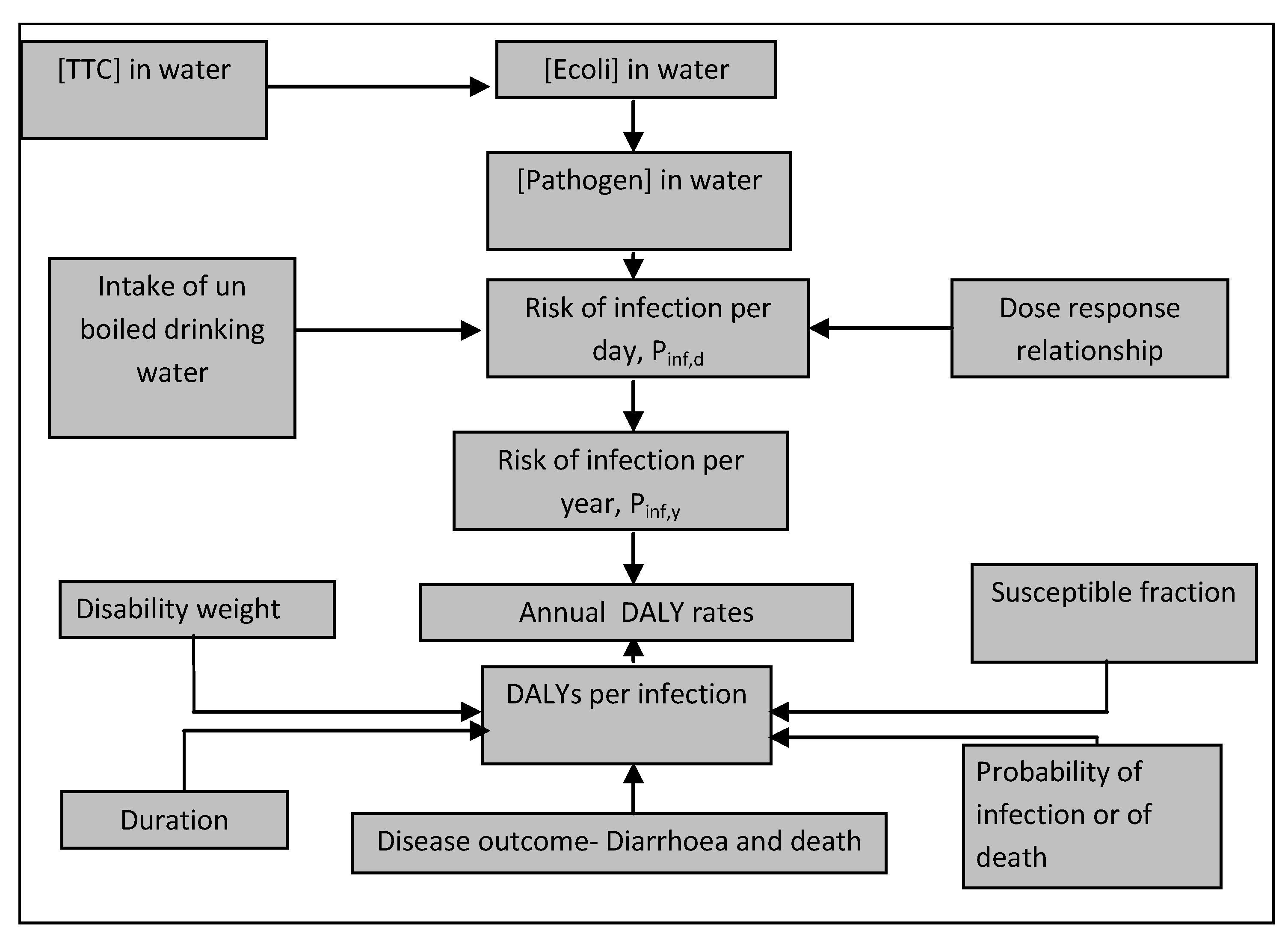
3.2. Water Analysis
Table 3 presents the arithmetic mean ± SD, median (95% CI) and percentiles (95% CI) for [TTC]
source, [TTC]
end-use and arsenic (As) in drinking water for the whole study area. We found [TTC]
end-use to be typically greater than [TTC]
source for most of the paired samples with almost no correlation between them (
Figure 3). The mean and median [TTC]
source for DTWs in this study (0 CFU/100mL) are very comparable to that observed by Howard
et al. [
10] in similar areas in Bangladesh. The observed median arsenic concentration, 28 µg/L is comparable to that previously observed by Mondal
et al. [
16] of 27.9 µg/L (n = 2171). We found no significant correlation between As and [TTC]
source (
Figure 4) and observed TTC and As in source water to be the highest for the treatment plants among the three different mitigation options. A Kruskal-Wallis test to determine the differences in [TTC]
source between the three different mitigation options, showed DTWs to have significantly lower [TTC]
source (
p < 0.05) compared to TP and TW.
Table 3.
Summary of water analyses.
Table 3.
Summary of water analyses.
| Parameter | [TTC]source | [TTC]end-use | [As] |
|---|
| Unit | CFU/100 mL | CFU/100 mL | µg/L |
|---|
| Number of samples | 102 | 102 | 102 |
| Mean ± S.D. | 5.6 ± 16 | 56 ± 162 | 40 ± 40 |
| Median (95% CI) | 0 (0–1) | 7 (2–18) | 28 (19–37) |
| 5th percentile | 0 (0–0) | 0 (0–0) | 0.76 (0–2) |
| 25th percentile | 0 (0–0) | 0 (0–2) | 7 (4–14) |
| 75th percentile | 2 (1–5) | 36 (24–69) | 57 (46–89) |
| 95th percentile | 41 (12–80) | 256 (126–843) | 112 (100–143) |
Table 4 presents [TTC]
end-use stratified by the different risk factors for this study. We found [TTC]
end-use was higher for households with positive [TTC]
source than for those with [TTC]
source = 0. [TTC]
end-use was found to be highest for the water sourced from TW for the studied area. It was also noted that people using TW mostly use the water for drinking, cooking and washing (75%) whereas people using the DTW and TP used the water either only for drinking (23%) or for drinking and cooking (75%). We also observed that [TTC]
end-use was higher for respondents living in mud houses having primary education and storing the water in big mouth containers such as buckets and
hari. But it was found to be lower for households cleaning the storage container weekly rather than daily for the overall data, perhaps reflecting differences in the sampled populations using daily and weekly cleaning regimes.
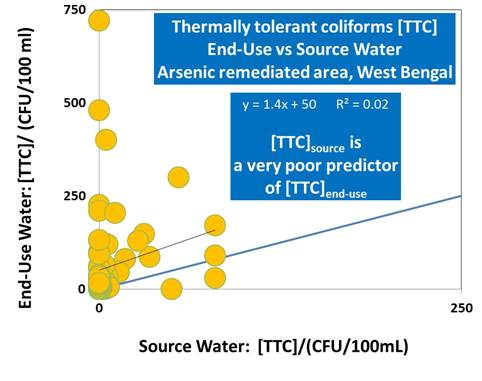
Figure 3.
Cross-plot of thermally tolerant coliforms [TTC] in supplied and end-use drinking water from households in Chakda Block, West Bengal, India. The correlation between [TTC]end-use and [TTC]source is extremely poor—thus [TTC]source is an extremely poor predictor of [TTC]end-use and hence of water-borne pathogen attributable health risks to householders. For the majority of households, [TTC]end-use >> [TTC]source suggesting that post-supply contamination, either during transport to the household or within the household is an important process.
Figure 3.
Cross-plot of thermally tolerant coliforms [TTC] in supplied and end-use drinking water from households in Chakda Block, West Bengal, India. The correlation between [TTC]end-use and [TTC]source is extremely poor—thus [TTC]source is an extremely poor predictor of [TTC]end-use and hence of water-borne pathogen attributable health risks to householders. For the majority of households, [TTC]end-use >> [TTC]source suggesting that post-supply contamination, either during transport to the household or within the household is an important process.
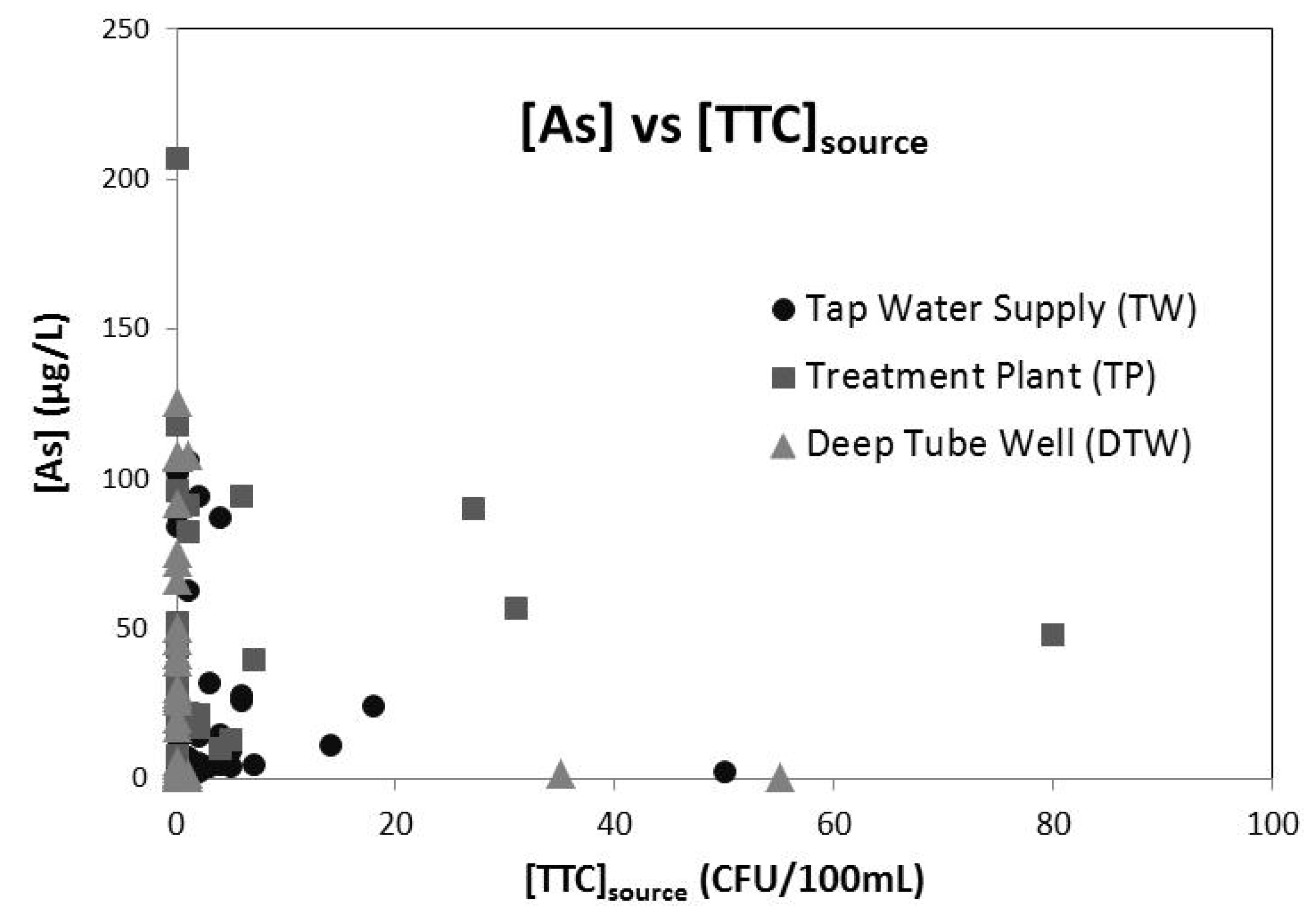
Figure 4.
Cross-plot of arsenic and thermally tolerant coliforms in water supplied to households in the Chakdha, West Bengal, India study area through three different arsenic mitigation options. There is no significant correlation between [As] and [TTC]source for any of Tap Water Supply (●; R2 = 0.034), Arsenic Treatment Plant (■, R2 = −0.002), Deep Tube Wells (▲, R2 = 0.064) of for the dataset as a whole (R2 = 0.003). R2 refers to the correlation for the simple least squares linear regression between [As] and [TTC]source for the relevant dataset.
Figure 4.
Cross-plot of arsenic and thermally tolerant coliforms in water supplied to households in the Chakdha, West Bengal, India study area through three different arsenic mitigation options. There is no significant correlation between [As] and [TTC]source for any of Tap Water Supply (●; R2 = 0.034), Arsenic Treatment Plant (■, R2 = −0.002), Deep Tube Wells (▲, R2 = 0.064) of for the dataset as a whole (R2 = 0.003). R2 refers to the correlation for the simple least squares linear regression between [As] and [TTC]source for the relevant dataset.
Table 4.
[TTC]end-use classified by independent or predictor variables.
Table 4.
[TTC]end-use classified by independent or predictor variables.
| Variable | Classification criteria | Mean [TTC]end-use | SD [TTC]end-use |
|---|
| [TTC]source | =0 | 45 | 114 |
| >0 | 77 | 207 |
| Mitigation type | TW (tap water) | 101 | 239 |
| TP (treatment plant) | 38 | 51 |
| DTW (deep tube well) | 21 | 55 |
| Highest level of education | Higher | 39 | 123 |
| Middle | 4.0 | 0.0 |
| Primary | 89 | 250 |
| Nil | 28 | 37 |
| Type of storage container | Bottle | 38 | 73 |
| Jug | 2.7 | 4.6 |
| Pitcher | 46 | 129 |
| Jar | 17 | 30 |
| Bucket | 129 | 301 |
| Hari | 260 | 198 |
| Cleaning frequency | Daily | 72 | 192 |
| Weekly | 34 | 80 |
Table 5 presents the results of fitting the regression model. The model, when refitted after dropping outliers and influential values, produced similar results. The results indicate that the linear term for [TTC]
source is significantly positive (
p-value = 0.002), that is, among those with positive [TTC]
source, [TTC]
end-use has a significant linear relationship with [TTC]
source after adjusting for other confounders. The estimate for the linear term is 0.05301 (95% confidence interval = (0.025, 0.0933)) indicating that if all other factors remaining constant, a unit increase in [TTC]
source will lead to a 1.054440 (=exp(0.05301)) fold increase in [TTC]
end-use. In general, we can say that an x unit increase in [TTC]
source will lead to an exp(0.05301x) fold increase in [TTC]
end-use if the source water is contaminated. It was further noted that DTW users have significantly lower [TTC]
end-use. Cross tabulations of the predictors revealed that DTW users are more likely to live in mud houses, and have higher education levels. None of the other interactions were statistically significant at the 5% level. However the direction of the estimated coefficients suggests that positive [TTC]
source leads to lowest [TTC]
end-use for those using water from treatment plants (TP) followed by tap water (TW) and then deep tube wells (DTW). Positive [TTC]
source leads to lower [TTC]
end-use for those living in mixed houses than for those living in mud houses. The main effect and interactions are marginally significant for the cleaning frequency of the storage container. If [TTC]
source = 0, then weekly cleaning leads to a 0.45 (=exp(−0.79202)) fold reduction in [TTC]
end-use whereas if [TTC]
source > 0, weekly cleaning leads to a 1.8 fold increase in [TTC]
end-use.
An analysis of variance showed that the maximum variation in [TTC]end-use was explained by the linear term for [TTC]source (15%) followed by the type of mitigation from where the water is sourced (10%) and the interaction between the type of house and whether or not [TTC]source > 0 (4%). While the model allowing for a nonlinear effect of [TTC]source was found to have better predictive power over the linear effect model (p value from Analysis of Deviance = 0.02), we observed no evidence of significant contrasts after correcting for multiple comparisons. Based on the results of the Tobit analysis we found a highly significant effect of the linear term for [TTC]source (p-value = 0.0004). The analysis did not find evidence for a significant effect of any of the other risk factors. Finally the output from application of the SIMEX algorithm showed that the interactions were no longer marginally significant but the other estimates and p-values continued to be similar.
Table 5.
Regression model a of [TTC]end-use.
Table 5.
Regression model a of [TTC]end-use.
| Variable | Estimate | Standard Error | t | Pr (>|t|) b |
|---|
| (Intercept) | 1.48749 | 1.32 | 1.125 | 0.26 |
| (if [TTC]source = 0) | 2.93330 | 1.37 | 2.145 | 0.035 * |
| ([TTC]source − 1) * (if [TTC]source > 0) | 0.05301 | 0.02 | 3.167 | 0.0021 ** |
| (Type of mitigation = TP) | −0.61338 | 0.43 | −1.415 | 0.16 |
| (Type of mitigation = DTW) | −1.75188 | 0.57 | −3.084 | 0.0027 ** |
| (Type of house = Mud) | −0.17066 | 0.53 | −0.322 | 0.75 |
| (Cleaning frequency = Weekly) | −0.79202 | 0.45 | −1.751 | 0.084 |
| (if [TTC]source > 0) * (Type of mitigation = TP) | −0.77046 | 1.10 | −0.703 | 0.48 |
| (if [TTC]source > 0) * (Type of mitigation = DTW) | 0.87905 | 1.08 | 0.813 | 0.42 |
| (if [TTC]source > 0) * (Type of house = Mud) | 2.23692 | 1.48 | 1.507 | 0.14 |
| (if [TTC]source > 0) * (Cleaning frequency = weekly) | 1.41970 | 0.80 | 1.764 | 0.081 |
3.4. Water Quality
Against the WHO [
26] guideline of absence of any fecal pollution as the standard for microbial water quality, the presence of TTC in 25% of the collected source water samples and 50% of the end-use household water samples confirms the potential risk of water-borne diseases from drinking water contamination for the studied area. We also found the arsenic concentration in the collected arsenic mitigated drinking water to be higher than the WHO provisional guideline value of 10 µg/L for 68% of the samples and higher than the Indian permissible limit of 50 µg/L for 29% of the samples, indicating that the water from the arsenic mitigation options neither completely arsenic free nor even compliant with these guidelines/regulations. The significant inverse correlation between As and pathogens (
E.coli) for shallow tube wells of Bangladesh observed by van Geen
et al. [
27] could be a potential concern for health risk substitution for arsenic mitigation options, but although we observed a very weak inverse relationship of As with [TTC]
source (
Figure 4) we found the relationship not to be significant.
Based on the regression analysis we observed that the contamination of end-use water largely depends on whether the source water is contaminated or not rather than any other predictors. Hence the fate of [TTC]
end-use is predicted in a different way for [TTC]
source = 0 and [TTC]
source > 0. Once the source water is contaminated, [TTC]
end-use increases exponentially irrespective of other factors. In a study in Sierra Leone, Clasen and Bastable [
28] observed a 200 fold increase in [TTC]
end-use with respect to [TTC]
source when the source was contaminated compared to when the source was not contaminated.
Significantly high [TTC]
end-use compared to [TTC]
source (
Figure 3) supports evidences from previous studies that have shown the importance of fecal contamination of drinking water after collection associated with household storage linked to water handling and hygiene practices. Eshcol
et al. [
29] illustrated 36% households have their water being contaminated by fecal coliforms on storage within 24–36 h of collection for a study at Andhra Pradesh, India (n = 50). We observed, the overall [TTC]
end-use to be higher for people living in mud houses (
Table 4). The type of house- mixed or mud as observed in this study, acts as a proxy for wealth, reflects the sense of personal hygiene, with mixed being better than mud suggests that contamination within the household is a likely factor in controlling the end-use water contamination.
The significant difference in [TTC]source between the three different mitigation options and TP and TW being significantly higher than DTW suggests that much of the pathogen loading in TP and TW is attributable to contamination arising from storage of waters during the treatment for arsenic removal and/or in the distribution network of tap water supply. The overall [TTC]end-use is found to be lower for DTW users, in spite of the fact that DTW users more likely dwell in mud houses.
Our study shows that wide-mouth open containers such as buckets or Hari had higher potential risk of contamination than narrow-mouth vessels such as bottles, jugs or pitchers (
Table 4)—we speculate that the contamination could arise while fetching water from wide-mouth containers by dipping. Clasen and Bastable [
28] indicated that using a tap or spigot to access water is protective of stored water quality compared with water accessed by dipping.
3.5. Disease Burden—Pathogens in Source vs. End-Use Water
DALYs arising from TTC in source water accounts for only 11% (174 µDALYs/person/year/ 1574 µDALYs/person/year) of the DALYs resulting from TTC in end-use household drinking water (
Table 7) indicating that the microbial contamination of source water is only a small factor for diarrheal disease incidence arising from drinking water from arsenic mitigated sources in the study area. Irrespective of whether water-borne pathogens originate in supplied water or from household contamination, calculated risks from water-borne pathogens (1574 µDALYs/person/year; or using modeled [TTC]
end-use, 1252 µDALYs/person/year) represent only around 10% of the total diarrheal disease burden (14,530 µDALYs/person/year) reported by GBD for India [
30] thus drinking water would seem to be a minor contributor to diarrheal diseases in Chakdha relative to other causes. This is consistent with Esrey’s [
8] large cross sectional study on 8 developing countries, which concluded that (i) calculated predictions of reduction in diarrheal disease incidence may be invalid where they are based only on the improvement of microbiological quality of (improved) supplied water; (ii) health benefits from improved water supply were less important than improvements in sanitation; and (iii) improved sanitation was the governing factor reducing diarrheal disease incidence. Pruss [
12] further noted that the transmission of water-borne-pathogens may be linked to a lack of water linked in turn to inadequate personal hygiene and use of contaminated water for cleaning [
12]. In this study, we observed that the [TTC]/100 mL in pond water (median 3490, interquartile range 1680–7125, n = 6) and dugwell water (median 1440, interquartile range 1120–2442, n = 5), both extensively used for washing and bathing in Chakdha block, were extremely high and may represent an important source of exposure to water-borne pathogens unrelated to the quality of either supplied or end-use drinking water.
The links between water and health are complex and errors may arise from: (i) for developing countries recall-data on diarrhoea prevalence from cross-sectional studies may be too insensitive with chances of misclassification [
8]; (ii) the GBD estimate is for the whole of India and there could be possible local variation in diarrheal disease incidences; and (iii) potential flaws of the DALY approach, given the present availability of data [
19]. Important but almost inevitable shortcomings are the uncertainties associated with the model input parameters which can be reduced with further research in the fields of country specific Pathogen:
E coli ratios and country specific probabilities of infection for reference pathogens as well a more quantitative understanding of differences in post-supply pathogen contamination arising from outside and inside households. Notwithstanding this, verification of the microbial quality of drinking-water by testing for TTC as an indicator of faecal pollution provides conclusive evidence of recent faecal pollution that should not be present in drinking-water. While TTC is a useful indicator, it has limitations. Enteric viruses and protozoa are more resistant to disinfection; consequently, the absence of TTC will not necessarily indicate freedom from these organisms [
26]. Under certain circumstances, such as high levels of viral and parasitic diseases in the community it may be desirable to include the estimation of more resistant microorganisms, such as bacteriophages and/or bacterial spores [
26] as indicator organisms. In addition, it is important to note that, although diarrheal diseases are probably the largest contributor to the disease burden from water, sanitation and hygiene, it cannot entirely be attributed to these factors because it is also transmitted through food and through air [
12].
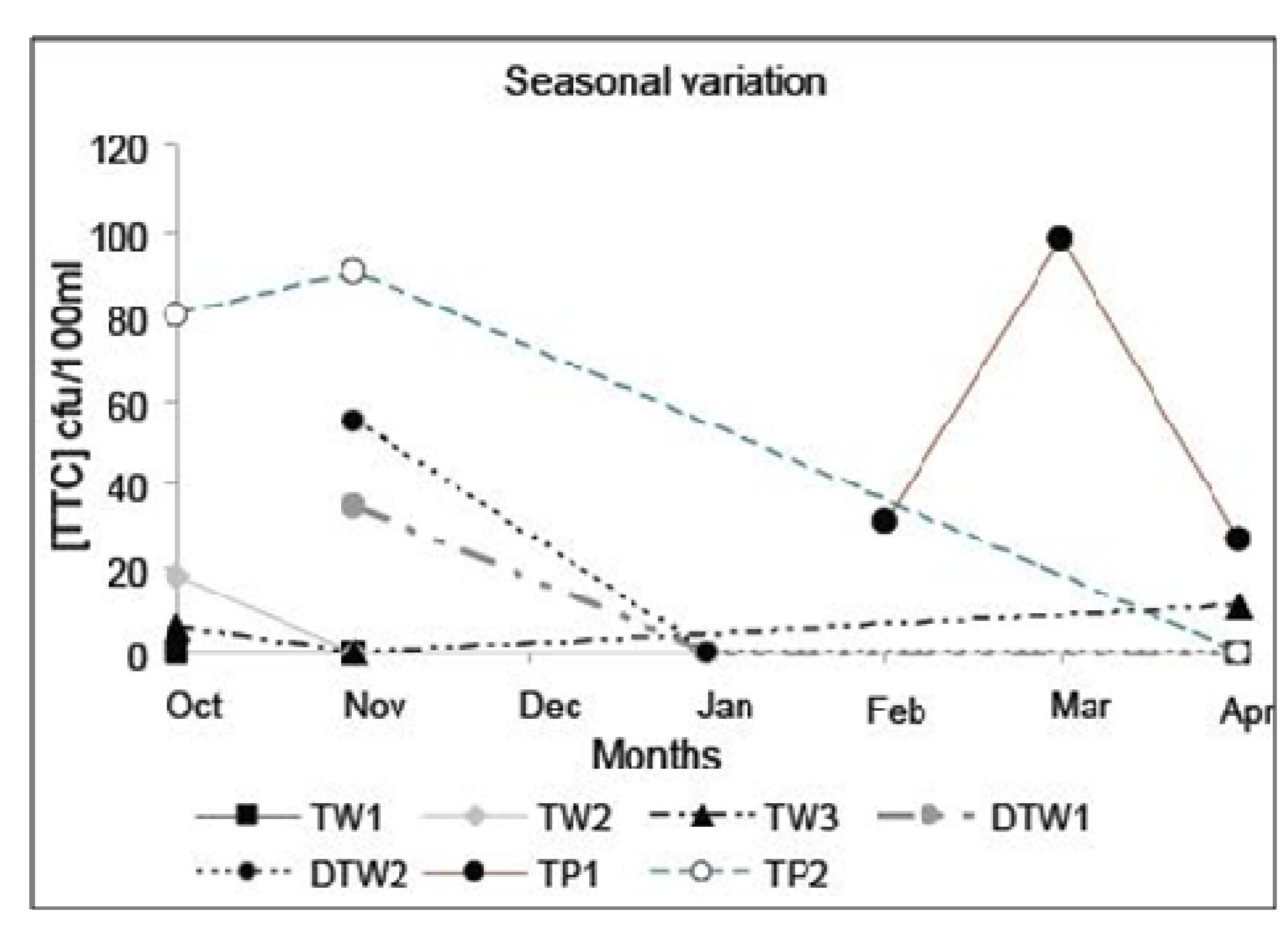
Lastly, we note that water quality can vary rapidly, and all systems are subject to occasional failures [
26] which can have a disproportionate impact on disease. Rainfall can greatly increase the levels of microbial contamination in source waters and waterborne outbreaks often occur following rainfall [
26] and this is apparent from the study on seasonal variation for the observed seven sources (
Figure 5). Results of the daily, continuous monitoring of Cryptosporidium in drinking water in the UK showed that the Cryptosporidium concentration generally followed a continuous trend, however, in some cases a trend break was detected which dominated the mean health risk hence frequent monitoring rather than the point data as obtained for this study would be required to detect such events [
30]. Thus, whilst we report here relative magnitudes of pathogen and arsenic attributable health risks calculated from our small study, the uncertainties on the calculated risks are considerable and further work, including stochastic treatment of the QMRA [
30] is required to obtain a more reliable result.
Notwithstanding these limitations to the model DALY calculations, because we do observe a statistically significant, though weak, relationship between microbiological quality of source and end-use water where [TTC]
source is > 0, implies that whilst sanitation and hygiene are important in combating diarrheal diseases, degradation of source water quality because of arsenic mitigation can result in increased diarrheal disease incidence. In the study, this is estimated to be 5% (25th and 75th percentile: 2%, 20%)—this is substantially lower than the 20% figure used by Lokuge
et al. [
11] to estimate increase in diarrheal incidences when there is a shift from existing water sources that are arsenic contaminated to arsenic mitigated options having potential for microbiological contamination. We also note that our datum is significantly higher than 0, reflecting that arsenic mitigated water supplies can be prone to microbiological contamination. This indicates that efforts to minimise microbiological contamination at source, for example chlorination of the source water for deactivating water-borne pathogens, done on a frequent and regular basis and accompanied by appropriate monitoring in order for its effectiveness, are indicated [
2]. Additional treatment at household level is indicated for the more effectively reduction of diarrheal disease incidences. Among the various approaches to household-based water treatment are heat and UV radiation, sedimentation, filtration and chemical treatment (coagulation, flocculation, precipitation, adsorption and disinfection) [
28].
Figure 5.
Seasonal variation in [TTC]source for water supplied from arsenic removal treatment plants (TW), deep tube wells (DTW) and tap waters (TP) in Chakdha Block, West Bengal, India. The lines are a visual aid to link samples from the same well but are not intended to indicate a determined trend. Nevertheless, the data suggest that other than the occasional spike (TP1, March), [TTC]source is most commonly highest in October/November near the end of the monsoon season (June–October).
Figure 5.
Seasonal variation in [TTC]source for water supplied from arsenic removal treatment plants (TW), deep tube wells (DTW) and tap waters (TP) in Chakdha Block, West Bengal, India. The lines are a visual aid to link samples from the same well but are not intended to indicate a determined trend. Nevertheless, the data suggest that other than the occasional spike (TP1, March), [TTC]source is most commonly highest in October/November near the end of the monsoon season (June–October).
3.6. Overall Change in Disease Burden Arising from Arsenic Mitigation
We observe (
Table 7) that DALYs calculated as arising from As in mitigated waters in this study (238 µDALYs/person/year) are less than half of those calculated for As in pre-mitigation wells (580 µDALYs/person/year) in the same area using data reported from previous studies [
16]. Both these values are substantially less those arising (1000 µDALYs/person/year) from unmitigated shallow wells in similar areas in Bangladesh. The arsenic mitigations considered here therefore have clearly resulted in a substantial reduction in drinking water arsenic attributable health risks in the study area.
Although DALYs arising from pathogens in end-use water (1574 µDALYs/person/year; or using modeled [TTC]end-use, 1252 µDALYs/person/year) are higher than any of the average arsenic-attributable values calculated, the regression model derived here demonstrates that only a small fraction of those DALYs, viz. 174 µDALYs/person/year, can be attributed to pathogens in the supplied water, as opposed to pathogens acquired post-supply, either outside or inside households. Thus, the combined DALYs arising from both [TTC]source and As in mitigated wells in this study (424 µDALYs/person/year) represents, on average, a reduction in disease burden as a result of the arsenic mitigation methods—we thus find no evidence in this small study for significant risk substitution resulting in a net increase in overall water-borne disease burden for the sequelae considered here.