Resistance of Two Mediterranean Cold-Water Coral Species to Low-pH Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Experimental Setup
2.2. Skeletal Measurements
2.3. Statistical Analysis
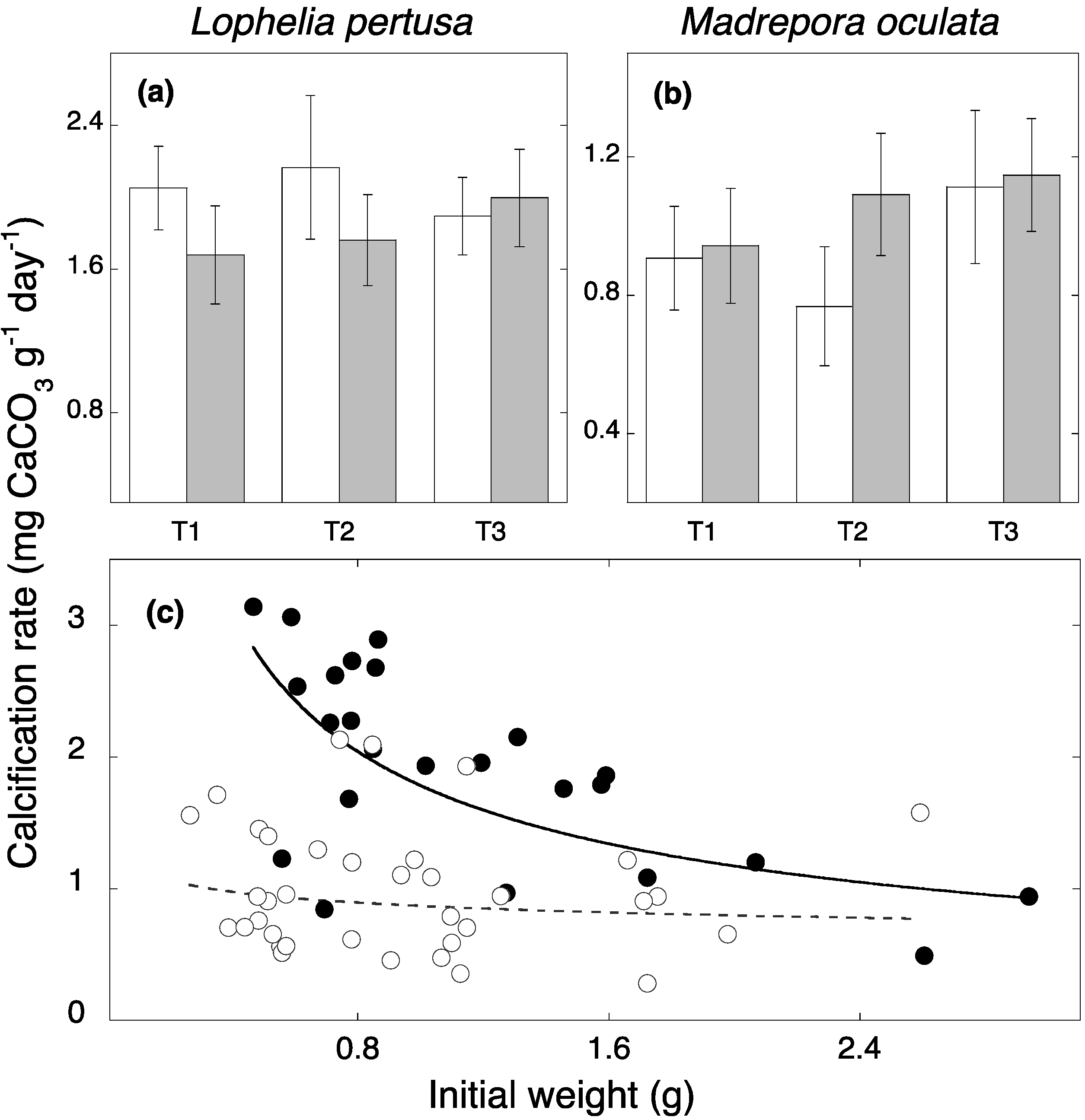
3. Results and Discussion
| Measured parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | pHT | TA | Sal | T | ||||
| Control | 8.092 ± 0.022 | 2540 ± 13 | 37.6 ± 0.1 | 12.3 ± 0.3 | ||||
| (8.061 − 8.112) | (2521 − 2551) | (37.5 − 37.8) | (11.9 − 12.5) | |||||
| High-CO2 | 7.808 ± 0.031 | 2541 ± 12 | 37.6 ± 0.1 | 12.3 ± 0.2 | ||||
| (7.787 − 7.854) | (2524 − 2551) | (37.5 − 37.7) | (12.0 − 12.5) | |||||
| Calculated parameters | ||||||||
| Treatment | pCO2 | χCO2 | DIC | [CO2]aq | [HCO3−] | [CO32−] | ΩC | ΩA |
| Control | 384 ± 23 | 389± 24 | 2286 ± 16 | 15.4 ± 0.9 | 2087 ± 20 | 184 ± 7 | 4.3 ± 0.2 | 2.8 ± 0.1 |
| High-CO2 | 809 ± 61 | 821 ± 61 | 2420 ± 18 | 32.4 ± 2.6 | 2283 ± 21 | 105 ± 7 | 2.5 ± 0.2 | 1.6 ± 0.1 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gattuso, J.P.; Hansson, L. Ocean Acidification: Background and History. In Ocean Acidification; Gattuso, J.P., Hansson, L., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 1–20. [Google Scholar]
- Khatiwala, S.; Tanhua, T.; Mikaloff-Fletcher, S.; Gerber, M.; Doney, S.C.; Graven, H.D.; Gruber, N.; McKinley, G.A.; Murata, A.; Ríos, A.F.; et al. Global ocean storage of anthropogenic carbon. Biogeosciences 2013, 10, 2169–2191. [Google Scholar] [CrossRef] [Green Version]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Touratier, F.; Goyet, C. Impact of the Eastern Mediterranean Transient on the distribution of anthropogenic CO2 and first estimate of acidification for the Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 1–15. [Google Scholar] [CrossRef]
- Schneider, A.; Wallace, D.W.R.; Körtzinger, A. Alkalinity of the Mediterranean sea. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar]
- Schneider, A.; Tanhua, T.; Körtzinger, A.; Wallace, D.W.R. High anthropogenic carbon content in the eastern Mediterranean. J. Geophys. Res. 2010. [Google Scholar] [CrossRef]
- Touratier, F.; Goyet, C. Decadal evolution of anthropogenic CO2 in the northwestern Mediterranean Sea from the mid-1990s to the mid-2000s. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 1708–1716. [Google Scholar] [CrossRef]
- Calvo, E.; Simó, R.; Coma, R.; Ribes, M.; Pascual, J.; Sabatés, A.; Gili, J.M.; Pelejero, C. Effects of climate change on Mediterranean marine ecosystems: The case of the Catalan Sea. Clim. Res. 2011, 50, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Guinotte, J.M.; Orr, J.C.; Cairns, S.S.; Freiwald, A.; Morgan, L.; George, R. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 2006, 4, 141–146. [Google Scholar] [CrossRef]
- Steinacher, M.; Joos, F.; Frölicher, T.L.; Plattner, G.K.; Doney, S.C. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences 2009, 6, 515–533. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, A.; Kawamiya, M.; Ishida, A.; Yamanaka, Y.; Watanabe, S. Impact of rapid sea-ice reduction in the Arctic Ocean on the rate of ocean acidification. Biogeosciences 2012, 9, 2365–2375. [Google Scholar] [CrossRef]
- Thresher, R.; Tilbrook, B.; Fallon, S.J.; Wilson, N.C.; Adkins, J. Effects of chronic low carbonate saturation levels on the distribution, growth and skeletal chemistry of deep-sea corals and other seamount megabenthos. Mar. Ecol. Prog. Ser. 2011, 442, 87–99. [Google Scholar] [CrossRef]
- Form, A.U.; Riebesell, U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Chang. Biol. 2012, 18, 843–853. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 2009, 37, 1131–1134. [Google Scholar] [CrossRef]
- Pelejero, C.; Calvo, E.; Hoegh-Guldberg, O. Paleo-perspectives on ocean acidification. Trends Ecol. Evol. 2010, 25, 332–344. [Google Scholar] [CrossRef]
- Wicks, L.; Roberts, J.M. Benthic invertebrates in a high-CO2 world. Oceanogr. Mar. Biol. Annu. Rev. 2012, 50, 127–188. [Google Scholar]
- Parker, L.; Ross, P.; Connor, W.; Pörtner, H.; Scanes, E.; Wright, J. Predicting the response of molluscs to the impact of ocean acidification. Biology 2013, 2, 651–692. [Google Scholar] [CrossRef]
- Maier, C.; Hegeman, J.; Weinbauer, M.G. Calcification of the cold-water coral Lophelia pertusa under ambient and reduced pH. Biogeosciences 2009, 6, 1671–1680. [Google Scholar] [CrossRef]
- Maier, C.; Watremez, P.; Taviani, M.; Weinbauer, M.G.; Gattuso, J.P. Calcification rates and the effect of ocean acidification on Mediterranean cold-water corals. Proc. R. Soc. Lond. B. Biol. Sci. 2012, 279, 1716–1723. [Google Scholar] [CrossRef]
- Maier, C.; Schubert, A.; Berzunza-Sánchez, M.M.; Weinbauer, M.G.; Watremez, P.; Gattuso, J.P. End of the century pCO2 levels do not impact calcification in Mediterranean cold-water corals. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Hennige, S.J.; Wicks, L.C.; Kamenos, N.A.; Bakker, D.; Findlay, H.S.; Dumousseaud, C.; Roberts, J.M. Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to Ocean Acidification. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013. [Google Scholar] [CrossRef]
- Olariaga, A.; Gori, A.; Orejas, C.; Gili, J.M. Development of an autonomous aquarium system for maintaining deep corals. Oceanography 2009, 22, 44–45. [Google Scholar] [CrossRef] [Green Version]
- Bramanti, L.; Movilla, J.; Guron, M.; Calvo, E.; Gori, A.; Dominguez-Carrió, C.; Grinyó, J.; López-Sanz, A.; Martínez-Quintana, A.; Pelejero, C.; et al. Detrimental effects of Ocean Acidification on the economically important Mediterranean red coral (Corallium rubrum). Glob. Chang. Biol. 2013, 19, 1897–1908. [Google Scholar] [CrossRef]
- Perez, F.F.; Fraga, F. A precise and rapid analytical procedure for alkalinity determination. Mar. Chem. 1987, 21, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Perez, F.F.; Rios, A.F.; Rellán, T.; Alvarez, M. Improvements in a fast potentiometric seawater alkalinity determination. Ciencias Mar. 2000, 26, 463–478. [Google Scholar]
- Clayton, T.D.; Byrne, R.H. Spectrophotometric seawater pH measurements: Total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep Sea Res. I 1993, 40, 2115–2129. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Maragos, J.E.; Franzisket, L. Coral Growth: Buoyant Weight Technique. In Coral Reef: Research Methods; Stoddart, D.R., Johannes, R.E., Eds.; United Nations Educational Scientific and Cultural Organization: Paris, France, 1978; pp. 529–541. [Google Scholar]
- Davies, P.S. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989, 101, 389–395. [Google Scholar] [CrossRef]
- Bucher, D.; Harriott, V.J.; Roberts, L.G. Skeletal micro-density, porosity and bulk density of acroporid corals. J. Exp. Mar. Bio. Ecol. 1998, 228, 117–136. [Google Scholar] [CrossRef]
- Movilla, J.; Orejas, C.; Calvo, E.; Gori, A.; López-Sanz, A.; Grinyó, J.; Dominguez-Carrió, C.; Pelejero, C. Differential response of two Mediterranean cold-water coral species to ocean acidification. Coral Reefs 2013. submitted. [Google Scholar]
- Movilla, J.; Calvo, E.; Pelejero, C.; Coma, R.; Serrano, E.; Fernández-Vallejo, P.; Ribes, M. Calcification reduction and recovery in native and non-native Mediterranean corals in response to ocean acidification. J. Exp. Mar. Bio. Ecol. 2012, 438, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; de Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003, 142, 419–426. [Google Scholar]
- Cohen, A.L.; Holcomb, M. Why corals care about ocean acidification: Uncovering the mechanism. Oceanography 2009, 22, 118–127. [Google Scholar] [CrossRef]
- Allemand, D.; Tambutté, É.; Zoccola, D.; Tambutté, S. Coral Calcification, Cells to Reefs. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Heidelberg, Germany, 2011; pp. 119–150. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Movilla, J.; Gori, A.; Calvo, E.; Orejas, C.; López-Sanz, À.; Domínguez-Carrió, C.; Grinyó, J.; Pelejero, C. Resistance of Two Mediterranean Cold-Water Coral Species to Low-pH Conditions. Water 2014, 6, 59-67. https://doi.org/10.3390/w6010059
Movilla J, Gori A, Calvo E, Orejas C, López-Sanz À, Domínguez-Carrió C, Grinyó J, Pelejero C. Resistance of Two Mediterranean Cold-Water Coral Species to Low-pH Conditions. Water. 2014; 6(1):59-67. https://doi.org/10.3390/w6010059
Chicago/Turabian StyleMovilla, Juancho, Andrea Gori, Eva Calvo, Covadonga Orejas, Àngel López-Sanz, Carlos Domínguez-Carrió, Jordi Grinyó, and Carles Pelejero. 2014. "Resistance of Two Mediterranean Cold-Water Coral Species to Low-pH Conditions" Water 6, no. 1: 59-67. https://doi.org/10.3390/w6010059
APA StyleMovilla, J., Gori, A., Calvo, E., Orejas, C., López-Sanz, À., Domínguez-Carrió, C., Grinyó, J., & Pelejero, C. (2014). Resistance of Two Mediterranean Cold-Water Coral Species to Low-pH Conditions. Water, 6(1), 59-67. https://doi.org/10.3390/w6010059





