Metal–Organic Framework-Functionalized Alumina Membranes for Vacuum Membrane Distillation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of MOF-Functionalized Hydrophobic Membranes
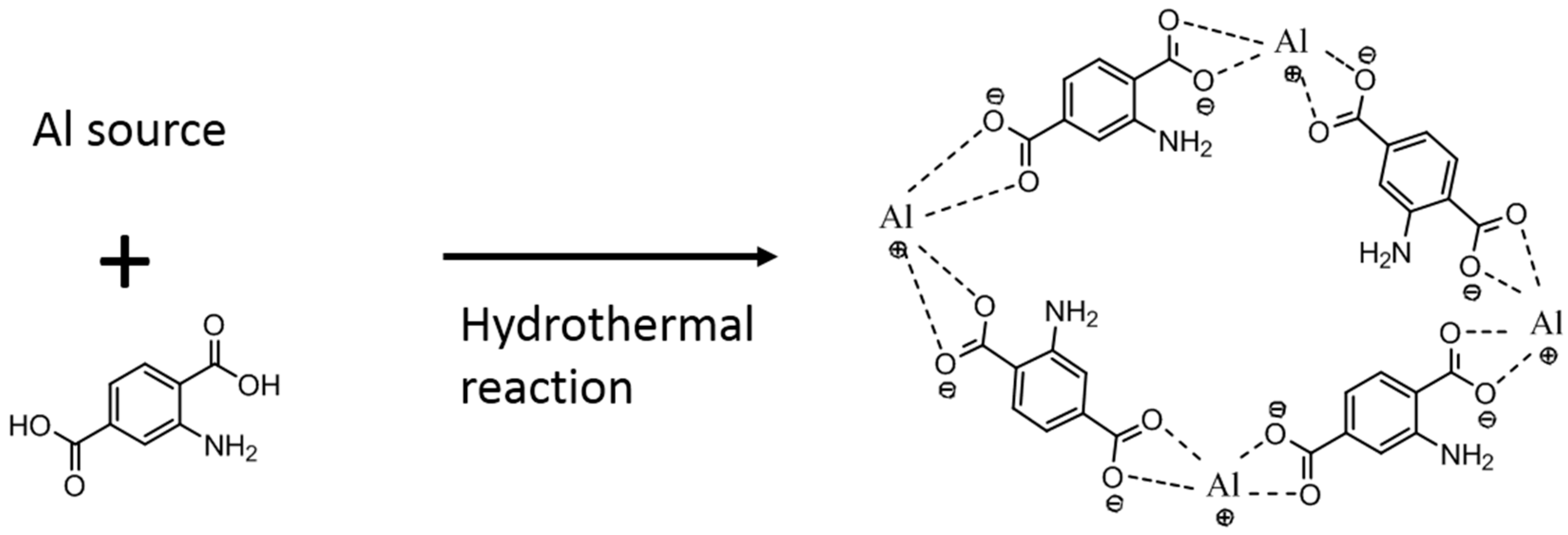
2.2.1. MOF Growth on the Alumina Support
2.2.2. Growth of the Hydrophobic Floss
2.3. Membrane Characterizations
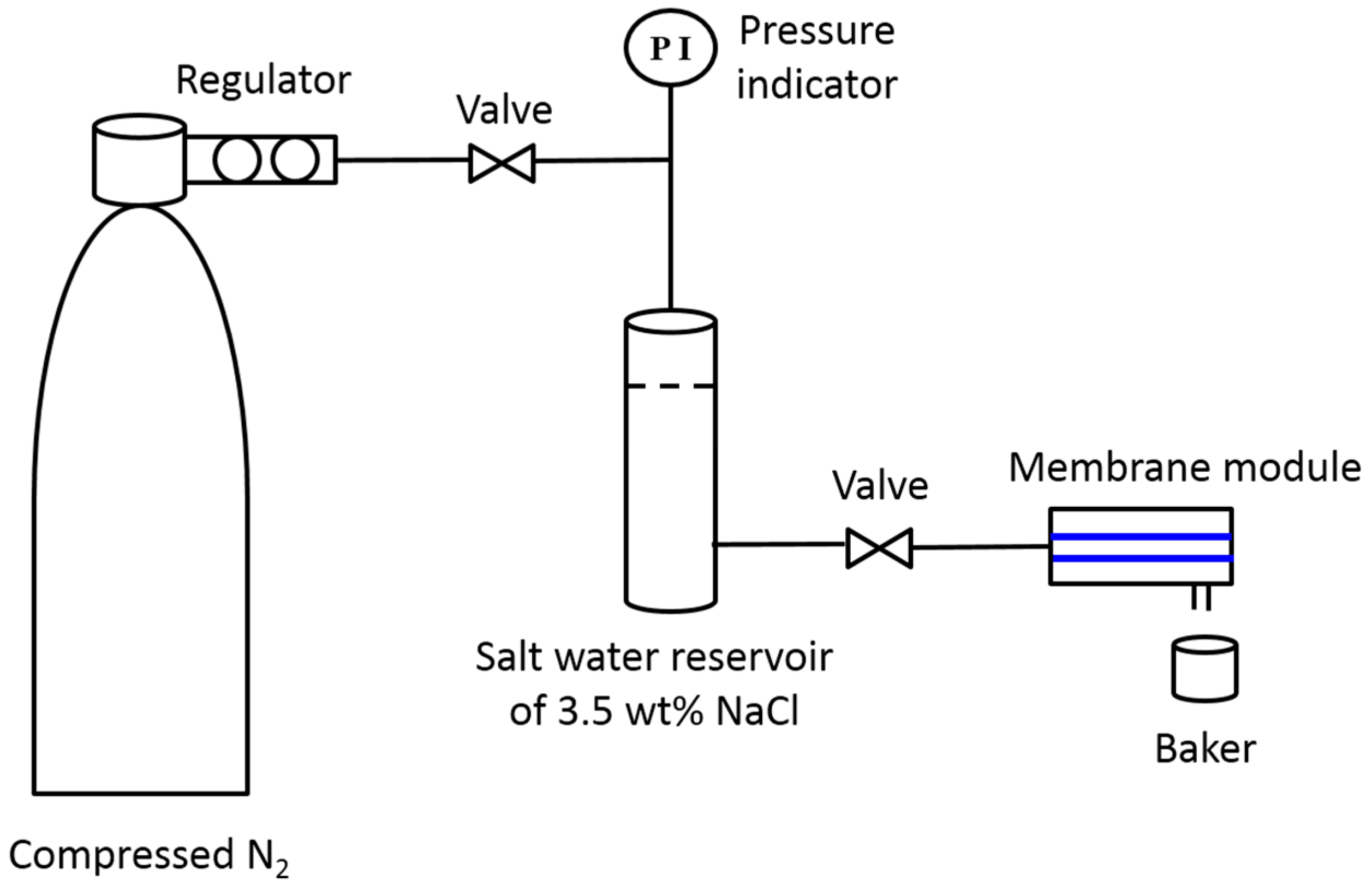
2.4. VMD Desalination Experiments
3. Results and Discussion
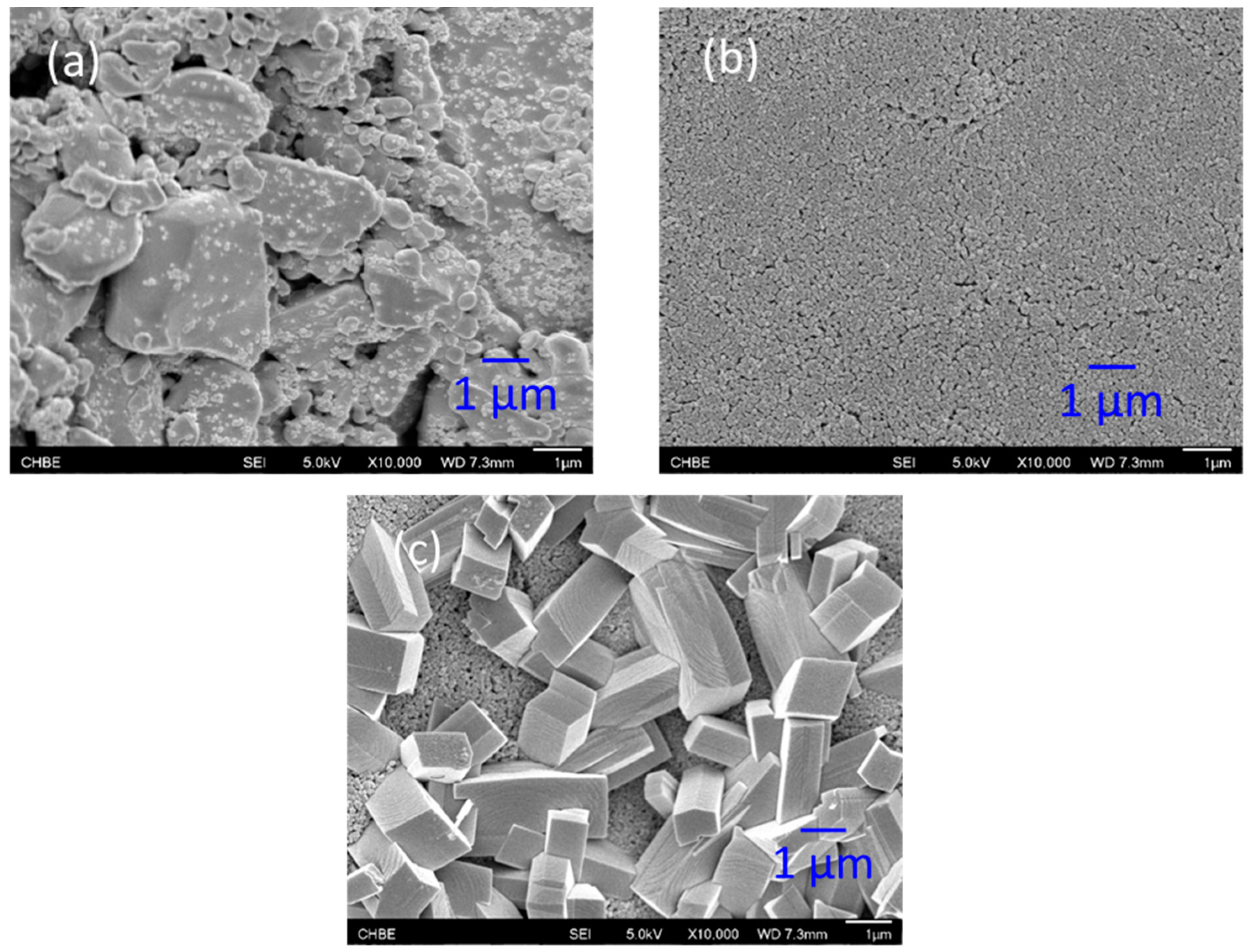
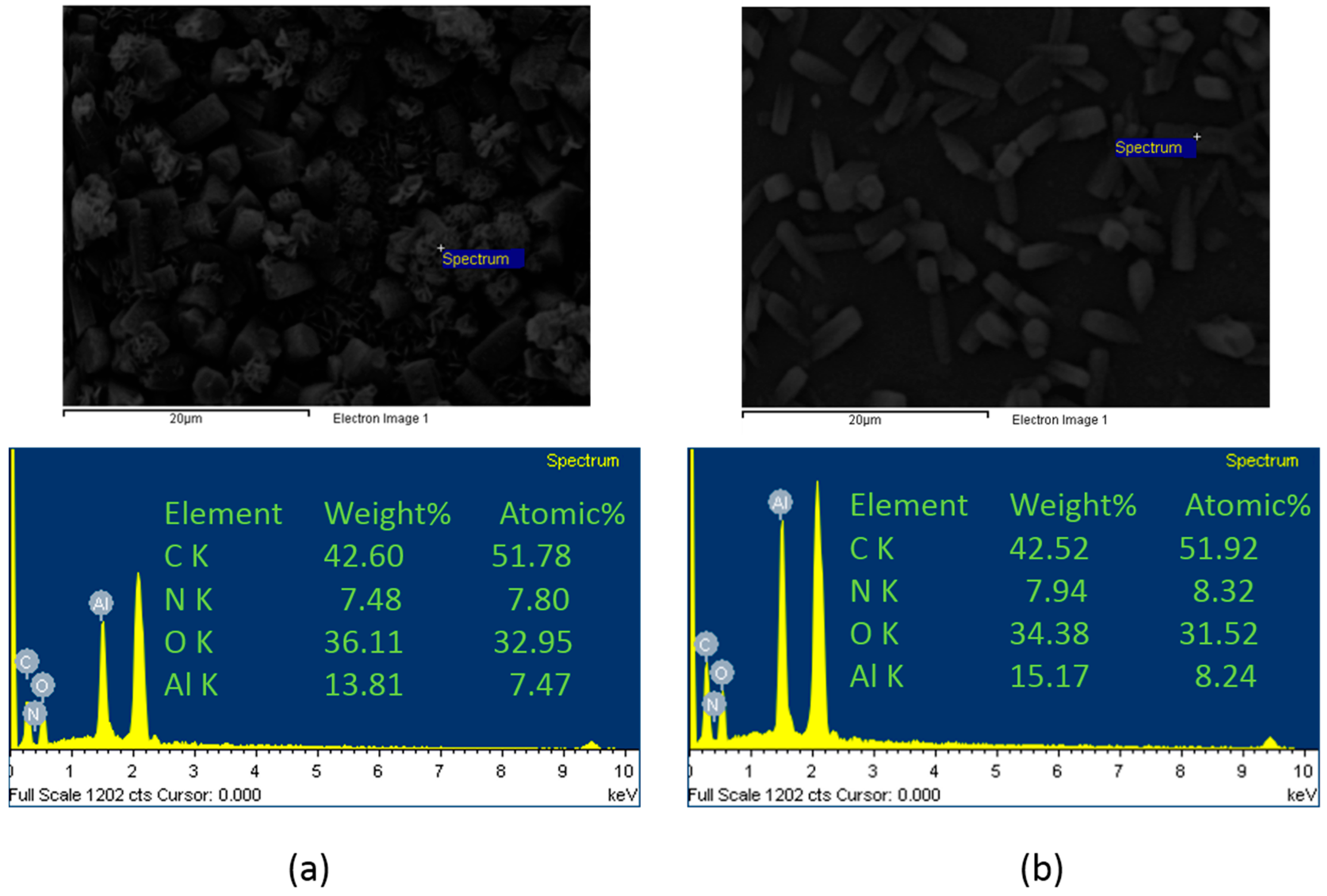
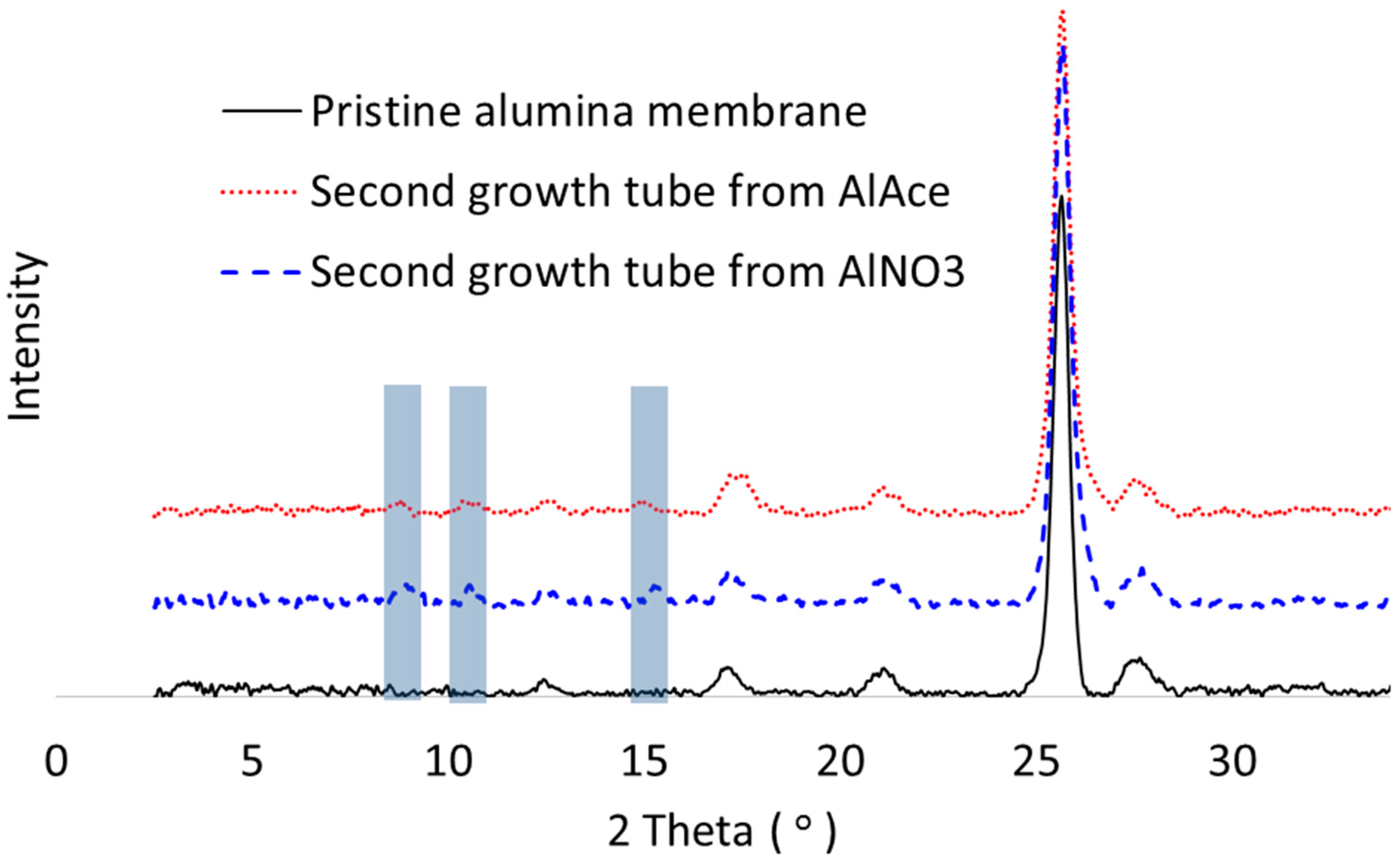
3.1. Growth of MOF on Alumina Membranes
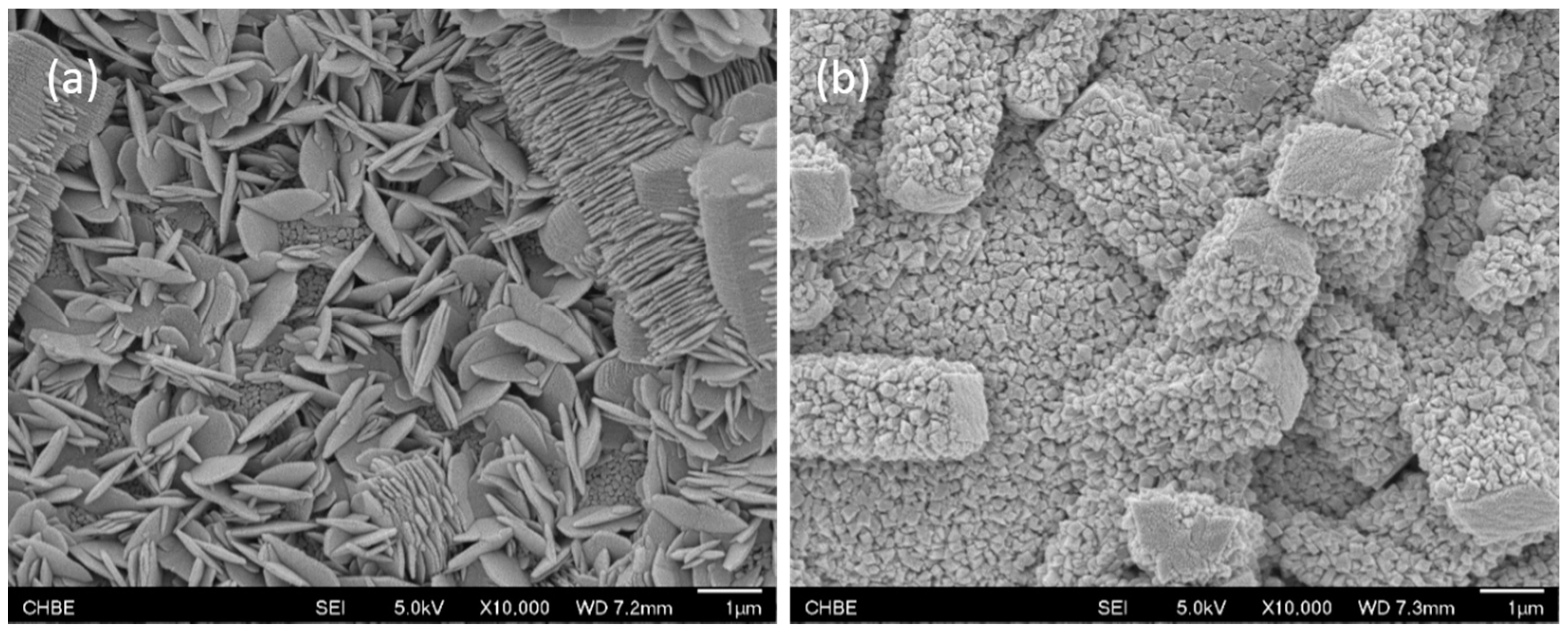
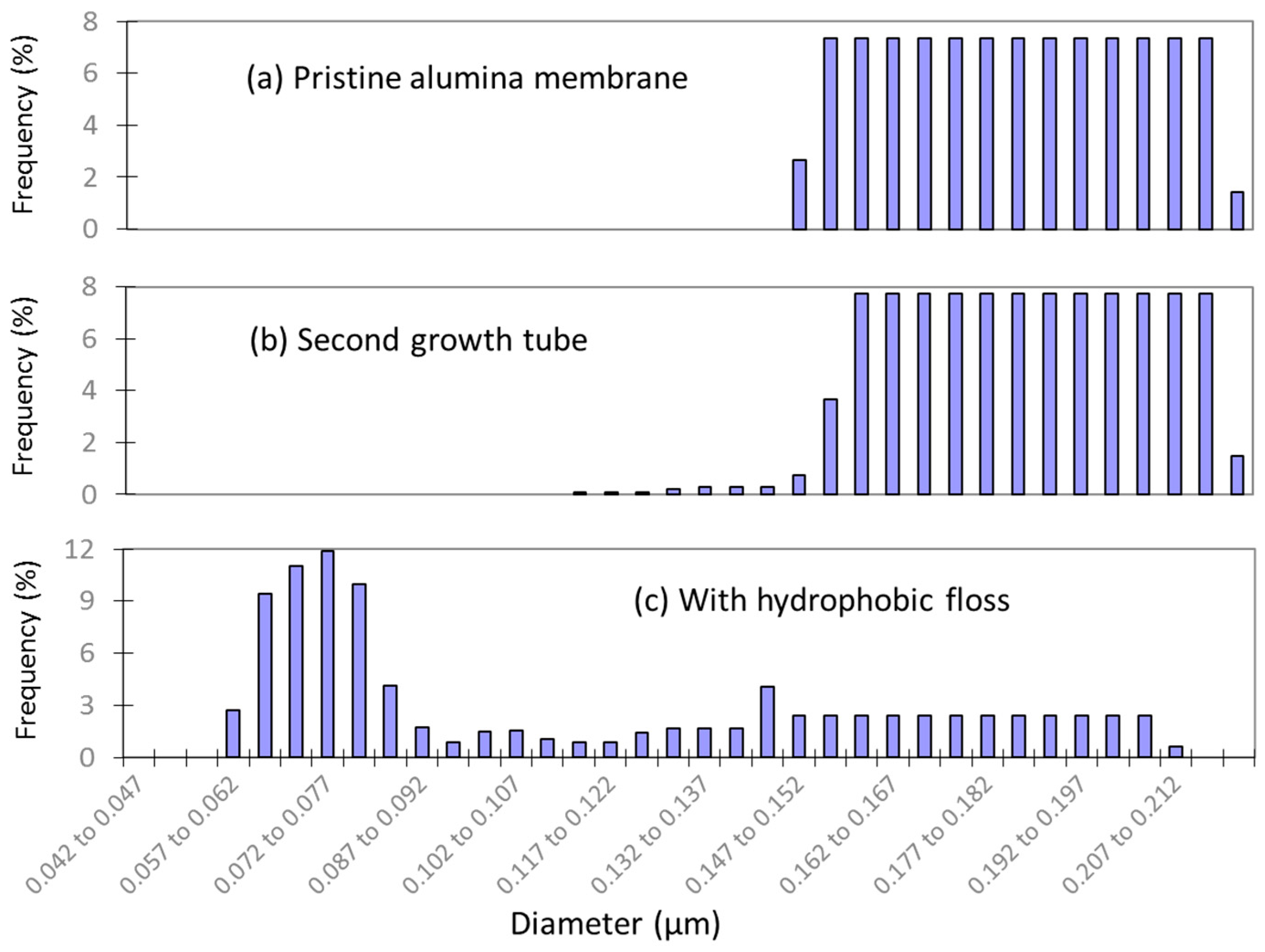
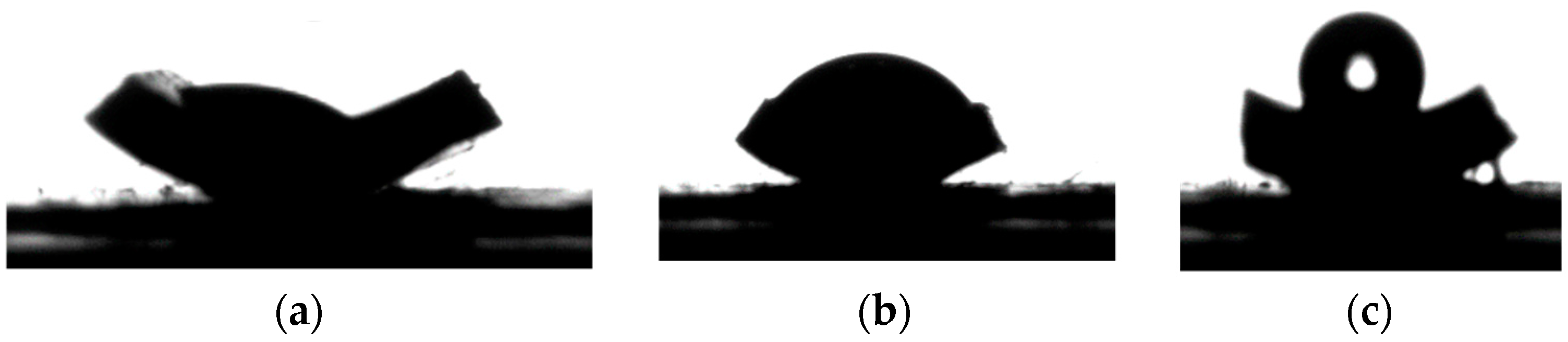
3.2. Growth of Hydrophobic Floss
3.3. Effect of Second MOF Growth Time on Membrane Performance
4. Conclusions
- (1)
- NH2-MIL-53(Al) MOF crystals have been successfully formed on alumina supports via hydrothermal reaction. The alumina substrate functions not only as a solid support, but also provides the Al sites for MOF growth. Thus, the crystals were intergrown from the support surface and immobilized on the surface.
- (2)
- Different MOF morphologies were achieved through the manipulation of different alumina sources in the second growth phase. The micro-petal crystal morphology from aluminum nitrate had a 2D-like structure, which possessed a high aspect ratio. However, the substrate surface was not fully covered by these crystals due to the distance among the petals. On the other hand, the ordered nanocrystals morphology from aluminum acetylacetonate covered the entire substrate surface and was able to reduce the membrane defects.
- (3)
- Hydrophobic membranes with better wetting resistance were produced after the growth of hydrophobic floss on the MOF functionalized surface. The LEP value of the newly developed membrane increased from 0 to 3 bars.
- (4)
- The VMD performance was improved through the optimization of reaction time for the second MOF growth phase. A shorter reaction duration results in a looser MOF layer, thus the higher permeation flux. The minimum reaction time for the second MOF growth phase was about 1 h. A further shortening the reaction time would lead to membrane defects.
Acknowledgment
Author Contributions
Conflicts of Interest
Abbreviations
| ATR | attenuated total reflection |
| DI | Deionized |
| EDX | energy dispersive spectrometry |
| FESEM | field emission scanning electron microscopy |
| FTIR | Fourier transform infrared |
| HKUST | Hong Kong University of Science and Technology |
| LEP | liquid entry pressure |
| MD | membrane distillation |
| MIL | Materials of Institut Lavoisier |
| MOF | Metal–Organic framework |
| NaCl | sodium chloride |
| PE | polyethylene |
| PMMA | poly(methyl methacrylate) |
| PMI | porometer |
| PP | polypropylene |
| PTFE | polytetrafluoro-ethylene |
| PVDF | polyvinylidene fluoride |
| SEM | scanning electron microscope |
| THF | tetrahydrofuran |
| VMD | vacuum membrane distillation |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| A | membrane area |
| cf | NaCl concentrations in feed |
| cp | NaCl concentrations in permeate |
| m | mass of the permeate sample |
| Jw | permeation flux |
| t | time |
| β | salt rejection |
References
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Qian, Y.; Badshah, A.; Nadeem, M.A.; Zhao, D. Highly porous carbon derived from MOF-5 as a support of ORR electrocatalysts or fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 17268–17275. [Google Scholar] [CrossRef] [PubMed]
- Jhung, S.H.; Lee, J.H.; Yoon, J.W.; Serre, C.; Ferey, G.; Chang, J.S. Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption ability. Adv. Mater. 2007, 19, 121–124. [Google Scholar] [CrossRef]
- Taylor, K.M.L.; Jin, A.; Lin, W. Surfactant-assisted synthesis of nanoscale gadolinium metal-organic frameworks for potential multimodal imaging. Angew. Chem. Int. Ed. 2008, 47, 7722–7725. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Lee, H.J.; Choi, G.; Choi, S.; Oh, M.J. Dual changes in conformation and optical properties of fluorophores within a metal-organic framework during framework construction and associated sensing event. Am. Chem. Soc. 2014, 136, 12201–12204. [Google Scholar] [CrossRef] [PubMed]
- Nalaparaju, A.; Zhao, X.S.; Jiang, J.W. Biofuel purification by pervaporation and vapor permeation in metal-organic frameworks: A computational study. Energy Environ. Sci. 2011, 4, 2107–2116. [Google Scholar] [CrossRef]
- Zhao, Y.; Seredych, M.; Zhong, Q.; Bandosz, T.J. Superior performance of copper based MOF and aminated graphite oxide composites as CO2 adsorbents at room temperature. ACS Appl. Mater. Interfaces 2016, 5, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 adsorption in a microporous metal-organic framework with open metal sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef] [PubMed]
- Latroche, M.; Surble, S.; Serre, C.; Mellot-Draznieks, C.; Llewellyn, P.L.; Lee, J.H.; Chang, J.S.; Sung, H.J.; Ferey, G. Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL101. Angew. Chem. Int. Ed. 2006, 4, 8227–8231. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Sholl, D.S. Selecting metal organic frameworks as enabling materials in mixed matrix membranes for high efficiency natural gas purification. Energy Environ. Sci. 2010, 3, 343–351. [Google Scholar] [CrossRef]
- Yang, T.X.; Xiao, Y.C.; Chung, T.S. Poly-/Metal-benzimidazole nano-composite membranes for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171–4180. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. Factors controlling successful formation of mixedmatrix gas separation materials. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Mahajan, R.; Koros, W.J. Factors controlling successful formation of mixedmatrix gas separation materials. Ind. Eng. Chem. Res. 2000, 39, 2692–2696. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Matrimid@/[Cu3(BTC)2] mixed-matrix membranes for gas separations. J. Membr. Sci. 2010, 362, 478–487. [Google Scholar] [CrossRef]
- Zacher, D.; Shekhah, O.; Woll, C.; Fischer, R.A. Thin films of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Lu, C.; Pei, C.; Xu, S.; Qiu, S. Polymer-supported and free-standing metal-organic framework membrane. Chem. Eur. J. 2012, 18, 10250–10253. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.T.Y.; Reithofer, M.R.; Chen, E.Y.; Menon, A.G.; Hor, T.S.A.; Xu, J.; Chin, J.M. Tuning omniphobicity via morphological control of metal-organic framework functionalized surfaces. J. Am. Chem. Soc. 2013, 135, 16272–16275. [Google Scholar] [CrossRef] [PubMed]
- Saeki, D.; Nagashima, Y.; Sawada, I.; Matsuyama, H. Effect of hydrophobicity of polymer materials used for water purification membranes on biofilm formation dynamics. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 622–628. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Rajabzadeh, S.; Nicolo, E.D.; Usuda, H.; Shaikh, A.R.; Matsuyama, H. Preparation and characterization of ECTFE hollow fiber membranes via thermally induced phase separation (TIPS). Polymer 2016, 97, 515–524. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Sun, X.; Ji, Z.; Luan, Z. Preparation and properties of PVDF composite hollow fiber membranes for desalination through direct contact membrane distillation. J. Membr. Sci. 2012, 405–406, 185–200. [Google Scholar] [CrossRef]
- Rafat, M.; De, D.; Khulbe, K.C.; Nguyen, T.; Matsuura, T. Surface characterization of hollow fiber membranes used in artificial kidney. J. Appl. Polym. Sci. 2006, 101, 4386–4400. [Google Scholar] [CrossRef]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.M.; Chen, E.Y.; Menon, A.G.; Tan, H.Y.; Hor, A.T.S.; Schreyer, M.K.; Xu, J. Tuning the aspect ratio of NH2-MIL-53(Al) microneedles and nanorods via coordination modulation. Cryst. Eng. Commun. 2013, 15, 654–657. [Google Scholar] [CrossRef]
- Shi, G.M.; Chung, T.S. Thin film composite membranes on ceramic for pervaporation dehydration of isopropanol. J. Membr. Sci. 2013, 448, 34–43. [Google Scholar] [CrossRef]
- Li, B.; Sirkar, K.K. Novel membrane and device for vacuum membrane distillation-based desalination process. J. Membr. Sci. 2005, 257, 60–75. [Google Scholar] [CrossRef]
- Tomaszewska, M. Membrane distillation. Environ. Prot. Eng. 1999, 25, 37–47. [Google Scholar]
- Garcia-Payo, M.C.; Izquierdo-Gil, M.A.; Fernandez-Pineda, C. Wetting study of hydrophobic membranes via liquid entry pressure measurements with aqueous alcohol solutions. J. Colloid Interface Sci. 2000, 230, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.W.; Jiang, L.Y. Optimization of morphology and performance of PVDF hollow fiber for direct contact membrane distillation using experimental design. Chem. Eng. Sci. 2013, 101, 130–143. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.D.; Duke, M.; Hoang, M.; Xie, Z.; Groth, A.; Tun, C.; Gray, S. Influence of module design and membrane compressibility on VMD performance. J. Membr. Sci. 2013, 442, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Sukitpaneenit, P.; Chung, T.S. Molecular design of the morphology and pore size of PVDF hollow fiber membranes for ethanol-water separation employing the modified pore-flow concept. J. Membr. Sci. 2011, 374, 67–82. [Google Scholar] [CrossRef]
- Zuo, J.; Bonyadi, S.; Chung, T.S. Exploring the potential of commercial polyethylene membranes for desalination by membrane distillation. J. Membr. Sci. 2016, 497, 239–247. [Google Scholar] [CrossRef]
- Kujawski, W.; Kujawa, J.; Wierzbowska, E.; Cerneaux, S.; Bryjak, M.; Kujawski, J. Influence of hydrophobization conditions and ceramic membranes pore size on the properties in vacuum membrane distillation of water-organic solvent mixtures. J. Membr. Sci. 2016, 499, 442–451. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation (review). J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Alsaadi, A.S.; Nunes, S.P.; Amy, G.L. Performance evaluation of the DCMD desalination process under bench scale and large scale module operating conditions. J. Membr. Sci. 2014, 455, 103–112. [Google Scholar] [CrossRef]
- Alsaadi, A.; Francis, L.; Amy, G.L.; Ghaffour, N. Experimental and theoretical analyses of temperature polarization effect in vacuum membrane distillation. J. Membr. Sci. 2014, 471, 138–148. [Google Scholar] [CrossRef]
- Fan, H.; Peng, Y. Application of PVDF membranes in desalination and comparison of the VMD and DCMD processes. Chem. Eng. Sci. 2012, 79, 94–102. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. A new-generation asymmetric multi-bore hollow fiber membrane for sustainable water production via vacuum membrane distillation. Environ. Sci. Technol. 2013, 47, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.B.; Wang, W.; Qiu, L.G.; Yuan, Y.P.; Peng, F.M.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Surfactant-assisted synthesis of lanthanide metal-organic framework nanorods and their fluorescence sensing of nitroaromatic explosives. Mater. Lett. 2011, 65, 1385–1387. [Google Scholar] [CrossRef]
- Yun, S.K.; Kim, J.W.; Jung, M.J.; Nho, Y.C.; Kang, P.H.; Lee, Y.S. An XPS study of oxyfluorinated multiwalled carbon nano tubes. Carbon Lett. 2007, 8, 292–298. [Google Scholar] [CrossRef]
- Nanse, G.; Papirer, E.; Fioux, P.; Moguet, F.; Tressaud, A. Fluorination of carbon blacks: An X-ray photoelectron spectroscopy study: I. A literature review of XPS studies of fluorinated carbons. XPS investigation of some reference compounds. Carbon 1997, 35, 175–194. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.S. Design and synthesis of a fluoro-silane amine monomer for novel thin film composite membranes to dehydrate ethanol via pervaporation. J. Mater. Chem. A 2013, 1, 9814–9826. [Google Scholar] [CrossRef]
- Mihaly, J.; Sterkel, S.; Ortner, H.M.; Kocsis, L.; Hajba, L.; Furdyga, E.; Mink, J. FTIR and FT-Raman spectroscopic study on polymer based high pressure digestion vessels. Croat. Chem. Acta 2006, 79, 497–501. [Google Scholar]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.D.; Duke, M.; Xie, Z.; Gray, S. Performance of asymmetric hollow fiber membranes in membrane distillation under various configurations and vacuum enhancement. J. Membr. Sci. 2010, 362, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Chandavasu, C.; Xanthos, M.; Sirkar, K.K.; Gogos, C.G. Fabrication of microporous polymeric membranes by melt processing of immiscible blends. J. Membr. Sci. 2003, 211, 167–175. [Google Scholar] [CrossRef]
- Maab, H.; Francis, I.; Al-saadi, A.; Aubry, C.; Ghaffour, N.; Amy, G.; Nunes, S.P. Synthesis and fabrication of nanostructured hydrophobic polyazole membranes for low-energy water recovery. J. Membr. Sci. 2012, 423–424, 11–19. [Google Scholar] [CrossRef]
- Tomaszewska, M. Preparation and properties of flat-sheet membranes from poly(vinylidene fluoride) for membrane distillation. Desalination 1996, 104, 1–11. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Preparation and characterization of polyvinylidene fluoride membranes for membrane distillation. Ind. Eng. Chem. Res. 2001, 40, 5710–5718. [Google Scholar] [CrossRef]
- Tang, N.; Jia, Q.; Zhang, H.; Li, J.; Cao, S. Preparation and morphological characterization of narrow pore size distributed polypropylene hydrophobic membranes for vacuum membrane distillation via thermally induced phase separation. Desalination 2010, 256, 27–36. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Criscuoli, A.; Al-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolo, E.; Al-harbi, O.A.; Drioli, E. Hollow fivers for seawater desalination from blends of PVDF with different molecular weights: Morphology, properties and VMD performance. Polymer 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Zhang, J.W.; Fang, H.; Wang, J.W.; Hao, L.Y.; Xu, X.; Chen, C.S. Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination. J. Membr. Sci. 2014, 450, 197–206. [Google Scholar] [CrossRef]
- Sun, A.C.; Kosar, W.; Zhang, Y.; Feng, X. Vacuum membrane distillation for desalination of water using hollow fiber membranes. J. Membr. Sci. 2014, 455, 131–142. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, L.; Zhang, J.W.; Xu, X.; Chen, C.S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]



| Membrane | Second MOF Growth Al Source | Second MOF Growth Time (h) | VMD Flux at 60 °C (L/m2-h) |
|---|---|---|---|
| Final hydrophobic membrane | Aluminum nitrate | 50 | Leak |
| Final hydrophobic membrane | Aluminum acetylacetonate | 50 | 6.2 |
| Membrane | Second MOF Growth Time (h) | Hydrophobic Floss Growth | LEP (Bar) |
|---|---|---|---|
| Second growth tube | 50 | No | 0 |
| Second growth tube | 50 | Yes | 3 |
| Membrane | Second MOF Growth Time (h) | VMD Flux at 50 °C (L/m2-h) | VMD Flux at 60 °C (L/m2-h) |
|---|---|---|---|
| Final hydrophobic membrane | 50 | 4.5 | 6.2 |
| Final hydrophobic membrane | 24 | 5.1 | 8.4 |
| Final hydrophobic membrane | 5 | 9.6 | 17.7 |
| Final hydrophobic membrane | 1 | 16.7 | 32.3 |
| Final hydrophobic membrane | 0.5 | 23.9 | Leak |
| Author | Membrane | Year | Feed | Feed Temperature (°C) | Flux (L/m2-h) |
|---|---|---|---|---|---|
| Khayet and Matsuura [53] | PVDF flat sheet | 2001 | Pure water | 25 | 17.0 |
| Tang et al. [54] | PP flat sheet | 2010 | 3 wt % NaCl | 70 | 24.8 |
| Alsaadi et al. [40] | PTFE flat sheet | 2014 | Seawater | 60 | 12.0 |
| Figoli et al. [55] | PVDF hollow fiber | 2014 | 3.5 wt % NaCl | 50 | 22.1 |
| Zhang et al. [56] | Si3N4 hollow fiber | 2014 | 4 wt % NaCl | 80 | 28.3 |
| Sun et al. [57] | PVDF hollow fiber | 2014 | Pure water | 80 | 1.9 |
| Wang et al. [58] | β-Sialon ceramic hollow fiber | 2016 | 2 wt % NaCl | 60 | 6.7 |
| This work | MOF-functionalized alumina tube | 2016 | 3.5 wt % NaCl | 60 | 32.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Chung, T.-S. Metal–Organic Framework-Functionalized Alumina Membranes for Vacuum Membrane Distillation. Water 2016, 8, 586. https://doi.org/10.3390/w8120586
Zuo J, Chung T-S. Metal–Organic Framework-Functionalized Alumina Membranes for Vacuum Membrane Distillation. Water. 2016; 8(12):586. https://doi.org/10.3390/w8120586
Chicago/Turabian StyleZuo, Jian, and Tai-Shung Chung. 2016. "Metal–Organic Framework-Functionalized Alumina Membranes for Vacuum Membrane Distillation" Water 8, no. 12: 586. https://doi.org/10.3390/w8120586






