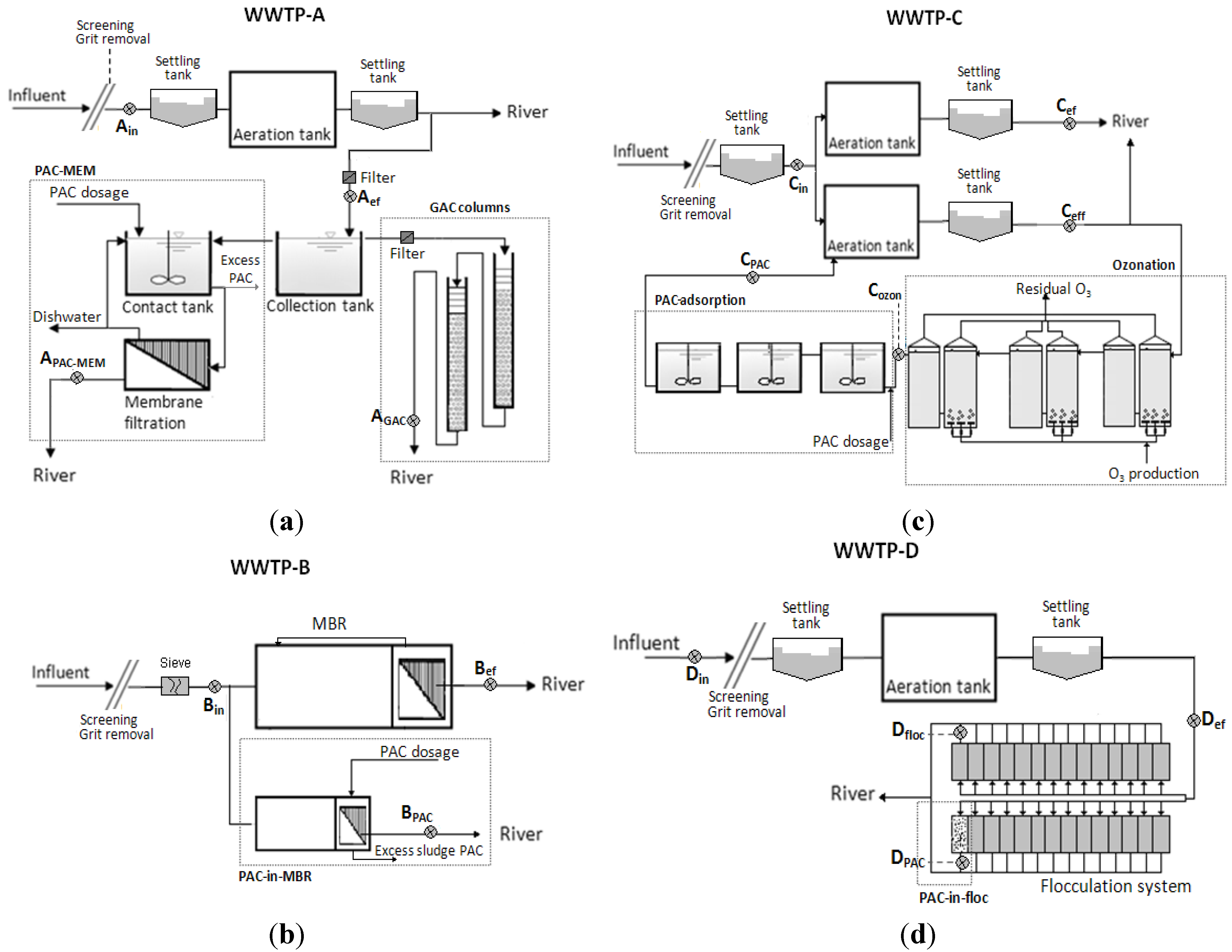
| Characteristics | WWTPs | |||||||
|---|---|---|---|---|---|---|---|---|
| WWTP-A | WWTP-B | WWTP-C | WWTP-D | |||||
| Treatment technology | Conventional activated sludge | Membrane bioreactor (MBR) | Conventional activated sludge | Conventional activated sludge | ||||
| Sampling period | 01/08/2011−06/09/2011 | 01/09/2011−06/10/2011 | 08/08/2011−18/08/2011 | 09/08/2011−15/08/2011 | ||||
| Population served (PE) | 74,000 | 69,000 | 50,000 | 370,000 | ||||
| Average flow (m3/a) | 6,000,000 | 5,500,000 | 6,000,000 | 47,000,000 | ||||
| Wastewater type | 98% R, 2% I | 98% R, 2% I | 92% R, 8% I | 81% R, 19% I | ||||
| Collection system | Combined | Combined | Combined | Combined | ||||
| HRT (h) | 18 | 28.5 | 38 | 51 | ||||
| SRTsludge (d) | 25–30 | 26 | 22 | 12 | ||||
| T (°C) | 19.4 | 17.5 | 19.7 | 18.4 | ||||
| Characteristics | Investigated projects at each WWTP | |||||||
| 1. PAC adsorption followed by membrane filtration (PAC-MEM) | MBR-PAC integrated system (PAC-in-MBR) | Ozonation followed by PAC-adsorption | PAC adsorption in flocculation system (PAC-in-floc) | |||||
| 2. GAC columns | ||||||||
| Dimension | Pilot-scale | Pilot-scale | Full-scale | Full-scale | ||||
| Project | PAC-MEM | GAC-columns | PAC-in-MBR | Ozonation | PAC-ad. | PAC-in-floc | ||
| AC type | Carbopal AP | Hydraffin XC30 | Carbopal AP | - | Norit SAE Super | Norit SAE Super | ||
| AC dosage (mg AC/L of wastewater) | 5 | - | 10 | - | 15 | 20 | ||
| Transferred ozone dose (mg O3/L of wastewater) | - | - | - | 0.6 | - | - | ||
| HRT (h) | 0.9 | - | 24 | 0.4 | 0.9 | 0.4 | ||
| SRTcarbon (day) | 1 | - | 25a | - | 22a | 0.5 | ||
| EBCT (h) | - | 0.4 | - | - | - | - | ||
2.4. Analytical Methods
3. Results and Discussion
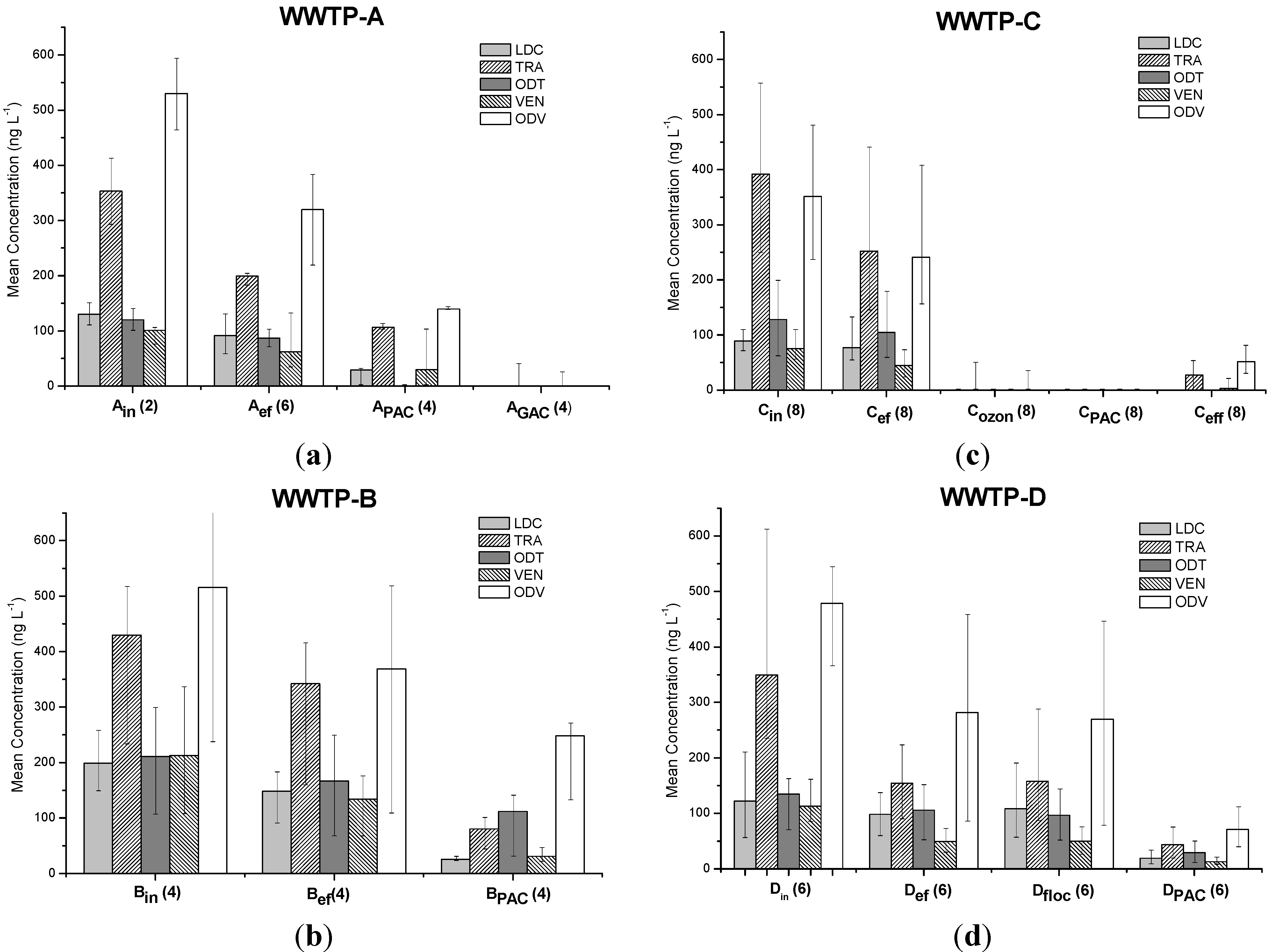
3.1. Occurrence and Removal of Target Analyses through Activated Sludge Treatment
| WWTP | % Removal | ||||
|---|---|---|---|---|---|
| LDC | TRA | ODT | VEN | ODV | |
| WWTP-A | 29 | 43 | 27 | 40 | 39 |
| WWTP-B | 25 | 20 | 21 | 37 | 29 |
| WWTP-C | 14 | 36 | 17 | 40 | 31 |
| WWTP-D | 35 | 56 | 21 | 56 | 41 |
3.2. Removal of Target Analytes through Unconventional Technologies
3.2.1. Adsorption Experiments
| Characteristics | Carbopal AP | Hydraffin XC30 | Norit SAE super |
|---|---|---|---|
| Type | PAC | GAC | PAC |
| Avg. particle size diameter (µm) | 33.6 | 1400 | 15 |
| SBET (m2 g−1) | 899 | 1036 | 965 |
| VT (cm3 g−1) | 0.524 | 0.619 | 0.69 |
| Wo (cm3 g−1) | 0.40 | 0.44 | 0.40 |
| Activated carbon | Freundlich isotherms constants | Langmuir isotherm constants | ||||
|---|---|---|---|---|---|---|
| KF (mg g−1/(mg L−1)1/n) | 1/n | r2 | qm (mg g−1) | KL (L mg−1) | r2 | |
| LDC | ||||||
| Carbopal APa | 137 | 0.13 | 0.773 | 215 | 0.81 | 0.998 |
| Hydraffin XC30-Aa | 56 | 0.31 | 0.918 | 196 | 0.17 | 0.997 |
| Hydraffin XC30-Bb | 89 | 0.27 | 0.851 | 246 | 0.28 | 0.999 |
| Norit SAE Supera | 156 | 0.06 | 0.980 | 204 | 0.79 | 0.999 |
| Activated carbon | TRA | |||||
| Carbopal APa | 46 | 0.15 | 0.964 | 84 | 0.36 | 0.995 |
| Hydraffin XC30-Aa | 25 | 0.23 | 0.965 | 76 | 0.10 | 0.999 |
| Hydraffin XC30-Bb | 29 | 0.23 | 0.948 | 85 | 0.15 | 0.999 |
| Norit SAE Supera | 61 | 0.08 | 0.987 | 87 | 0.39 | 0.999 |
3.2.2. Projects PAC-MEM and GAC-columns
| Treatment system | LDC | TRA | ODT | VEN | ODV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| %RUP | %Rtotal | %RUP | %Rtotal | %RUP | %Rtotal | %RUP | %Rtotal | %RUP | %Rtotal | |
| WWTP-A | ||||||||||
| PAC-MEM | 68 | 77 | 47 | 70 | >80 | >88 | 51 | 67 | 56 | 74 |
| GAC columns | >72 | >93 | >90 | >97 | >80 | >88 | >70 | >90 | >92 | >98 |
| WWTP-B | ||||||||||
| PAC-in-MBR | - | 87 | - | 81 | - | 47 | - | 85 | - | 52 |
| WWTP-C | ||||||||||
| Ozonation | >89 | >91 | >95 | >97 | >84 | >87 | >80 | >88 | >95 | >97 |
| PAC-adsorption | nc | >91 | nc | >97 | nc | >87 | nc | >88 | nc | >97 |
| Secondary clarifier | nc | >91 | nc | 93 | nc | >87 | nc | >88 | nc | 85 |
| WWTP-D | ||||||||||
| Flocculation system | 4 | 37 | 2 | 55 | 5 | 28 | 2 | 56 | 5 | 44 |
| PAC-in-floc | 76 | 84 | 72 | 88 | 73 | 79 | 73 | 87 | 71 | 85 |
3.2.3. Project PAC-in-MBR
3.2.4. Project Ozonation Followed by PAC-Adsorption
3.2.5. Project PAC-in-floc
3.2.6. Mechanisms for Adsorption onto AC and Ozonation
3.3. Evaluation of other Parameters Relevant to the Wastewater Treatment

4. Conclusions
Acknowledgments
References
- Clara, M.; Strenn, M.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Smook, T.M.; Zho, H.; Zytner, R.G. Removal of ibuprofen from wastewater: Comparing biodegradation in conventional, membrane bioreactor, and biological nutrient removal treatment systems. Water Sci. Technol. 2008, 57, 1–8. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Wastewater treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro River Basin (Northeast Spain). Environ. Toxicol.Chem. 2007, 26, 1553–1562. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving water. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Palmer, P.M.; Wilson, L.R.; O’Keefe, P.; Sheridan, R.; King, T.; Chen, C.-Y. Sources of pharmaceutical pollution in the New York City watershed. Sci. Total Environ. 2008, 394, 90–102. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.; Andrews, D.M. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.; Püttmann, W. Occurrence and removal of lidocaine, tramadol, venlafaxine and their metabolites in German wastewater treatment plants. Environ. Sci. Pollut. Res. 2012, 19, 689–699. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The ocurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total Environ. 2008, 397, 158–166. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S. Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef]
- Nentwig, G.; Oetken, O.; Oehlmann, J. Effects of pharmaceuticals on aquatic invertebrates—The example of carbamazepine and clofibric acid. In Pharmaceuticals in the Environment, Sources, Fate, Effects and Risks, 2nd; Kümmerer, K., Ed.; Springer-Verlag: Berlin, Germany, 2004; pp. 195–207. [Google Scholar]
- Schulte-Oehlmann, U.; Oetken, M.; Bachmann, J.; Oehlmann, J. Effects of ethinyloestradiol and methyltestosterone in prosobranch snails. In Pharmaceuticals in the Environment, Sources, Fate, Effects and Risks, 2nd; Kümmerer, K., Ed.; Springer-Verlag: Berlin, Germany, 2004; pp. 233–247. [Google Scholar]
- Fong, P.P.; Hoy, C.M. Antidepressants (venlafaxine and citalopram) cause foot detachment from the substrate in freshwater snails at environmentally relevant concentrations. Mar. Freshw. Behav. Phy. 2012, 45, 145–153. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; Mcardell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar]
- Huber, M.M.; Göbel, A.; Joss, A.; Herrmann, N.; Löffler, D.; MCardell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Dosoretz, C.G.; Böddeker, K. Removal of trace organics from water using a pumped bed-membrane bioreactor with powdered activated carbon. J. Memb. Sci. 2004, 239, 81–90. [Google Scholar] [CrossRef]
- Li, X.; Hai, F.I.; Nghiem, L.D. Simultaneous activated carbon adsorption within a membrane bioreactor for an enhanced micropollutant removal. Bioresour. Technol. 2011, 102, 5319–5324. [Google Scholar] [CrossRef]
- Ternes, T.A.; Joss, A. Human Pharmaceuticals, Hormones and Fragrances: The Challenge of Micropollutants in Urban Water Management; IWA Publishing: London, UK, 2006. [Google Scholar]
- Bandosz, T.J. Activated carbon surfaces in environmental remediation. In Interface Science and Technology; Elservier: Amsterdam, The Netherlands, 2006; Volume 7. [Google Scholar]
- Kim, S.H.; Shon, H.K.; Ngo, H.H. Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J. Ind. Eng. Chem. 2010, 16, 344–349. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; Mcdowell, D.; Sacher, F.; Brauch, H.J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Mestre, A.S.; Haro, M.; Ania, C.O. Advanced Methods for the removal of acetaminophen from water. In Acetaminophen, properties, clinical uses and adverse effects; Javaherian, A., Latifpour, P., Eds.; Nova Science Publishers Inc.: Hauppauge, New York, NY, USA, 2012. [Google Scholar]
- Ania, C.O.; Pelayo, J.G.; Bandosz, T.J. Reactive adsorption of penicillin in activated carbons. Adsorption 2011, 17, 421–429. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Impact of wastewater treatment plant discharge of lidocaine, tramadol, venlafaxine and their metabolites on the quality of surface waters and groundwate. J. Environ. Monitor. 2012, 14, 1391–1399. [Google Scholar] [CrossRef]
- Lajaunesse, A.; Gagnon, C.; Sauvé, S. Determination of basic antidepressants and their n-desmethyl metabolites in raw sewage and wastewater using solid phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vadja, A.M. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar]
- Reungoat, J.; Macova, M.; Escher, B.I.; Carswell, S.; Mueller, J.F.; Keller, J. Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water Res. 2010, 44, 625–637. [Google Scholar] [CrossRef]
- Lee, C.O.; Howe, K.J.; Thomson, B.M. Ozone and biofiltration as an alternative to reverse osmosis for removing PPCPs and micropollutants from treated wastewater. Water Res. 2012, 46, 1005–1014. [Google Scholar] [CrossRef]
- Dubinin, M.M. Microporous structures of carbonaceous adsorbents. In Characterization of Porous Solids; Greeg, S.J., Sing, K.S.W., Stoecki, H.F., Eds.; Society of Chemical Industry: London, UK, 1979. [Google Scholar]
- German Institute for Standardization, Limits of Detection, Identification and Quantitation (in German); DIN 32645; German Institute for Standardization: Berlin, Germany, 1994.
- German Federal Ministry of Justice, Regulation on Requirements for the Discharge of Wastewater into Surface Waters (in German); German Federal Ministry of Justice: Berlin, Germany, 1997.
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Siegrist, H.; Joss, A.; Alder, A.; Gobel, A.; Keller, E.; McArdell, C.; Ternes, T.A. Micropollutants—New requirements for wastewater treatment (in German). EAWAG News 2003, 57, 7–10. [Google Scholar]
- Larsen, T.A.; Lienert, J.; Joss, A.; Siegrist, H. How to avoid pharmaceuticals in the aquatic environment. J. Biotechnol. 2004, 113, 295–304. [Google Scholar]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. The occurrence and removal of selected pharmaceutical compounds in a sewage treatment works utilising activated sludge treatment. Environ. Pollut. 2007, 145, 738–744. [Google Scholar] [CrossRef]
- Gomez, M.J.; Bueno, M.J.M.; Lacorte, S.; Fernandez-Alba, A.R.; Aguera, A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef]
- Wang, L.; Govind, R. Sorption of toxic organic compounds on wastewater solids: Mechanism and modeling. Environ. Sci. Technol. 1993, 27, 152–158. [Google Scholar] [CrossRef]
- Ania, C.O.; Bandosz, T.J. Importance of structural and chemical heterogeneity of activated carbon surfaces for adsorption of dibenzothiophene. Langmuir 2005, 21, 7752–7759. [Google Scholar] [CrossRef]
- Pipe-Martin, C.; Reungoat, J.; Keller, J. Dissolved Organic Carbon Removal by Biological Treatment; CRC for Water Quality Research Australia: Adelaide, Australia, 2010. [Google Scholar]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of trace organic contaminants by a membrane bioreactor-granular activated carbon (MBR-GAC) system. Bioresour. Technol. 2011, 113, 169–173. [Google Scholar]
- Grünebaum, T. FinalReport Phase 1: Elimination of Drug Residues in Sewage Treatment Plants (in German); Ministry for Climate Protection, Environment, Agriculture, Nature Conservation and Consumer Protection of the German State of North Rhine-Westphalia: Düsseldorf, Germany, 2011. [Google Scholar]
- Schmidt, C.K.; Brauch, H.-J. N,N-dimethylsulfamide as precursor for N-nitrosodimethylamine (NDMA) formation upon ozonation and its fate during drinking water treatment. Environ. Sci. Technol. 2008, 42, 6340–6346. [Google Scholar] [CrossRef]
- SRC PhysProp Database. Available online: http://www.syrres.com/what-we-do/databaseforms.aspx?id=386 (accessed on 3 September 2012).
- Tzvetkov, M.V.; Saadatmand, A.R.; Lötsch, J.; Tegeder, I.; Stingl, J.C.; Brockmöller, J. Genetically polymorphic OCT1: Another piece in the puzzle of the variable pharmacokinetics and pharmacodynamics of the opioidergic drug tramadol. Clin. Pharmacol. Ther. 2011, 90, 143–150. [Google Scholar] [CrossRef]
- Ellingrod, V.L.; Perry, P.J. Venlafaxine: A heterocyclic antidepressant. Am. J. Hosp. Pharm. 1994, 51, 3033–3046. [Google Scholar]
- Molconvert, version 5.8.2; Molecule File Conversion with MolConverter; ChemAxon: Budapest, Hungary, 2012.
- Babić, S.; Horvat, A.J.M.; Pavlović, D.M.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- Cabrita, I.; Ruiz, B.; Mestre, A.S.; Fonseca, I.M.; Carvalho, A.P.; Ania, C.O. Removal of an analgesic using activated carbons prepared from urban and industrial residues. Chem. Eng. J. 2010, 163, 249–255. [Google Scholar] [CrossRef]
- Ng, A.S.; Stenstrom, M.K. Nitrification in powdered activated carbon-activated sludge process. J. Environ. Eng. 1987, 113, 1285–1301. [Google Scholar] [CrossRef]
- Serrano, D.; Suárez, S.; Lema, J.M.; Omil, F. Removal of persistent pharmaceutical micropollutants from sewage by addition of PAC in a sequential membrane bioreactor. Water Res. 2011, 45, 5323–5333. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rúa-Gómez, P.C.; Guedez, A.A.; Ania, C.O.; Püttmann, W. Upgrading of Wastewater Treatment Plants Through the Use of Unconventional Treatment Technologies: Removal of Lidocaine, Tramadol, Venlafaxine and Their Metabolites. Water 2012, 4, 650-669. https://doi.org/10.3390/w4030650
Rúa-Gómez PC, Guedez AA, Ania CO, Püttmann W. Upgrading of Wastewater Treatment Plants Through the Use of Unconventional Treatment Technologies: Removal of Lidocaine, Tramadol, Venlafaxine and Their Metabolites. Water. 2012; 4(3):650-669. https://doi.org/10.3390/w4030650
Chicago/Turabian StyleRúa-Gómez, Paola C., Arlen A. Guedez, Conchi O. Ania, and Wilhelm Püttmann. 2012. "Upgrading of Wastewater Treatment Plants Through the Use of Unconventional Treatment Technologies: Removal of Lidocaine, Tramadol, Venlafaxine and Their Metabolites" Water 4, no. 3: 650-669. https://doi.org/10.3390/w4030650





