The Origin of the Natural Water Chemical Composition in the Permafrost Region of the Eastern Slope of the Polar Urals
Abstract
:1. Introduction
2. Site Description and Methods
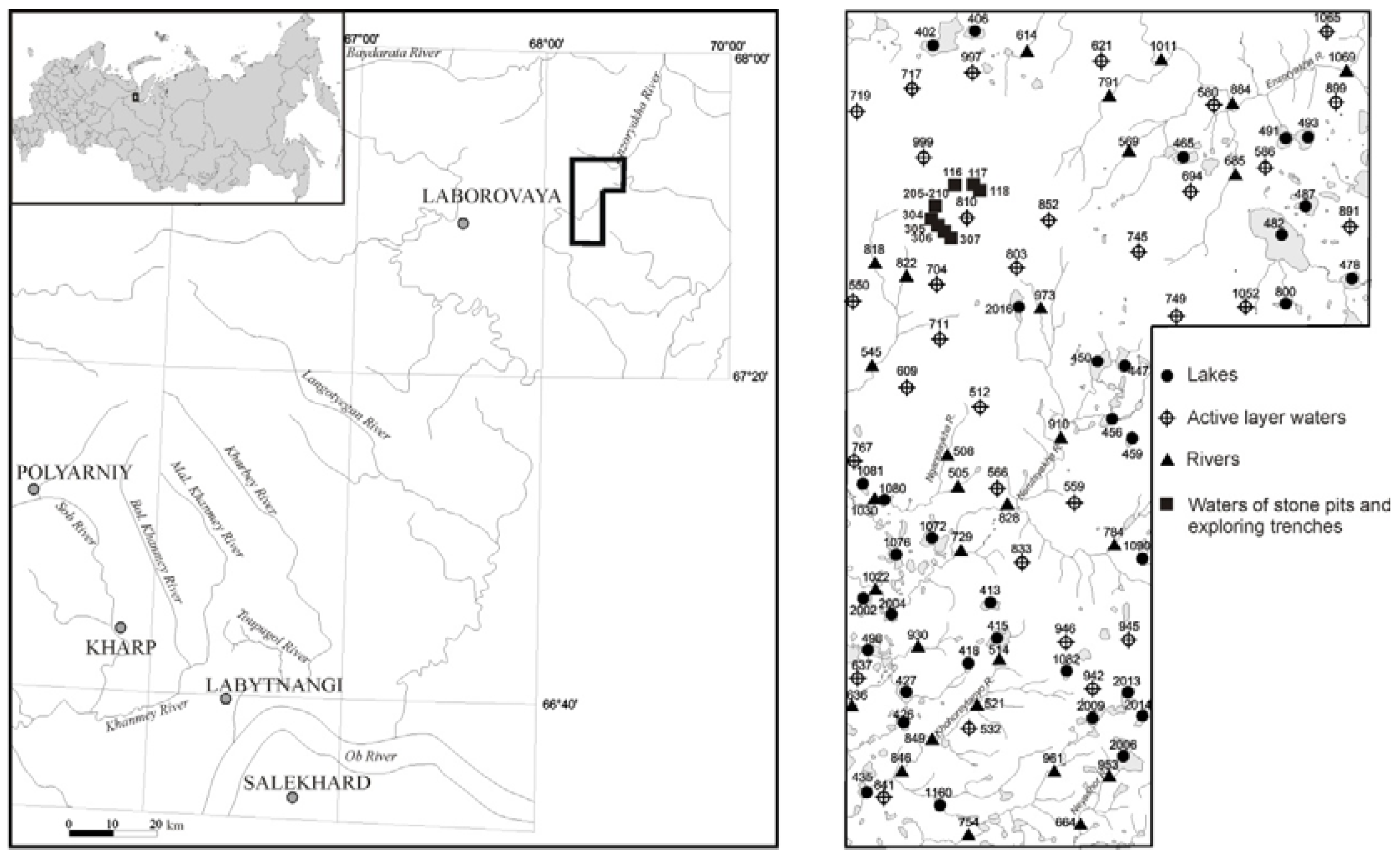
2.1. Study Area
2.2. Sampling and Analysis
3. Results and Discussion
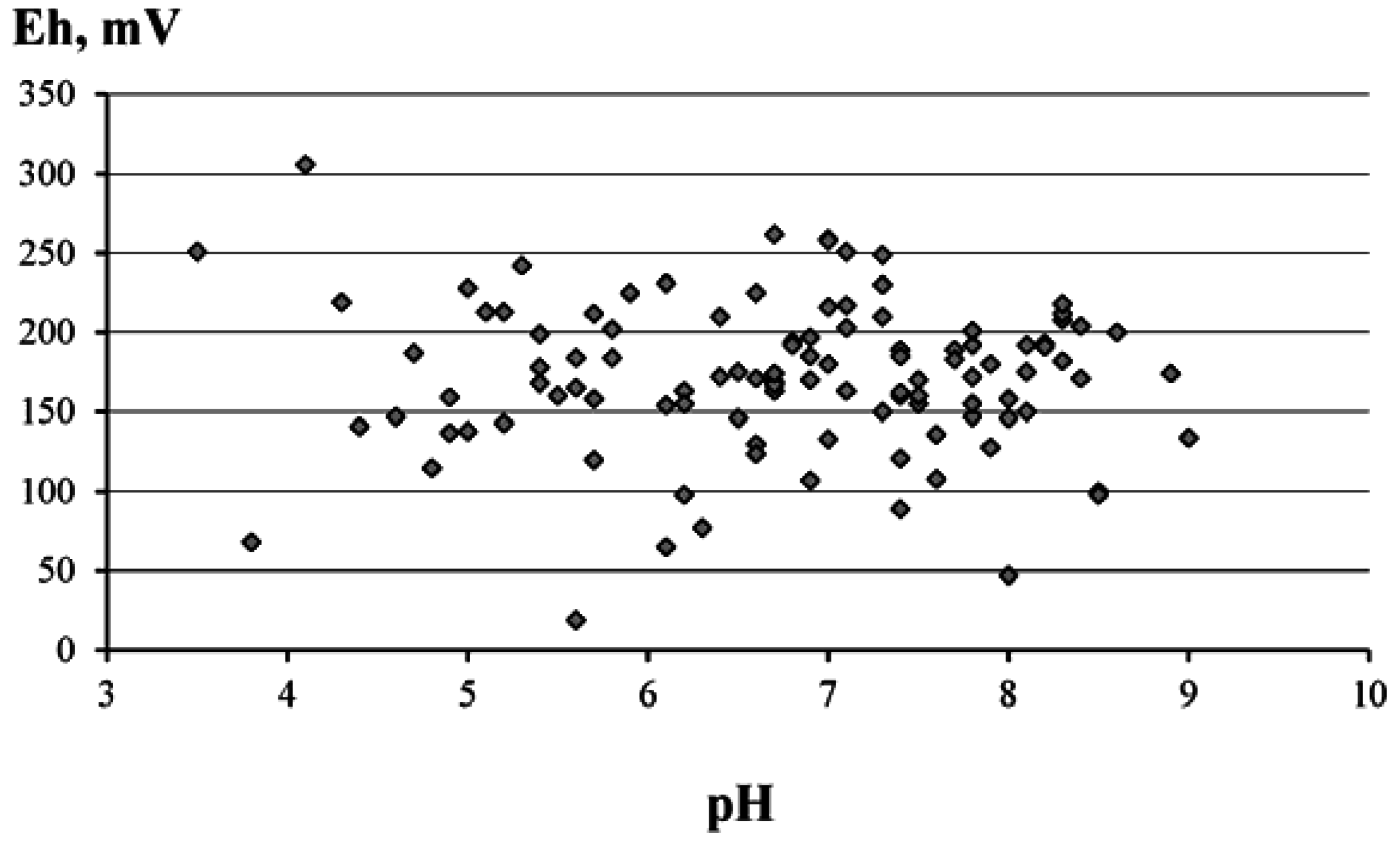
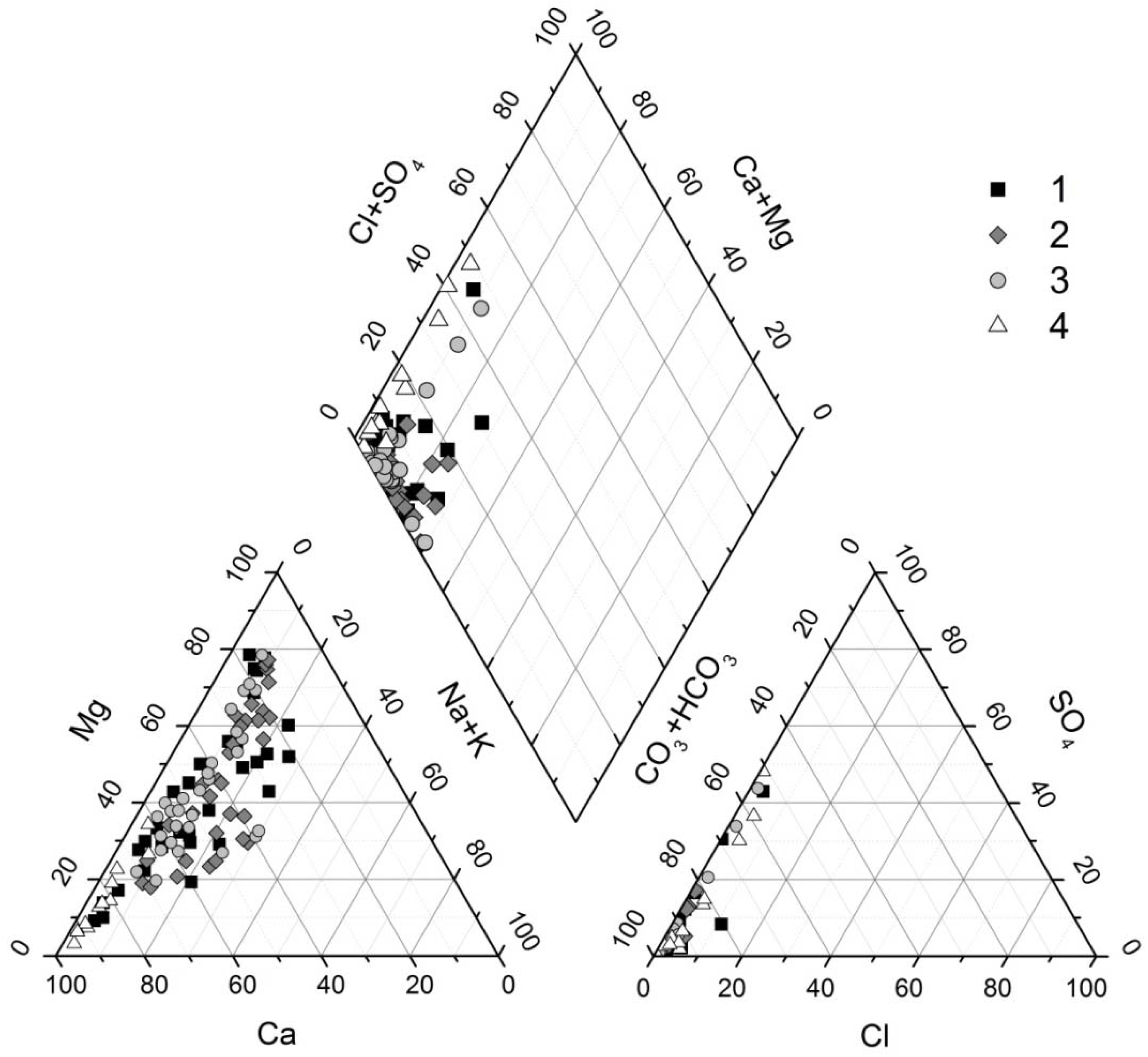
3.1. General Characteristics of Waters
3.2. Chemical Composition of Water Types
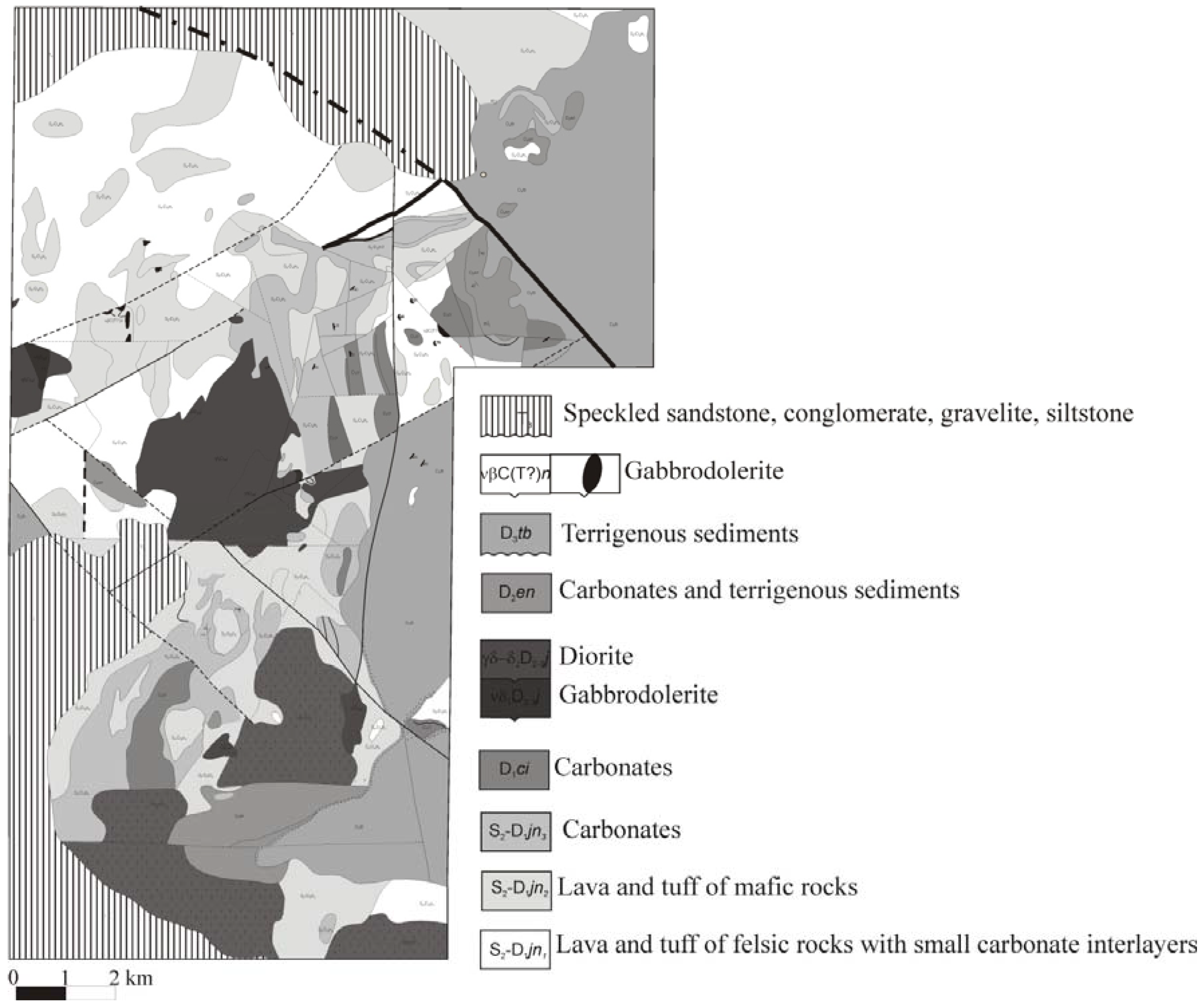
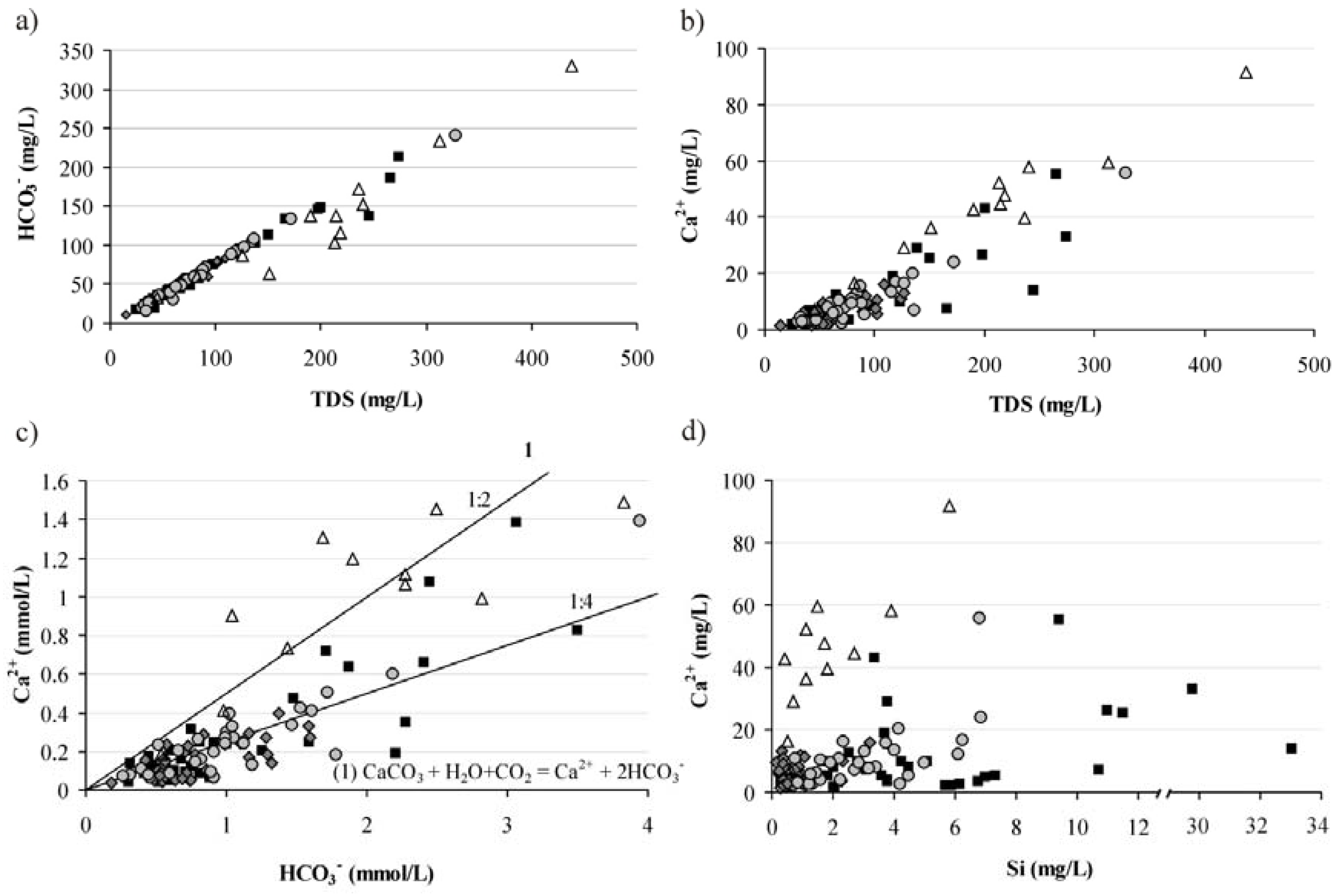
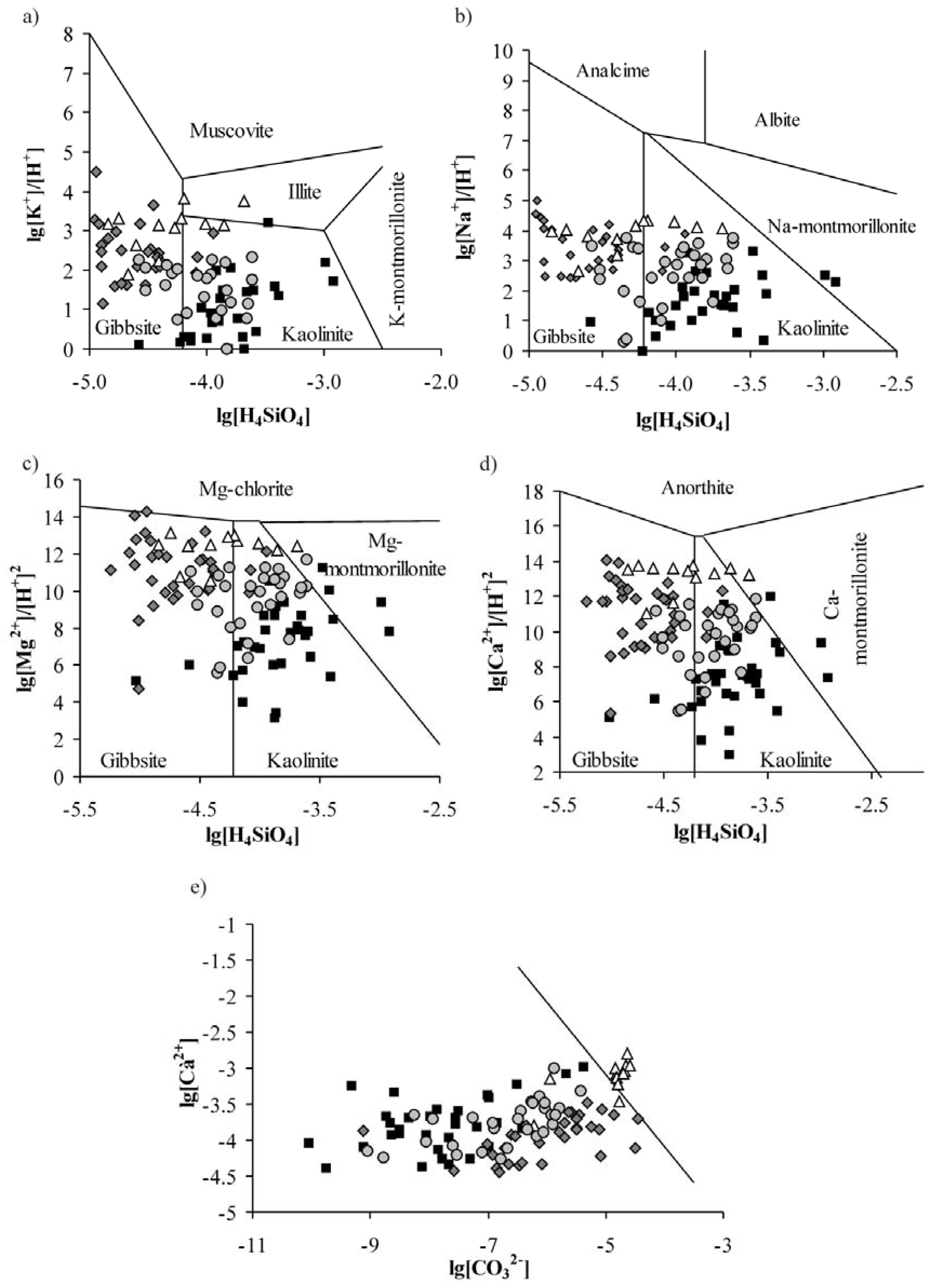
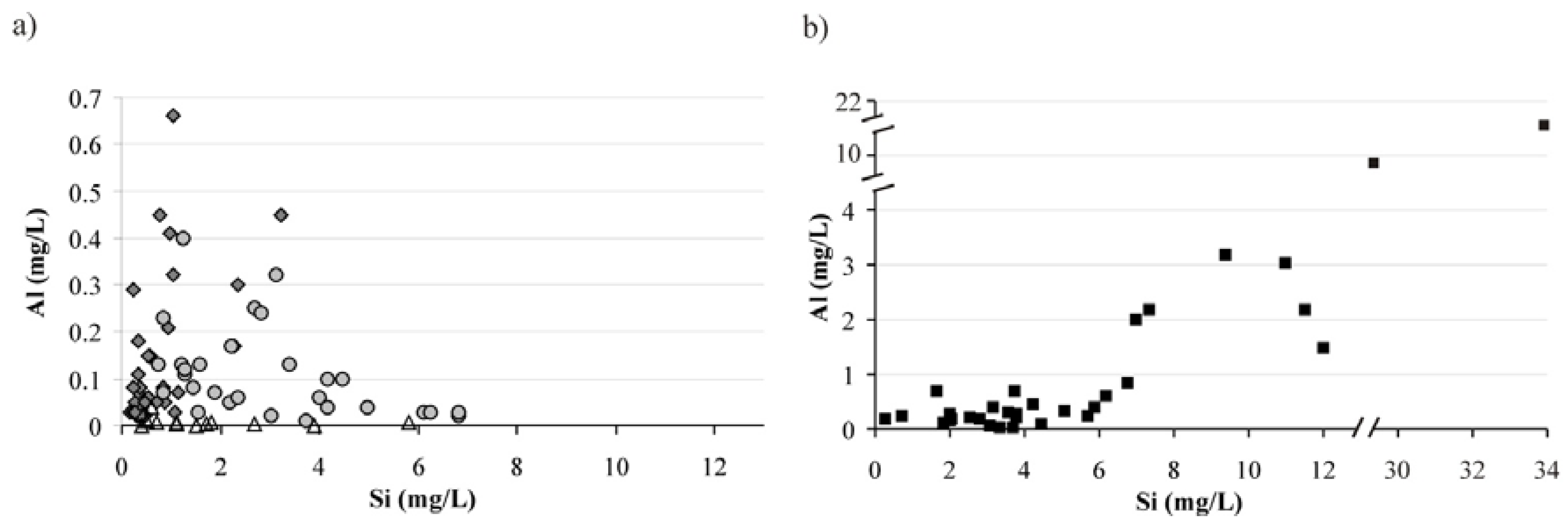
3.3. Water-Rock Interaction
3.4. Mechanisms of Water Chemical Composition Formation
4. Conclusions
Acknowledgments
Conflicts of Interest
Appendix A.
| Type | Sample | pH | Eh | HCO3 | Cl | SO4 | Ca | Mg | Na | K | TDS | Si | Al | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active layer waters | 512 | 5.8 | 202 | 35.4 | 0.2 | 0.5 | 2.5 | 5.0 | 1.2 | 0.4 | 44 | 5.88 | 0.39 | 0.06 | 0.60 |
| 532 | 6.3 | 77 | 213.4 | 0.3 | 0.5 | 33.2 | 19.3 | 4.1 | 3.4 | 274 | 28.75 | 9.52 | 0.26 | 17.45 | |
| 550 | 5.6 | 184 | 49.4 | 4.0 | 4.0 | 3.5 | 7.4 | 1.7 | 6.0 | 76 | 6.76 | 0.85 | 2.40 | 3.80 | |
| 559 | 7.5 | 155 | 186.7 | 1.9 | 12.0 | 55.4 | 5.7 | 1.7 | 2.1 | 266 | 9.38 | 3.19 | 1.20 | 2.75 | |
| 566 | 6.6 | 130 | 134.2 | 0.6 | 0.5 | 7.5 | 21.2 | 2.1 | 0.4 | 166 | 12.0 | 1.50 | 0.08 | 0.91 | |
| 580 | 3.5 | 251 | 38.2 | 0.3 | 0.5 | 4.2 | 3.8 | 1.7 | 1.5 | 50 | 3.75 | 0.69 | 0.71 | 6.21 | |
| 586 | 4.3 | 219 | 49.3 | 0.3 | 1.0 | 10.1 | 3.4 | 0.5 | 1.2 | 66 | 1.66 | 0.70 | 0.11 | 9.79 | |
| 609 | 6.1 | 231 | 53.3 | 1.5 | 1.0 | 2.6 | 8.9 | 1.3 | 0.9 | 69 | 6.16 | 0.62 | 1.56 | 3.20 | |
| 621 | 4.1 | 306 | 18.4 | 0.3 | 0.5 | 1.8 | 1.9 | 1.2 | 0.4 | 24 | 2.01 | 0.26 | 0.15 | 3.49 | |
| 637 | 6.2 | 163 | 89.7 | 0.3 | 0.5 | 19.2 | 5.4 | 1.5 | 0.5 | 117 | 3.68 | 0.04 | 1.39 | 6.88 | |
| 694 | 7.3 | 249 | 148.9 | 0.3 | 2.0 | 43.2 | 2.8 | 2.3 | 0.2 | 200 | 3.34 | 0.04 | 0.03 | 0.33 | |
| 704 | 6.7 | 262 | 38.0 | 0.3 | 6.0 | 8.2 | 2.9 | 2.0 | 0.9 | 58 | 4.46 | 0.08 | 0.19 | 0.14 | |
| 711 | 5.9 | 225 | 36.2 | 0.3 | 0.5 | 2.1 | 5.5 | 1.1 | 0.1 | 45 | 5.69 | 0.25 | 0.05 | 0.42 | |
| 717 | 5.4 | 199 | 45.8 | 0.3 | 4.0 | 5.1 | 4.4 | 4.0 | 2.0 | 66 | 6.99 | 1.99 | 0.24 | 1.88 | |
| 719 | 5.3 | 242 | 138.5 | 0.3 | 48.0 | 14.0 | 20.8 | 15.9 | 7.3 | 245 | 33.83 | 21.21 | 6.95 | 23.37 | |
| 745 | 5.2 | 143 | 27.1 | 0.9 | 0.5 | 5.6 | 1.1 | 1.5 | 1.3 | 38 | 3.57 | 0.29 | 1.77 | 6.02 | |
| 749 | 4.9 | 137 | 36.4 | 0.3 | 3.0 | 8.2 | 2.3 | 0.9 | 1.0 | 52 | 2.02 | 0.16 | 0.52 | 1.30 | |
| 767 | 5.5 | 160 | 31.9 | 0.4 | 0.5 | 1.9 | 4.9 | 0.8 | 0.2 | 40 | 2.05 | 0.19 | 0.05 | 0.35 | |
| 803 | 6.5 | 146 | 27.2 | 0.2 | 3.0 | 7.1 | 1.4 | 1.0 | 0.1 | 40 | 3.06 | 0.06 | 0.27 | 1.74 | |
| 810 | 5.2 | 213 | 19.1 | 0.7 | 12.0 | 5.5 | 3.2 | 0.6 | 0.7 | 42 | 7.32 | 2.17 | 0.34 | 5.09 | |
| 833 | 4.4 | 141 | 146.4 | 0.3 | 7.0 | 26.5 | 13.3 | 2.2 | 1.5 | 197 | 10.97 | 3.04 | 11.34 | 108.64 | |
| 841 | 6.1 | 154 | 113.8 | 0.3 | 1.0 | 25.6 | 6.4 | 1.7 | 0.8 | 150 | 11.50 | 2.17 | 2.78 | 218.26 | |
| 852 | 5.4 | 178 | 45.6 | 0.3 | 3.0 | 12.6 | 1.7 | 0.4 | 1.1 | 65 | 2.53 | 0.20 | 1.69 | 8.61 | |
| 891 | 5.0 | 228 | 41.5 | 0.3 | 0.5 | 6.4 | 3.2 | 2.2 | 0.5 | 54 | 0.73 | 0.24 | 0.37 | 0.77 | |
| 899 | 5.4 | 168 | 55.2 | 0.3 | 0.5 | 10.0 | 3.3 | 3.1 | 0.3 | 72 | 2.79 | 0.18 | 0.69 | 1.92 | |
| 942 | 6.7 | 163 | 43.3 | 0.3 | 0.5 | 3.7 | 4.6 | 3.3 | 0.4 | 56 | 3.79 | 0.26 | 0.30 | 1.51 | |
| 945 | 5.7 | 120 | 76.3 | 0.3 | 0.5 | 8.3 | 8.6 | 3.0 | 0.4 | 97 | 3.14 | 0.39 | 0.86 | 12.70 | |
| 946 | 5.6 | 19 | 96.6 | 0.3 | 0.5 | 10.0 | 10.8 | 4.5 | 0.6 | 123 | 5.05 | 0.34 | 0.72 | 1.71 | |
| 997 | 4.7 | 187 | 25.3 | 0.3 | 0.5 | 3.6 | 2.6 | 0.7 | 0.1 | 33 | 0.26 | 0.19 | 0.10 | 2.22 | |
| 999 | 5.6 | 165 | 29.2 | 0.3 | 0.5 | 5.4 | 1.9 | 1.2 | 0.2 | 38 | 1.81 | 0.12 | 0.26 | 0.58 | |
| 1052 | 3.8 | 68 | 104.2 | 0.3 | 0.5 | 28.9 | 2.1 | 2.0 | 0.4 | 138 | 3.76 | 0.22 | 2.56 | 15.19 | |
| 1065 | 5.0 | 138 | 61.3 | 0.3 | 0.5 | 9.8 | 3.6 | 5.0 | 0.4 | 80 | 4.21 | 0.45 | 0.18 | 8.36 | |
| Mean | 6.0 | 173 | 57.5 | 0.4 | 1.5 | 8.6 | 4.5 | 1.9 | 0.4 | 80.0 | 3.69 | 0.19 | 0.26 | 1.58 | |
| Lakes | 402 | 7.0 | 216 | 33.6 | 0.3 | 4.0 | 5.4 | 2.9 | 2.8 | 0.5 | 50 | 1.03 | 0.66 | 0.04 | 0.64 |
| 406 | 8.9 | 174 | 60.5 | 0.3 | 10.0 | 9.5 | 5.4 | 6.5 | 0.5 | 93 | 0.25 | 0.29 | 0.06 | 1.08 | |
| 413 | 8.3 | 208 | 97.5 | 0.3 | 0.5 | 10.9 | 10.4 | 4.5 | 0.4 | 124 | 0.31 | 0.02 | 0.03 | 0.20 | |
| 415 | 7.7 | 189 | 71.0 | 0.3 | 0.5 | 11.8 | 5.3 | 3.2 | 0.3 | 92 | 0.93 | 0.21 | 0.02 | 0.50 | |
| 418 | 8.1 | 175 | 96.9 | 0.3 | 2.0 | 13.2 | 9.2 | 4.9 | 0.5 | 127 | 0.34 | 0.04 | 0.02 | 0.32 | |
| 426 | 7.9 | 180 | 33.7 | 0.3 | 0.5 | 4.9 | 2.9 | 1.5 | 0.5 | 44 | 0.47 | 0.02 | 0.01 | 0.09 | |
| 427 | 7.8 | 192 | 78.2 | 0.3 | 0.5 | 10.8 | 4.7 | 8.3 | 0.3 | 103 | 0.35 | 0.07 | 0.01 | 0.30 | |
| 435 | 7.4 | 160 | 67.2 | 0.3 | 0.5 | 9.8 | 6.1 | 2.6 | 0.3 | 86 | 2.34 | 0.30 | 0.03 | 0.66 | |
| 447 | 7.8 | 172 | 51.0 | 0.3 | 0.5 | 11.3 | 2.6 | 1.5 | 0.2 | 67 | 1.08 | 0.03 | 0.01 | 0.20 | |
| 450 | 7.8 | 147 | 42.9 | 0.3 | 0.5 | 7.9 | 2.9 | 1.6 | 0.2 | 56 | 0.88 | 0.05 | 0.01 | 0.21 | |
| 456 | 7.5 | 170 | 31.7 | 0.3 | 0.5 | 4.2 | 2.9 | 1.7 | 0.2 | 41 | 1.13 | 0.07 | 0.03 | 0.47 | |
| 459 | 7.4 | 162 | 45.1 | 0.3 | 2.0 | 2.2 | 7.5 | 1.4 | 0.3 | 59 | 1.13 | 0.07 | 0.01 | 0.35 | |
| 465 | 7.7 | 183 | 35.4 | 0.3 | 4.0 | 9.5 | 1.6 | 1.3 | 0.5 | 53 | 0.16 | 0.03 | 0.01 | 0.14 | |
| 478 | 8.0 | 146 | 44.1 | 0.3 | 0.5 | 9.1 | 1.9 | 2.8 | 0.1 | 58 | 0.35 | 0.02 | 0.04 | 0.21 | |
| 482 | 8.1 | 150 | 33.8 | 0.3 | 0.5 | 6.6 | 1.7 | 2.1 | 0.2 | 45 | 0.40 | 0.02 | 0.04 | 0.17 | |
| 487 | 6.5 | 175 | 10.9 | 0.3 | 0.5 | 1.6 | 0.7 | 1.1 | 0.2 | 15 | 0.27 | 0.05 | 0.02 | 0.23 | |
| 491 | 8.5 | 100 | 34.0 | 0.3 | 2.0 | 6.5 | 1.7 | 2.9 | 0.5 | 48 | 0.26 | 0.03 | 0.05 | 0.25 | |
| 493 | 7.8 | 201 | 34.4 | 0.30 | 0.5 | 5.9 | 1.7 | 2.9 | 0.5 | 46 | 0.25 | 0.03 | 0.05 | 0.30 | |
| 498 | 7.0 | 259 | 29.6 | 0.3 | 0.5 | 2.1 | 3.8 | 1.2 | 0.9 | 38 | 1.05 | 0.32 | 0.06 | 0.69 | |
| 800 | 4.6 | 147 | 25.9 | 0.3 | 0.5 | 6.0 | 0.9 | 1.2 | 0.1 | 34 | 0.27 | 0.03 | 0.04 | 0.10 | |
| 1072 | 9.0 | 134 | 43.8 | 0.3 | 0.5 | 3.6 | 5.0 | 2.4 | 1.2 | 56 | 0.32 | 0.11 | 0.11 | 0.62 | |
| 1076 | 7.1 | 203 | 47.3 | 0.3 | 0.5 | 7.5 | 3.1 | 3.1 | 1.0 | 62 | 0.53 | 0.06 | 0.08 | 0.14 | |
| 1080 | 6.7 | 169 | 80.6 | 0.3 | 0.5 | 5.5 | 10.1 | 5.1 | 0.3 | 102 | 0.83 | 0.08 | 0.02 | 0.47 | |
| 1081 | 6.7 | 167 | 38.7 | 0.3 | 0.5 | 1.9 | 5.9 | 1.3 | 0.3 | 48 | 0.58 | 0.15 | 0.01 | 0.50 | |
| 1082 | 6.4 | 172 | 52.3 | 0.3 | 0.5 | 4.1 | 6.5 | 2.9 | 0.2 | 66 | 0.36 | 0.08 | 0.03 | 0.31 | |
| 1090 | 6.8 | 194 | 33.0 | 0.3 | 1.0 | 1.7 | 5.30 | 1.1 | 0.2 | 43 | 0.46 | 0.05 | 0.02 | 0.35 | |
| 1160 | 6.9 | 197 | 32.9 | 0.3 | 0.5 | 3.5 | 3.69 | 1.3 | 0.4 | 42 | 2.27 | 0.17 | 0.05 | 0.62 | |
| 2002 | 7.0 | 258 | 45.7 | 0.3 | 0.5 | 2.0 | 7.14 | 1.4 | 0.5 | 57 | 0.72 | 0.05 | 0.05 | 0.27 | |
| 2004 | 7.1 | 251 | 41.7 | 0.3 | 0.5 | 2.2 | 6.02 | 1.8 | 0.4 | 52 | 0.35 | 0.18 | 0.05 | 0.51 | |
| 2006 | 8.5 | 98 | 34.4 | 0.3 | 1.0 | 2.7 | 4.75 | 1.2 | 0.6 | 45 | 0.98 | 0.41 | 0.20 | 1.22 | |
| 2009 | 6.7 | 174 | 32.1 | 0.3 | 0.5 | 2.8 | 4.00 | 1.3 | 0.4 | 41 | 0.53 | 0.15 | 0.01 | 0.31 | |
| 2013 | 8.0 | 47 | 79.0 | 0.3 | 1.0 | 7.4 | 10.14 | 2.5 | 0.6 | 101 | 0.76 | 0.45 | 0.09 | 1.80 | |
| 2014 | 7.8 | 155 | 70.7 | 0.3 | 0.5 | 6.6 | 8.52 | 3.1 | 0.1 | 89 | 0.23 | 0.08 | 0.03 | 0.29 | |
| 2016 | 7.9 | 128 | 84.0 | 0.3 | 0.5 | 15.9 | 5.78 | 2.4 | 0.5 | 109 | 3.21 | 0.45 | 0.08 | 0.81 | |
| Mean | 7.3 | 175 | 47.0 | 0.3 | 0.2 | 5.4 | 3.7 | 2.0 | 0.3 | 57 | 0.67 | 0.08 | 0.03 | 0.40 | |
| Rivers | 505 | 6.6 | 225 | 55.5 | 0.2 | 1.0 | 2.6 | 8.9 | 1.6 | 0.01 | 70 | 4.18 | 0.10 | 0.01 | 0.25 |
| 508 | 7.1 | 217 | 72.2 | 0.2 | 0.5 | 5.4 | 10.0 | 2.2 | 0.1 | 90 | 4.48 | 0.10 | 0.00 | 0.18 | |
| 569 | 7.0 | 133 | 60.8 | 0.3 | 1.0 | 12.1 | 3.42 | 3.3 | 0.03 | 81 | 6.10 | 0.03 | 0.02 | 0.22 | |
| 614 | 7.3 | 210 | 63.2 | 0.3 | 1.0 | 13.3 | 3.6 | 2.3 | 0.2 | 84 | 3.01 | 0.02 | 0.02 | 0.26 | |
| 636 | 6.9 | 185 | 36.1 | 0.3 | 0.5 | 3.5 | 4.2 | 1.6 | 0.6 | 46 | 0.85 | 0.23 | 0.10 | 0.78 | |
| 685 | 6.9 | 107 | 240.3 | 0.3 | 9.0 | 55.9 | 9.9 | 12.0 | 0.3 | 328 | 6.81 | 0.02 | 0.20 | 1.08 | |
| 754 | 4.8 | 115 | 19.1 | 0.3 | 4.0 | 3.2 | 2.5 | 0.7 | 0.4 | 30 | 1.22 | 0.13 | 0.10 | 0.82 | |
| 784 | 4.9 | 159 | 27.4 | 0.2 | 0.5 | 2.6 | 3.6 | 0.8 | 0.1 | 35 | 1.29 | 0.11 | 0.03 | 0.55 | |
| 791 | 5.7 | 212 | 24.2 | 0.3 | 0.5 | 4.3 | 1.7 | 1.2 | 0.1 | 32 | 2.23 | 0.17 | 0.01 | 0.71 | |
| 822 | 5.7 | 158 | 31.4 | 0.5 | 13.0 | 9.5 | 2.9 | 2.0 | 0.1 | 59 | 4.96 | 0.04 | 0.00 | 0.08 | |
| 930 | 7.0 | 180 | 104.5 | 0.3 | 0.5 | 20.3 | 7.6 | 1.8 | 0.1 | 135 | 4.16 | 0.04 | 0.04 | 0.25 | |
| 961 | 6.6 | 171 | 27.3 | 0.3 | 1.0 | 3.1 | 3.1 | 1.4 | 0.3 | 37 | 0.85 | 0.07 | 0.04 | 0.67 | |
| 1030 | 6.2 | 98 | 108.4 | 0.3 | 0.5 | 7.2 | 15.1 | 3.9 | 0.5 | 136 | 2.69 | 0.25 | 0.09 | 0.88 | |
| 729 | 6.9 | 170 | 92.8 | 0.3 | 0.5 | 16.9 | 7.4 | 1.6 | 0.1 | 119 | 6.25 | 0.03 | 0.01 | 0.10 | |
| 514 | 7.4 | 185 | 64.2 | 0.3 | 0.5 | 10.8 | 4.8 | 2.9 | 0.3 | 83 | 0.74 | 0.13 | 0.06 | 0.54 | |
| 521 | 7.4 | 89 | 89.3 | 0.3 | 0.5 | 13.5 | 7.8 | 3.6 | 0.3 | 115 | 3.99 | 0.06 | 0.07 | 0.34 | |
| 545 | 7.6 | 108 | 133.4 | 0.2 | 0.5 | 24.2 | 10.1 | 3.5 | 0.2 | 172 | 6.82 | 0.03 | 0.01 | 0.09 | |
| 664 | 5.8 | 184 | 54.3 | 0.3 | 2.0 | 3.9 | 8.1 | 1.6 | 0.4 | 71 | 1.59 | 0.13 | 0.06 | 1.49 | |
| 818 | 6.6 | 124 | 40.1 | 0.3 | 3.0 | 8.1 | 3.0 | 1.7 | 0.1 | 56 | 3.40 | 0.13 | 0.02 | 0.13 | |
| 828 | 5.1 | 213 | 60.2 | 0.3 | 1.0 | 10.8 | 4.7 | 1.9 | 0.2 | 79 | 2.20 | 0.05 | 0.01 | 0.18 | |
| 846 | 6.8 | 192 | 68.3 | 0.3 | 0.5 | 9.6 | 6.4 | 2.8 | 0.4 | 88 | 2.82 | 0.24 | 0.03 | 0.49 | |
| 849 | 7.5 | 160 | 55.4 | 0.3 | 1.0 | 7.8 | 5.5 | 2.1 | 0.2 | 72 | 3.14 | 0.32 | 0.03 | 0.54 | |
| 884 | 7.1 | 163 | 50.1 | 0.3 | 0.5 | 6.5 | 3.2 | 5.5 | 0.3 | 66 | 1.43 | 0.08 | 0.04 | 0.64 | |
| 910 | 7.6 | 136 | 48.8 | 0.3 | 3.0 | 10.6 | 3.3 | 1.6 | 0.1 | 68 | 1.56 | 0.03 | 0.04 | 0.26 | |
| 953 | 6.4 | 210 | 15.9 | 0.3 | 10.0 | 2.9 | 3.4 | 0.9 | 0.7 | 34 | 1.23 | 0.40 | 0.17 | 1.41 | |
| 973 | 7.3 | 230 | 62.2 | 1.1 | 3.0 | 15.7 | 2.9 | 1.9 | 0.02 | 87 | 3.73 | 0.01 | 0.01 | 0.25 | |
| 1011 | 7.3 | 150 | 47.3 | 0.3 | 0.5 | 5.9 | 3.1 | 5.2 | 0.2 | 62 | 1.27 | 0.12 | 0.05 | 0.85 | |
| 1022 | 6.2 | 155 | 98.1 | 0.3 | 0.5 | 16.4 | 8.1 | 2.8 | 0.4 | 126 | 2.34 | 0.06 | 0.04 | 0.31 | |
| 1069 | 6.1 | 65 | 60.3 | 0.3 | 0.5 | 9.7 | 3.3 | 5.4 | 0.3 | 79 | 1.89 | 0.07 | 0.03 | 0.50 | |
| Mean | 6.9 | 170 | 64.8 | 0.3 | 1.8 | 10.9 | 4.2 | 1.9 | 0.2 | 80 | 2.32 | 0.13 | 0.026 | 0.4 | |
| Waters of stone pits and exploration trenches | 116 | 8.2 | 193 | 138.6 | 4.4 | 18.0 | 44.6 | 4.9 | 3.0 | 0.4 | 214 | 2.7 | 0.003 | 0.00003 | 0.56 |
| 117 | 7.4 | 121 | 63.4 | 0.6 | 47.0 | 36.2 | 1.8 | 1.5 | 0.3 | 151 | 1.1 | 0.006 | 0.0004 | 0.41 | |
| 118 | 8.0 | 158 | 330.4 | 1.9 | 1.0 | 91.7 | 8.5 | 2.9 | 2.3 | 438 | 5.8 | 0.007 | 0.258 | 1.09 | |
| 205 | 8.3 | 212 | 102.8 | 4.4 | 50.0 | 52.3 | 2.4 | 0.7 | 0.3 | 213 | 1.1 | 0.002 | 0.003 | 0.60 | |
| 206 | 8.6 | 200 | 59.8 | 1.9 | 1.0 | 16.4 | 2.4 | 0.7 | 0.2 | 82 | 0.5 | 0.011 | 0.004 | 0.22 | |
| 207 | 8.1 | 192 | 172.0 | 1.9 | 7.0 | 39.6 | 9.8 | 4.1 | 2.1 | 236 | 1.8 | 0.008 | 0.106 | 0.88 | |
| 208 | 8.4 | 204 | 116.1 | 4.4 | 42.0 | 47.9 | 4.9 | 2.2 | 0.3 | 218 | 1.7 | 0.003 | 0.002 | 0.60 | |
| 209 | 8.3 | 218 | 152.4 | 4.4 | 22.0 | 58.0 | 1.2 | 1.6 | 0.3 | 240 | 3.9 | 0.001 | 0.002 | 0.80 | |
| 304 | 8.2 | 191 | 233.3 | 1.9 | 5.0 | 59.5 | 10.9 | 2.2 | 0.3 | 313 | 1.5 | 0.001 | 0.004 | 0.12 | |
| 305 | 7.4 | 189 | 32.9 | 0.8 | 1.0 | 7.2 | 2.4 | 0.4 | 0.1 | 45 | 0.6 | 0.039 | 0.012 | 0.65 | |
| 306 | 8.3 | 182 | 138.4 | 1.9 | 3.5 | 42.6 | 2.4 | 1.2 | 0.3 | 190 | 0.4 | 0.001 | 0.012 | 0.21 | |
| 307 | 8.4 | 171 | 87.6 | 1.9 | 5.0 | 29.3 | 1.2 | 0.6 | 0.1 | 126 | 0.7 | 0.007 | 0.006 | 0.05 | |
| Mean | 8.1 | 186 | 135.6 | 2.5 | 16.8 | 43.8 | 4.4 | 1.8 | 0.6 | 206 | 1.8 | 0.007 | 0.034 | 0.52 |
References
- Pašava, J.; Knésl, I.; Vymazalová, A.; Vavřín, I.; Gurskaya, L.I.; Kolbantsev, L.R. Geochemistry and mineralogy of platinum-group elements (PGE) in chromites from Centralnoye I, Polar Urals, Russia. Geosci. Front. 2011, 2, 81–85. [Google Scholar] [CrossRef]
- Soloviev, S.G.; Kryazhev, S.G.; Dvurechenskaya, S.S. Geology, mineralization, stable isotope geochemistry, and fluid inclusion characteristics of the Novogodnee–Monto oxidized Au–(Cu) skarn and porphyry deposit, Polar Ural, Russia. Miner. Deposita 2013, 48, 603–627. [Google Scholar] [CrossRef]
- Vasil’ev, N.V.; Chevychelov, V.Y.; Zaraisky, G.P.; Borodulin, G.P.; Udoratina, O.V. Tantalum-Niobium mineralization of the Taikeu ore cluster, the Polar Urals. Geol. Ore Depos. 2009, 51, 537–548. [Google Scholar] [CrossRef]
- Ugolini, F.C. Pedogenic zonation in the well-drained soils of the arctic regions. Quat. Res. 1986, 26, 100–120. [Google Scholar] [CrossRef]
- Hodson, A.; Tranter, M.; Vatne, G. Contemporary rates of chemical denudation and atmospheric CO2 sequestration in glacier basins: An Arctic perspective. Earth Surf. Process. Landf. 2000, 25, 1447–1471. [Google Scholar] [CrossRef]
- Reynolds, R.C., Jr.; Johnson, N.M. Chemical weathering in the temperate glacial environment of the Northern Cascade Mountains. Geochim. Cosmochim. Acta 1972, 36, 537–554. [Google Scholar] [CrossRef]
- Drever, J.I.; Zobrist, J. Chemical weathering of silicate rocks as a function of elevation in the southern Swiss Alps. Geochim. Cosmochim. Acta 1992, 56, 3209–3216. [Google Scholar] [CrossRef]
- Anderson, S.P.; Drever, J.I.; Humphrey, N.F. Chemical weathering in glacial environments. Geology 1997, 25, 399–402. [Google Scholar] [CrossRef]
- Anderson, S.P.; Drever, J.I.; Frost, C.D.; Holden, P. Chemical weathering in the foreland of a retreating glacier. Geochim. Cosmochim. Acta 2000, 64, 1173–1189. [Google Scholar] [CrossRef]
- Anderson, S.P.; Longacre, S.A.; Kraal, E.R. Patterns of water chemistry and discharge in the glacier-fed Kennicott River, Alaska: Evidence for subglacial water storage cycles. Chem. Geol. 2003, 202, 297–312. [Google Scholar] [CrossRef]
- Hodson, A.; Tranter, M.; Gurnell, A.; Slark, M.; Hagen, J.O. The hydrochemistry of Bayelva, a high Arctic proglacial stream in Svalbard. J. Hydrol. 2002, 257, 91–114. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. Northern latitude chemical weathering rates: Clues from the Mackenzie River Basin, Canada. Geochim. Cosmochim. Acta 2003, 67, 1305–1329. [Google Scholar] [CrossRef]
- Dragon, K.; Marciniak, M. Chemical composition of groundwater and surface water in the Arctic environment (Petuniabukta region, central Spitsbergen). J. Hydrol. 2010, 386, 160–172. [Google Scholar] [CrossRef]
- Cooper, R.J.; Wadhama, J.L.; Trantera, M.; Hodgkinsb, R.; Petersc, N.E. Groundwater hydrochemistry in the active layer of the proglacial zone, Finsterwalderbreen, Svalbard. J. Hydrol. 2002, 269, 208–223. [Google Scholar] [CrossRef]
- Savichev, O.G.; Kolesnichenko, L.G.; Saifulina, E.V. The ecologo-geochemical state of water bodies in the Taz-Yenisei interfluve. Geogr. Nat. Resour. 2011, 32, 333–336. [Google Scholar] [CrossRef]
- Kokelj, S.V.; Smith, C.A.S.; Burn, C.R. Physical and chemical characteristics of the active layer and permafrost, Herschel Island, western Arctic Coast, Canada. Permafr. Periglac. Process. 2002, 13, 171–185. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. The global control of silicate weathering rates and the coupling with physical erosion: New insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 2002, 196, 83–98. [Google Scholar] [CrossRef]
- Lobbes, J.M.; Fitznar, H.P.; Kattner, G. Biogeochemical characteristics of dissolved and particulate organic matter in Russian rivers entering the Arctic Ocean. Geochim. Cosmochim. Acta 2000, 64, 2973–2983. [Google Scholar] [CrossRef]
- Kawahigashi, M.; Kaiser, K.; Kalbitz, K.; Rodionov, A.; Guggenberger, G. Dissolved organic matter in small streams along a gradient from discontinuous to continuous permafrost. Glob. Chang. Biol. 2004, 10, 1576–1586. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M.; Chin, Y.P.; Miller, P.; Jaros, C.L. Chemical characteristics of fulvic acids from Arctic surface waters: Microbial contributions and photochemical transformations. J. Geophys. Res. 2007, 112, G04S51. [Google Scholar] [CrossRef]
- Holmes, R.M.; McClelland, J.W.; Raymond, P.A.; Frazer, B.B.; Peterson, B.J.; Stieglitz, M. Lability of DOC transported by Alaskan rivers to the Arctic Ocean. Geophys. Res. Lett. 2008, 35, L03402. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Aiken, G.R.; Wickland, K.P.; Striegl, R.G.; Hernes, P.J. Seasonal and spatial variability in dissolved organic matter quantity and composition from the Yukon River basin, Alaska. Glob. Biogeochem. Cycles 2008, 22, GB4002. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Aiken, G.R.; Butler, K.D.; Dornblaser, M.M.; Striegl, R.G.; Hernes, P.J. Utilizing chromophoric dissolved organic matter measurements to derive export and reactivity of dissolved organic carbon exported to the Arctic Ocean: A case study of the Yukon River, Alaska. Geophys. Res. Lett. 2009, 36, L06401. [Google Scholar] [CrossRef]
- Balcarczyk, K.L.; Jones, J.B., Jr.; Jaffeґ, R.; Maie, N. Stream dissolved organic matter bioavailability and composition in watersheds underlain with discontinuous permafrost. Biogeochemistry 2009, 94, 255–270. [Google Scholar] [CrossRef]
- Shirokova, L.S.; Pokrovsky, O.S.; Kirpotin, S.N.; Desmukh, C.; Pokrovsky, B.G.; Audry, S.; Viers, J. Biogeochemistry of organic carbon, CO2, CH4, and trace elements in thermokarst water bodies in discontinuous permafrost zones of Western Siberia. Biogeochemistry 2013, 113, 573–593. [Google Scholar] [CrossRef]
- Niu, F.; Lin, Z.; Liu, H.; Lu, J. Characteristics of thermokarst lakes and their influence on permafrost in Qinghai–Tibet Plateau. Geomorphology 2011, 132, 222–233. [Google Scholar] [CrossRef]
- Karlsson, J.M.; Lyon, S.W.; Destoun, G. Thermokarst lake, hydrological flow and water balance indicators of permafrost change in Western Siberia. J. Hydrol. 2012, 464–465, 459–466. [Google Scholar] [CrossRef]
- Kirpotin, S.N.; Polishchuk, Y.M.; Bryksina, N.A. Dynamics of areas of thermokarst lakes in perennial and nonperennial cryolithic zones of West Siberia under global warming condition. Vestn. Tomsk. Gos. Univ. 2008, 311, 185–189. (In Russian) [Google Scholar]
- Kuzin, A.V.; Tulenina, Y.V. Permafrost thickness on the eastern slope of the Polar Urals by the VES data. Environ. Geosci. 2011, 5, 474–476. (In Russian) [Google Scholar]
- Dobinski, W. Permafrost. Earth Sci. Rev. 2011, 108, 158–169. [Google Scholar] [CrossRef]
- Glavtyumengeologiya. The Results of Geological Survey of the Basin Area of Schuchya River (the Polar Urals); Report of the Schuchya Geological Field Company; Glavtyumengeologiya: Tyumen, Russia, 1973. (In Russian) [Google Scholar]
- Bukaty, M.B. Software development for solution of hydro-geological problems. Bull. Tomsk Polytech. Univ. 2002, 305, 348–365. (In Russian) [Google Scholar]
- Shvartsev, S.L. Geochemistry of fresh groundwater in the main landscape zones of the Earth. Geochem. Int. 2008, 46, 1285–1398. [Google Scholar] [CrossRef]
- Kambalina, M.G.; Scvortsova, L.N.; Mazurova, I.S.; Guseva, N.V.; An, V.V. Study of aqueous chemical forms of silicon in organic-rich waters. Procedia Chem. 2014, 10, 36–42. [Google Scholar] [CrossRef]
- Kataeva, M.N.; Alexeeva-Popova, N.V.; Drozdova, I.V.; Beljaeva, A.I. Chemical composition of soils and plant species in the Polar Urals as influenced by rock type. Geoderma 2004, 122, 257–268. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Shvartsev, S.L.; Ryzhenko, B.N.; Alekseev, V.A.; Dutova, E.M.; Kondrateva, I.A.; Kopylova, Y.; Lepokurova, O.E. Geological Evolution and Self-Organization of the Water–Rock System; Publishing House of Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2007. (In Russian) [Google Scholar]
- Guseva, N.V.; Kopylova, Y.G.; Khvashchevskaya, A.A. Geochemical types of natural waters in the interfluve of the Enzor’yakhi and the Yun’yakhi (eastern slope of the Polar Urals). Water Resour. 2013, 40, 417–425. [Google Scholar] [CrossRef]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals and Equilibria; Harper & Row: New York, NY, USA, 1965. [Google Scholar]
- Borovitskiy, V.I. Trace elements behavior in active layer and hydrogeochemical prospecting ore deposits in permafrost region. In Proceedings of the 5-th Meeting of Groundwater of Siberia and the Far East, Irkutsk, Russia, 21–26 June 1967; SB AS USSR: Irkutsk, Russia, 1967; pp. 109–110. (In Russian)[Google Scholar]
- Vozhov, V.I. The results of hydrogeochemical research in Talnahskoye Cu-Ni deposit. In Proceedings of the Conference of Hydrogeochemical and Paleohydrogeological Research Methods for Ore Deposit Prospecting, Tomsk, Russia, 15–18 February 1965; Tomsk University Publishing House: Tomsk, Russia, 1969; pp. 95–98. (In Russian)[Google Scholar]
- Klaminder, J.; Grip, H.; Morth, C.-M.; Laudon, H. Carbon mineralization and pyrite oxidation in groundwater: Importance for silicate weathering in boreal forest soils and stream base-flow chemistry. Appl. Geochem. 2011, 26, 319–325. [Google Scholar] [CrossRef]
- Dongarrà, G.; Manno, E.; Sabatino, G.; Varrica, D. Geochemical characteristics of waters in mineralised area of Peloritani Mountains (Sicily, Italy). Appl. Geochem. 2009, 24, 900–914. [Google Scholar] [CrossRef]
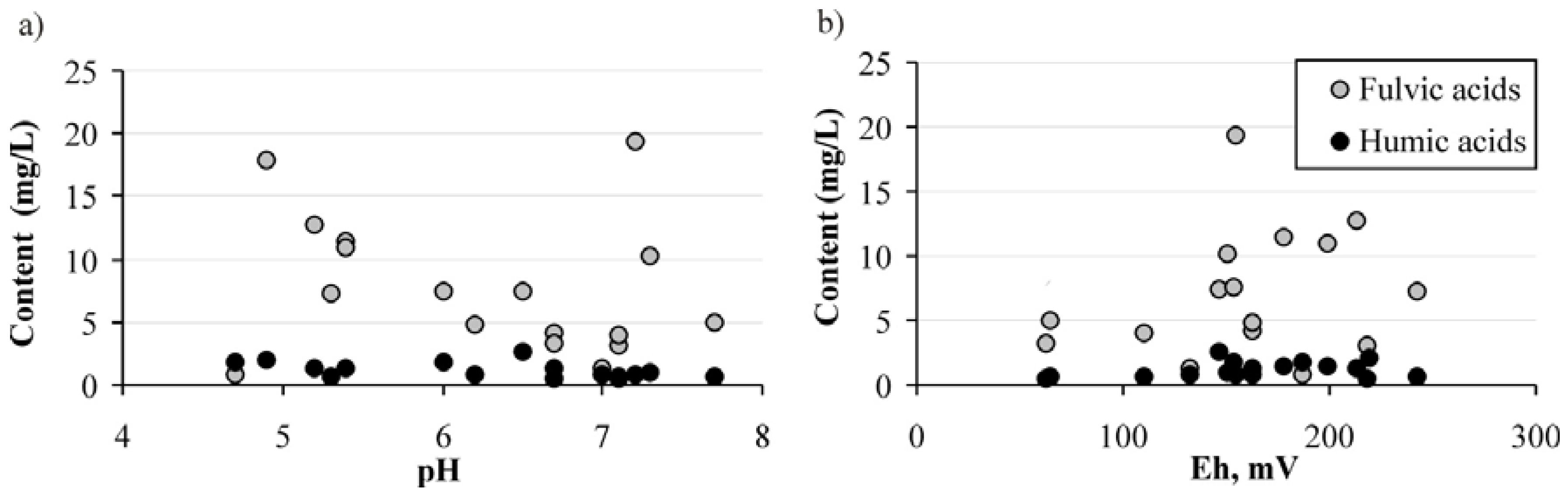

| Water Type | Eh | pH | Fulvic Acids (mg/L) | Humic Acids (mg/L) |
|---|---|---|---|---|
| river | 150 | 7.3 | 10.2 | 0.9 |
| river | 155 | 7.2 | 19.4 | 0.7 |
| river | 133 | 7.00 | 1.3 | 0.8 |
| river | 111 | 7.10 | 4.0 | 0.6 |
| river | 64 | 7.70 | 5.0 | 0.7 |
| waters of active layer | 125 | 6.50 | 7.4 | 2.7 |
| waters of active layer | 163 | 6.7 | 4.2 | 1.3 |
| waters of active layer | 178 | 5.4 | 11.5 | 1.3 |
| waters of active layer | 199 | 5.4 | 10.90 | 1.4 |
| waters of active layer | 213 | 5.2 | 12.8 | 1.3 |
| waters of active layer | 163 | 6.2 | 4.8 | 0.8 |
| waters of active layer | 219 | 4.9 | 17.8 | 2.0 |
| waters of active layer | 242 | 5.3 | 7.2 | 0.6 |
| waters of active layer | 218 | 7.1 | 3.1 | 0.5 |
| waters of active layer | 153 | 6.0 | 7.5 | 1.8 |
| waters of active layer | 62 | 6.70 | 3.3 | 0.5 |
| Average for waters of the Vasugan swamp [33] | 25.1 | 7.06 | ||
| Elements | Waters of Active Layer | Rivers | Lakes | Waters of Stone Pits and Exploring Trenches | Average of World Rivers [33] | Elements | Waters of Active Layer | Rivers | Lakes | Waters of Stone Pits and Exploring Trenches | Average of World Rivers [33] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li | 2.5 | Br | 20.0 | ||||||||
| Be | - | Rb | 2.0 | ||||||||
| B | - | 20 | Sr | 50.0 | |||||||
| Sc | 0.004 | Y | 0.7 | ||||||||
| Ti | 3.0 | Zr | 2.6 | ||||||||
| V | 1.0 | Nb | 0.001 | ||||||||
| Cr | 1.0 | Mo | 1.0 | ||||||||
| Co | 0.3 | Ru | - | ||||||||
| Ni | 2.5 | Rh | - | ||||||||
| Cu | 7.0 | Pd | - | ||||||||
| Zn | 20.0 | Ag | 0.2 | ||||||||
| Ga | - | 0.1 | Cd | 0.2 | |||||||
| Ge | - | 0.07 | Sn | 0.04 | |||||||
| As | 2.0 | Sb | 1.0 | ||||||||
| Se | 0.2 | Te | - | ||||||||
| Cs | 0.03 | Pt | - | ||||||||
| Ba | 30.0 | Au | 0.002 | ||||||||
| La | 0.05 | Hg | 0.07 | ||||||||
| Eu | 0.001 | Tl | 1.0 | ||||||||
| Lu | 0.001 | Pb | 1.0 | ||||||||
| Hf | - | Bi | - | ||||||||
| Ta | - | - | Th | 0.1 | |||||||
| W | 0.03 | U | 0.5 | ||||||||
| Re | - | - | - | - | - | - | - |
| Element | HCO3− | Cl− | SO42− | Ca2+ | Mg2+ | Na+ | K+ | TDS | Si | Al | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCO3− | 1 | |||||||||||
| Cl− | 0.15 | 1 | ||||||||||
| SO42− | 0.16 | 0.01 | 1 | |||||||||
| Ca2+ | 0.87 | 0.16 | 0.35 | 1 | ||||||||
| Mg2+ | 0.56 | 0.11 | 0.22 | 0.18 | 1 | |||||||
| Na+ | 0.54 | −0.05 | 0.39 | 0.45 | 0.24 | 1 | ||||||
| K+ | 0.23 | 0.13 | 0.29 | 0.15 | 0.28 | 0.24 | 1 | |||||
| TDS | 0.97 | 0.15 | 0.39 | 0.90 | 0.55 | 0.60 | 0.28 | 1 | ||||
| Si | 0.43 | 0.12 | 0.26 | 0.30 | 0.47 | 0.24 | 0.58 | 0.45 | 1 | |||
| Al | 0.19 | 0.0003 | 0.26 | 0.14 | 0.27 | 0.14 | 0.61 | 0.24 | 0.85 | 1 | ||
| Mn | 0.15 | 0.20 | 0.10 | 0.10 | 0.19 | 0.03 | 0.40 | 0.16 | 0.32 | 0.30 | 1 | |
| Fe | 0.08 | 0.01 | 0.08 | 0.08 | 0.08 | −0.0004 | 0.27 | 0.09 | 0.30 | 0.33 | 0.39 | 1 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guseva, N. The Origin of the Natural Water Chemical Composition in the Permafrost Region of the Eastern Slope of the Polar Urals. Water 2016, 8, 594. https://doi.org/10.3390/w8120594
Guseva N. The Origin of the Natural Water Chemical Composition in the Permafrost Region of the Eastern Slope of the Polar Urals. Water. 2016; 8(12):594. https://doi.org/10.3390/w8120594
Chicago/Turabian StyleGuseva, Natalia. 2016. "The Origin of the Natural Water Chemical Composition in the Permafrost Region of the Eastern Slope of the Polar Urals" Water 8, no. 12: 594. https://doi.org/10.3390/w8120594
APA StyleGuseva, N. (2016). The Origin of the Natural Water Chemical Composition in the Permafrost Region of the Eastern Slope of the Polar Urals. Water, 8(12), 594. https://doi.org/10.3390/w8120594





