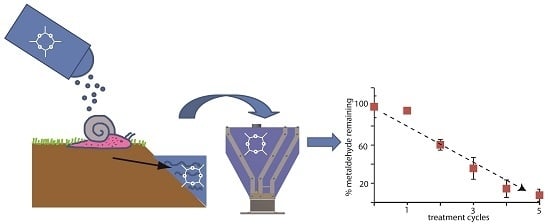
1. Introduction
Access to clean drinking water is a basic human need but as many as 750 million people in the world do not have access to it [
1]. Freshwater sources are often contaminated by industrial waste, pesticides, fertilizers, and other pollutants such as microorganisms, heavy metals and other hazardous chemicals which make water unfit for human consumption and which can be a key factor in water-related diseases [
2]. The pesticide, metaldehyde, is one such drinking water pollutant for which there are not only concerns about its health impacts [
3] but for which there are considerable practical difficulties in its efficient removal [
4].
Metaldehyde, the more widely used name for 2,4,6,8-tetramethyl-1,3,5,7-tetraoxacyclooctane, is a molluscicide extensively used worldwide, particularly in wet regions [
5]. In the United Kingdom (UK) alone between 2008 and 2012, over 1400 tonnes of metaldehyde have been used [
6]. Metaldehyde is long-lived in the environment, soluble in water with a solubility of 200 mg/L at 20 °C, and hence highly mobile in aqueous environments [
7]. However, leaching of metaldehyde in water sources during wet seasons in particular can pose a serious threat to water quality since, so far, existing technologies, such as adsorption on activated carbon, and advanced oxidation processes have shown to be ineffective in comprehensively reducing the amount of metaldehyde to European and UK regulatory limits of 0.1 μg/L in water [
8,
9,
10]. In 2011, most regions in the UK failed to comply with this limit [
10,
11].
The efficient removal of any pesticide from water is dependent on their chemical and physical characteristics. Metaldehyde is a cyclic tetramer of acetaldehyde and this makes chlorination or ozonation treatment methods ineffective [
12]. Hall
et al. [
13] further suggested that the low K
ow of 0.12 for metaldehyde is the reason why many current treatment processes are unable to adequately remove metaldehyde. Photocatalysis using nano-sized zinc oxide composites [
14] and advanced oxidation processes using UV/TiO
2 and UV/H
2O
2 [
15,
16] are promising for removal of micro-pollutants but they are costly and have yet to show that they can meet the regulatory limit set for metaldehyde. Recently, Busquets
et al. [
7] reported that metaldehyde can successfully be removed down to regulatory limits using an adsorption process based on phenolic carbon. However, this process is not chemical free and there is concern over the possibility of phenolic components from the adsorbent leaching out into water. An adsorption process developed by Veolia Water Solutions & Technologies (Pairs, France) and Affinity Water [
17] can also reduce metaldehyde to below regulatory levels. However, this process makes use of Powdered Activated Carbon (PAC), which is costly, and the process is solely based on adsorption thus leading to the requirement of disposing of a substantial amount of PAC waste.
Recently, one of us (Nigel Brown) has been involved in the development of a new, coupled adsorption and electrochemical destruction technology, the Arvia
TM (Cheshire, UK) Process, for the removal of organic contaminants from waters [
18,
19]. The process uses a low capacity graphitic material (a graphite intercalation compound; Nyex
TM (Cheshire, UK), a material comprising both graphitic edges and basal planes which are believed to provide a range of adsorption sites) as an effective adsorbent that can be rapidly and cost-effectively regenerated electrochemically [
20,
21,
22]. Although a relatively low sorption capacity material, the lack of intra-granular porosity provides for both rapid sorption and regeneration kinetics, whilst the relatively high electrical conductivity means that electrochemical regeneration could potentially be carried out using relatively little energy [
23]. The treatment process proceeds via a batch process comprising treatment cycles with three main stages, (i) adsorption; (ii) sedimentation; and (iii) electrochemical regeneration, with the efficiency of adsorption of the contaminant on the Nyex
TM in the case of low/trace concentrations and the amount of voltage applied to the system during the electrochemical regeneration step at higher concentrations being the limiting steps, respectively. The process has shown to be effective in removing some organic pollutants, such as Acid Violet 17, from wastewaters [
18,
19]. However, it remains unclear if this adsorption and electrochemical regeneration process can be used to remove metaldehyde from drinking waters to below drinking water standards.
Thus the aims of this study were (i) to determine if metaldehyde in drinking waters can be effectively removed/destroyed using this novel coupled adsorption and electrochemical destruction technology; (ii) to identify the optimum conditions for its removal; (iii) to determine if any significant concentrations of harmful breakdown products are formed; and (iv) since upland peat areas are an important source of drinking water in many parts of the EU and the UK, to assess the efficiency of metaldehyde removal for such relatively high natural organic matter (NOM) waters.
2. Materials/Reagents and Sample Preparation
Analytical grade metaldehyde (98% purity) was purchased from Lonza Chemical; D5-atrazine (99% purity), ethyl acetate, methanol, formic acid and sodium thiosulfate pentahydrate were all purchased from Sigma-Aldrich (Irvine, UK). Nyex
TM 1102, provided by Arvia
TM Technology Limited, was similar to previously characterized batches of Nyex
TM 100 and Nyex
TM 1000, contained above 90% carbon and had a particle size range of 100 to 700 µm [
18,
19,
20,
21,
22,
23]. The Nyex
TM was washed with tap water several times prior to use. ENVI-18 cartridges for extracting the samples were purchased from Kinesis (Bothell, WA, USA). The sodium thiosulfate solution was prepared by dissolving 0.9 g of sodium thiosulfate crystals in 50 mL deionised water [
24]. Metaldehyde stock solutions (~15,000 µg/L) were prepared by mixing metaldehyde in deionised water for 72 h. A range of metaldehyde test solutions (from 12,000 to 10 μg/L) were prepared by diluting these stock solutions with 18 MΩ·cm
−1 deionised water. Experiments without a regeneration stage were also performed to evaluate the adsorptive capacity of the system alone. High NOM peat water samples were obtained on 17 October 2013 from the Crowden Great Brook stream (Peak District; about 25 km east of Manchester, UK). Peat water test solutions were prepared by mixing 500 mL of the peat water and 500 mL of the metaldehyde stock solution.
2.1. Adsorption and Organic Destruction Experiments
Adsorption isotherm experiments were conducted by mixing (magnetic stirrer; 700 rpm; for 60 min) the Nyex
TM 1102 adsorbent (10 g) with metaldehyde solutions (100 mL; concentrations between 12,000 and 100 μg/L) in a temperature controlled laboratory at 20 ± 2 °C. The mixing time of 60 min had previously been determined from a kinetic study (
Figure 1A) to be a sufficient time to reach equilibrium. Aliquots (10 mL) were collected every 10 min, filtered (0.45 µm nylon syringe filter) and analysed by liquid chromatography mass spectrometry (LCMS) after the addition of 10 µL of the sodium thiosulfate solution to ensure that any chlorine was removed from the samples. This is necessary, as, during the regeneration phase, chlorine may be formed due to the use of the acidified sodium chloride solution as a catholyte.
The amount of metaldehyde,
qe (µg/g), adsorbed on the adsorbent was determined using Equation (1):
where
C0 is the initial concentration of metaldehyde in solution (µg/L),
Ce is the concentration of metaldehyde remaining in solution (µg/L),
m is the mass of adsorbent used (g), and
V is the volume of metaldehyde solution (L) for the whole experiment. Corrections were not made for any changes in adsorbent/solution ratios after taking sacrificial samples, because thorough mixing ensured that these ratios were conserved during sampling.
The observed metaldehyde/Nyex
TM adsorption data were tested against a Freundlich isotherm model given by Equations (2) and (3):
and
and for which the Freundlich model parameters, n and
Kf, may accordingly be derived from a best fit regression line on a log (q
e)
vs. log (C
e) plot.
The data were also fitted to a Langmuir adsorption isotherm model given by Equations (4) and (5).
where
and
are the model constants (with
equivalent to the Langmuir constant, α) and are derivable from a best fit regression line to a plot of 1/q
e vs. 1/C
e.
Metaldehyde removal/destruction experiments were conducted using the same laboratory scale sequential batch equipment as described by Asghar
et al. [
18] in a temperature controlled laboratory at 20 ± 2 °C. Details of the experimental set up, including schematics, can be found in Asghar
et al. [
18] and Mohammed
et al. [
19]. For the optimization experiments, the cell was loaded with 200 g of pre-cleaned wet Nyex
TM (approximately 100 g dry weight) and 1 L of metaldehyde test solution with known concentration was added, and the experiment was conducted for 5 treatment cycles. Under standard conditions, a treatment cycle consists of: (i) a mixing phase (15 min), during which the Nyex
TM and the test solution were well mixed by blowing air through it; (ii) a settling phase (10 min); and (iii) regeneration phase (15 min) during which a current of 0.5 A (10 mA/cm
2) was passed through the adsorbent bed to destroy the adsorbed metaldehyde. After each cycle, three 10 mL aliquots were collected, filtered (Whatman 541) and analysed in triplicate by liquid chromatography mass spectrometry (LCMS) after the addition of 10 μL of the sodium thiosulfate solution. The peat water experiments were performed using the same procedure.
In case of the low concentration experiments a similar set up was used but with 1.5 L of metaldehyde test solution (approximately 10 μg/L) and the experiments was conducted for 7 treatment cycles. Aliquots (200 mL) were collected after Cycle 2 and 4 and a 900 mL aliquot was obtained after the last treatment cycle. All the aliquots were filtered (Whatman 541), and concentrated using the solid phase extraction (SPE) method developed by Kinesis [
25]. In short, SPE columns (TELOS
TM ENV 200 mg/6 mL) were conditioned with ethyl acetate (2 column volumes), methanol (2 column volumes) and deionised water (1 column volume) while making sure the pre-conditioned columns did not dry out. Metaldehyde was extracted by passing the aliquot through the column at a flow rate of approximately 30 to 35 mL/min before drying the SPE column under vacuum until the sorbent became a uniform light brown colour. The dried columns were soaked in 2 mL of ethyl acetate for 5 min, the elute was collected and a further 2 mL ethyl acetate was added and allowed to elute. The combined samples were blown down from 100 to 25 µL and analysed using gas chromatography mass spectrometry (GCMS). The detection limit reached using this protocol was 0.01 μg/L. To ensure repeatability and determine recovery rates, control metaldehyde solutions were subjected to the same extraction procedure and analysed by gas chromatography mass spectrometry (GCMS).
2.2. Liquid Chromatography Mass Spectrometry (LCMS) and Chromatography Mass Spectrometry (GCMS) Analysis
LCMS analyses was performed using an Agilent 1200 HPLC instrument coupled to an Agilent 6130 quadrupole mass spectrometer equipped with a multimode source operated in atmospheric-pressure chemical ionization (APCI) positive ion mode based on a protocol adapted from the Environment Agency for determination of metaldehyde in water [
24]. Metaldehyde was analysed using a normal phase LCMS Kinetex C18 HPLC column (3 μm, >150 mm × 2.1 mm internal diameter; Phenomenex) and a guard column of the same material. LCMS settings were as follows: nebulizer pressure 20 psig, vaporiser temperature 250 °C, drying gas (N2) flow 6 L/min and temperature 200 °C, capillary voltage 2 kV and corona current 5 μA. In order to increase sensitivity/reproducibility, ion scanning was performed in single ion monitoring (SIM) mode using acetaldehyde [M + Na]+ and metaldehyde [M + Na]+ ions, ion peaks at
m/z 67 and 199, respectively. Separation was achieved at 30 °C with a flow rate of 0.4 mL·min
−1 and using the following gradient profile: 60% A and 40% B (0 min), 60% A and 40% B (1 min), 40% A and 60% B (6 min), 40% A and 60% B (9.5 min), 40% A and 60% B (12 min); where A = Water containing 0.25% formic acid and B = Methanol containing 0.25% formic acid; all HPLC grade. The sample injection rate was 10 µL and the retention time of metaldehyde was 6.9 min.
GCMS was performed using an Agilent 789A GC interfaced to an Agilent 5975C MSD mass spectrometer operating with electron ionisation at 40 eV and scanning from
m/z 50 to 600 at 2.7 scans/s based on a protocol adapted from the Environment Agency for determination of metaldehyde in water [
24]. The GC was equipped with an Agilent 7683B auto sampler and a programmable temperature variation (PTV) inlet. The samples were injected in pulsed spit-less injection mode (1 µL; inlet pressure of 25 psi for 0.25 min) and separated on an HP-5 capillary column (J&W scientific column 5% diphenyldimethyl polysiloxane; length 30 m, I.D. 250 µm, film thickness 0.25 µm). The samples were run at a constant flow (1 mL/min) with He as a carrier gas. The heated interface temperature was set to 280 °C, with the mass source temperature at 230 °C and the MS quadrupole at a temperature of 150 °C. The samples were injected at 35 °C and the oven was programmed to 260 °C at 20 °C/min at which it was held isothermally for 0.5 min. Peaks were acquired both in scan and SIM mode at ion peaks of
m/z = 45 and 89.
3. Results and Discussion
3.1. Adsorption Experiments
Initial adsorption experiments using metaldehyde concentrations ranging from 250 to 12,000 μg/L were undertaken to determine the maximum metaldehyde uptake by the NyexTM and the minimum contact time required to achieve equilibrium.
For both the 2000 μg/L and 12,000 μg/L metaldehyde solutions, equilibrium was reached within 20 min (
Figure 1A).
Figure 1.
(A) Kinetics of metaldehyde adsorption onto NyexTM 1102. Metaldehyde remaining expressed as a percentage of the initial metaldehyde concentrations of 250, 2000 or 12,000 μg/L as indicated; (B) Adsorption isotherm of metaldehyde on NyexTM 1102. Equilibration time 60 min; initial pH 6–7. Bars indicate the error; when they are not visible the error bar is smaller than the symbol used.
Figure 1.
(A) Kinetics of metaldehyde adsorption onto NyexTM 1102. Metaldehyde remaining expressed as a percentage of the initial metaldehyde concentrations of 250, 2000 or 12,000 μg/L as indicated; (B) Adsorption isotherm of metaldehyde on NyexTM 1102. Equilibration time 60 min; initial pH 6–7. Bars indicate the error; when they are not visible the error bar is smaller than the symbol used.
In the case of the lowest initial concentration, 250 μg/L, equilibrium was not reached until 50 min. However, a relatively larger proportion of metaldehyde in the starting solution was removed in the case of the lowest concentration (45%) compared to 25% for the 2000 μg/L and 12% for the 12,000 μg/L solutions. The maximum amount of metaldehyde that could be adsorbed on the Nyex
TM based on the adsorption isotherm (
Figure 1B) was found to be approximately 18 µg metaldehyde/g Nyex
TM. This clearly shows that, despite Nyex
TM being a largely non-porous material, metaldehyde can nevertheless be adsorbed on Nyex
TM and thus could potentially be removed using the combined adsorption and electrochemical regeneration process. However, the adsorption capacity is not as high as for some other adsorbent materials. Busquet
et al. [
7] for instance showed that the adsorption capacity of phenolic carbon is about 76 mg metaldehyde/g adsorbent, much higher than that observed here.
The adsorption data fits poorly to a Freundlich isotherm model (
Figure 2A)––an apparently first order linear trend of log (q
e) against log (C
e) at low metaldehyde concentrations then deviates substantially from this with a plateauing of log (q
e) values once initial metaldehyde concentrations are sufficiently high to approach the saturation limit (1.26 = log (~18 μg/g)) of the Nyex
TM.
Figure 2.
(
A) Freundlich log-log plot of solid-phase concentration (q
e)
versus liquid-phase concentration (C
e) at equilibrium. Dashed vertical line represent mean metaldehyde removed per treatment cycle as determined from
Figure 3A; (
B) Langmuir plot for the metaldehyde/Nyex
TM 1102 system, where q
e and C
e are in µg/g and µg/L, respectively. Bars indicate the error, when they are not visible the error bar is smaller than the symbol used.
Figure 2.
(
A) Freundlich log-log plot of solid-phase concentration (q
e)
versus liquid-phase concentration (C
e) at equilibrium. Dashed vertical line represent mean metaldehyde removed per treatment cycle as determined from
Figure 3A; (
B) Langmuir plot for the metaldehyde/Nyex
TM 1102 system, where q
e and C
e are in µg/g and µg/L, respectively. Bars indicate the error, when they are not visible the error bar is smaller than the symbol used.
In contrast, the adsorption data fit very well to the Langmuir model (
Figure 2B) with an excellent linear correlation (
R2 = 0.99) a Langmuir constant, α, of 0.08 µg/g and 1/
Q0 of 0.011 g/µg. This strong linear correlation is consistent with a monolayer coverage of the adsorbent material, indicating that the adsorption of metaldehyde is limited by the number of adsorption sites on the Nyex
TM and explaining the relative low adsorption capacity compared to other adsorbent materials.
3.2. Optimisation of Parameters for Metaldehyde Removal
To determine if metaldehyde can also be removed/destroyed during the electrochemical destruction/regeneration process, additional experiments were performed at a relatively high starting concentration of 8000 μg/L metaldehyde. The high concentration used was to ensure that the Nyex
TM was (completely) saturated. These experiments showed that >90% of 8000 μg/L metaldehyde in water can be removed within five treatment cycles (
Figure 3A).
Figure 3.
Metaldehyde remaining (expressed as a percentage of initial concentration ~8000 µg/L) after successive treatment cycles (A) with and without regeneration (15 min; current 0.5 A) and mixing (15 min); under (B) varying mixing times at constant regeneration times of 15 min and current of 0.5 A; (C) varying regeneration times at constant mixing time of 15 min and current of 0.5 A; and (D) varying current at constant mixing time (15 min) and regeneration time (15 min; current 0.1 A, 0.25 A and 0.5 A). Dotted arrows indicate generalized trends, when there are multiple comparable trends only a single arrow is given. Bars indicate the error, when they are not visible the error bar is smaller than the symbol used.
Figure 3.
Metaldehyde remaining (expressed as a percentage of initial concentration ~8000 µg/L) after successive treatment cycles (A) with and without regeneration (15 min; current 0.5 A) and mixing (15 min); under (B) varying mixing times at constant regeneration times of 15 min and current of 0.5 A; (C) varying regeneration times at constant mixing time of 15 min and current of 0.5 A; and (D) varying current at constant mixing time (15 min) and regeneration time (15 min; current 0.1 A, 0.25 A and 0.5 A). Dotted arrows indicate generalized trends, when there are multiple comparable trends only a single arrow is given. Bars indicate the error, when they are not visible the error bar is smaller than the symbol used.
A broadly linear relation was observed between percentage of metaldehyde removed and the number of treatment cycles for the first four treatment cycles, indicating that on average about 1500 μg/L metaldehyde was removed per treatment cycle. This limiting value for metaldehyde removal is equivalent to ~15 µg metaldehyde/g Nyex
TM, broadly consistent with the previously determined 18 µg metaldehyde/g Nyex
TM adsorption capacity (
Figure 1B). After the fourth treatment cycle, the relationship is no longer linear and would suggest that as the concentration of metaldehyde decreases so does the removal rate. A control experiment using the same set up but without the regeneration stage indicated a much lower metaldehyde removal after five treatment cycles (45%;
Figure 3A). This study demonstrated that the adsorbent material is effectively regenerated. Previous work on adsorption and electrochemical regeneration of organic loaded on the Nyex
TM would suggest that metaldehyde is destroyed by conversion to carbon dioxide via a reaction represented by Equation (6) [
25] and this is supported by the absence of the breakdown product acetaldehyde (
Section 3.3).
This frees the active sites on the NyexTM surface for further metaldehyde adsorption during the next cycle.
To determine the optimal experimental conditions, a series of tests were performed on the mixing and regeneration times as well as the current used during the adsorption and electrochemical regeneration process. Varying the mixing time indicated no significant differences in metaldehyde removal if a mixing time of ≥5 min is used (
Figure 3B). However, lowering the mixing time to 1 min resulted in a significant reduction in the removal rate with less than 10% metaldehyde being removed after five cycles. This indicates that a mixing time of 1 min is not enough for effective adsorption of metaldehyde on Nyex
TM and also confirms that adsorption is a prerequisite for effective oxidation of metaldehyde, that is, there is no indirect electrochemical oxidation in the bulk phase.
Altering the regeneration time to 5 min caused a significant drop in metaldehyde removal to 52%. Increasing the regeneration time to 30 min caused only a slight drop in metaldehyde removal indicating that maximal removal can be achieved using a regeneration time of 15 min (
Figure 3C). Reduction in performance when the regeneration time was increased from 15 to 30 min could be due to over treatment of the Nyex
TM. Mehta and Flora [
26] found that electrochemically regenerating activated carbon for extended periods adversely affected the adsorptive capacity and postulated that to be due to a decrease in surface carbonyl groups or to changes in surface area. Lowering the current used caused a significant drop in metaldehyde removal rate resulting in about 60% of metaldehyde being removed in five cycles at 0.1 A and 0.25 A, respectively, compared to more than 90% at 0.5 A (
Figure 3D). This result suggests that at a current of 0.1 A, the voltage generated is insufficient to drive the oxidation reaction (
Table 1) with a minimum voltage required for effective regeneration of the adsorbent, this value being in excess of 3.0 V.
Table 1.
Typical voltages observed at fixed applied current for regeneration and % metaldehyde remaining after five cycles, respectively.
Table 1.
Typical voltages observed at fixed applied current for regeneration and % metaldehyde remaining after five cycles, respectively.
| Current/A | Voltage/V | Metaldehyde Remaining |
|---|
| 0.50 | 3.8 | 8% |
| 0.25 | 2.9 | 63% |
| 0.10 | 2.4 | 57% |
| 0.00 | 0.0 | 56% |
Combined these experiments showed that (i) metaldehyde is actively being destroyed and the drop in metaldehyde concentration observed is not just caused by adsorption on the NyexTM and (ii) maximum metaldehyde removal rates are achieved using a current of 0.5 A, a regeneration time of 15 min and a minimum mixing time of 5 min.
3.3. Evaluation of the Coupled Adsorption and Electrochemical Regeneration Technology for Metaldehyde Removal at Low Concentrations
To determine if this coupled adsorption and electrochemical regeneration process could be used to reduce metaldehyde in water supply systems to below EU and UK standards, additional low concentration experiments were performed. The starting metaldehyde concentration of 11 µg/L was chosen to be comparable in magnitude to the highest concentrations of metaldehyde observed in the UK post-treatment water supply systems,
viz. ~8 µg/L [
24]. These experiments show that metaldehyde (from a starting concentration of 11 μg/L) was clearly removed to residual concentrations well below EU/UK regulatory limits of 0.1 μg/L (
Figure 4).
Figure 4.
Metaldehyde remaining of triplicate spikes (initial concentration 11 µg/L) after successive treatment cycles. Mixing times, 15 min; regeneration times, 15 min; current, 0.5 A. Bars indicate the error, when they are not visible the error bar is smaller than the symbol used. Metaldehyde concentrations in all the spikes were reduced to below the EU and UK regulatory limit of 0.1 μg/L [
10] (dashed line) after seven treatment cycles.
Figure 4.
Metaldehyde remaining of triplicate spikes (initial concentration 11 µg/L) after successive treatment cycles. Mixing times, 15 min; regeneration times, 15 min; current, 0.5 A. Bars indicate the error, when they are not visible the error bar is smaller than the symbol used. Metaldehyde concentrations in all the spikes were reduced to below the EU and UK regulatory limit of 0.1 μg/L [
10] (dashed line) after seven treatment cycles.
Additional spiking experiments, using the same adsorbent material but with a fresh batch of metaldehyde solution, showed that the process is repeatable over time and that there was no overloading of the adsorbent material at these comparatively low concentrations. In the later cycles the final concentrations were actually below the detection limit of the equipment used,
i.e., below 0.01 µg/L. Closer analysis of the results revealed that the process was more effective with increasing number of treatment cycles. The EU/UK regulatory limit of 0.1 μg/L was met within four treatment cycles compared to seven cycles for the initial spike experiment. A possible explanation could be that the Nyex
TM adsorbent once regenerated a number of times is more active, enhancing the adsorption as well as the regeneration process. Brown
et al. [
20] showed that the adsorptive capacity of Nyex
TM is enhanced on regeneration, possibly due to the breakup of the edges of the graphene layers in the adsorbent molecules, thereby increasing surface roughness. This enhances the contribution from the edges and which would account for the greater adsorption observed for successive spiking experiments. In addition, during the analyses of all cycles, no toxic metaldehyde breakdown by-products, in particular the hazardous metaldehyde monomer unit, acetaldehyde [
27], were observed suggesting that complete oxidation of the metaldehyde took place.
The experiments on the adsorption and electrochemical regeneration process showed that metaldehyde removal is efficient and that no waste adsorbent is generated, with the adsorbent material able to be continuously regenerated and used for subsequent adsorption, although the energy consumption is relatively high. This represents a major advantage over processes that are based solely on adsorption where spent adsorbent material has to be disposed of, usually in landfills. Such disposal could potentially lead to leaching of metaldehyde back to the environment or simply to substantially greater treatment costs. For other adsorbent materials used to remove metaldehyde from drinking water, degradation may also result in the formation of complex chemicals such as phenolic carbon [
7], and functionalised resins [
28].
To our knowledge, though there are adsorption processes that have been reported to remove metaldehyde to residual concentrations in drinking water below the EU and UK regulatory limits, no process has previously reported the destruction of metaldehyde to concentrations meeting EU and UK regulatory limits. This is the first time that metaldehyde removal and destruction has been shown using this adsorption and electrochemical regeneration process under lab conditions. Spiking experiments has shown the process is repeatable and has the potential for operation without changing the adsorbent material. However, large-scale continuous treatment at higher flow rates and greater energy efficiency is needed to completely demonstrate that this adsorption and electrochemical regeneration process is viable on an industrial scale.
3.4. Evaluation of Environmental Parameters on the Adsorption and Electrochemical Regeneration Technology
Drinking water sources often have different pH depending on the nature of the water source; water from peat sources are acidic in nature [
29], whereas hard water rich in clay and limestone have an alkaline pH. To test the effect of pH on the removal rate of metaldehyde (average starting concentration of 8000 μg/L) by the adsorption and electrochemical regeneration process, experiments were conducted at pH 3 and 10 and compared to those at pH 6–7. In all cases, linear metaldehyde removal was observed but the combined adsorption and electrochemical regeneration process was much more effective at low and neutral pH than under alkaline conditions (
Figure 5A).
A possible explanation could be that, under alkaline conditions, the adsorption stage is hindered due to the interaction of OH− ions with the adsorbent material, which has an overall positive charge, thereby reducing the density of sites available for metaldehyde sorption. Under acidic or neutral conditions no such interaction occurs and maximum adsorption of metaldehyde is possible.
All previous experiments have been conducted using synthetic deionised water. To test the adsorption and electrochemical regeneration process under “natural” conditions peat water containing 10 mg/L Total Organic Carbon (TOC) was used to prepare a metaldehyde test solution (see
Section 2.1) and compared to the standard test solutions prepared in deionised water. A similar trend for metaldehyde removal was obtained in either case; however, the removal efficiency was reduced in the presence of high NOM peat water (decrease of about 20%,
Figure 5B). A possible explanation could be that for the peat water/metaldehyde solution, competition for active binding sites on Nyex
TM occurs due to the presence of other organic components in peat water. However, it clearly showed that even in the presence of other organics, the adsorption and electrochemical regeneration process can remove/destroy more metaldehyde compared to advanced oxidation processes, which are dependent on the TOC levels [
15].
Figure 5.
Metaldehyde remaining (expressed as a percentage of the mean starting concentration of 8000 µg/L) after successive treatment cycles using the coupled adsorption and electrochemical regeneration technology (A) for different initial pHs and (B) in the presence and in the absence of other organics in peat water. Dotted arrows indicate generalised trends. Bars indicate the error; when they are not visible, the error bar is smaller than the symbol used. All experiments were carried out with mixing and regeneration times of 15 min and a current of 0.5 A.
Figure 5.
Metaldehyde remaining (expressed as a percentage of the mean starting concentration of 8000 µg/L) after successive treatment cycles using the coupled adsorption and electrochemical regeneration technology (A) for different initial pHs and (B) in the presence and in the absence of other organics in peat water. Dotted arrows indicate generalised trends. Bars indicate the error; when they are not visible, the error bar is smaller than the symbol used. All experiments were carried out with mixing and regeneration times of 15 min and a current of 0.5 A.