Preliminary Study on the Removal of Steroidal Estrogens Using TiO2-Doped PVDF Ultrafiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membranes
2.3. Cross-Flow Experiment
2.4. Quantification
3. Results and Discussion
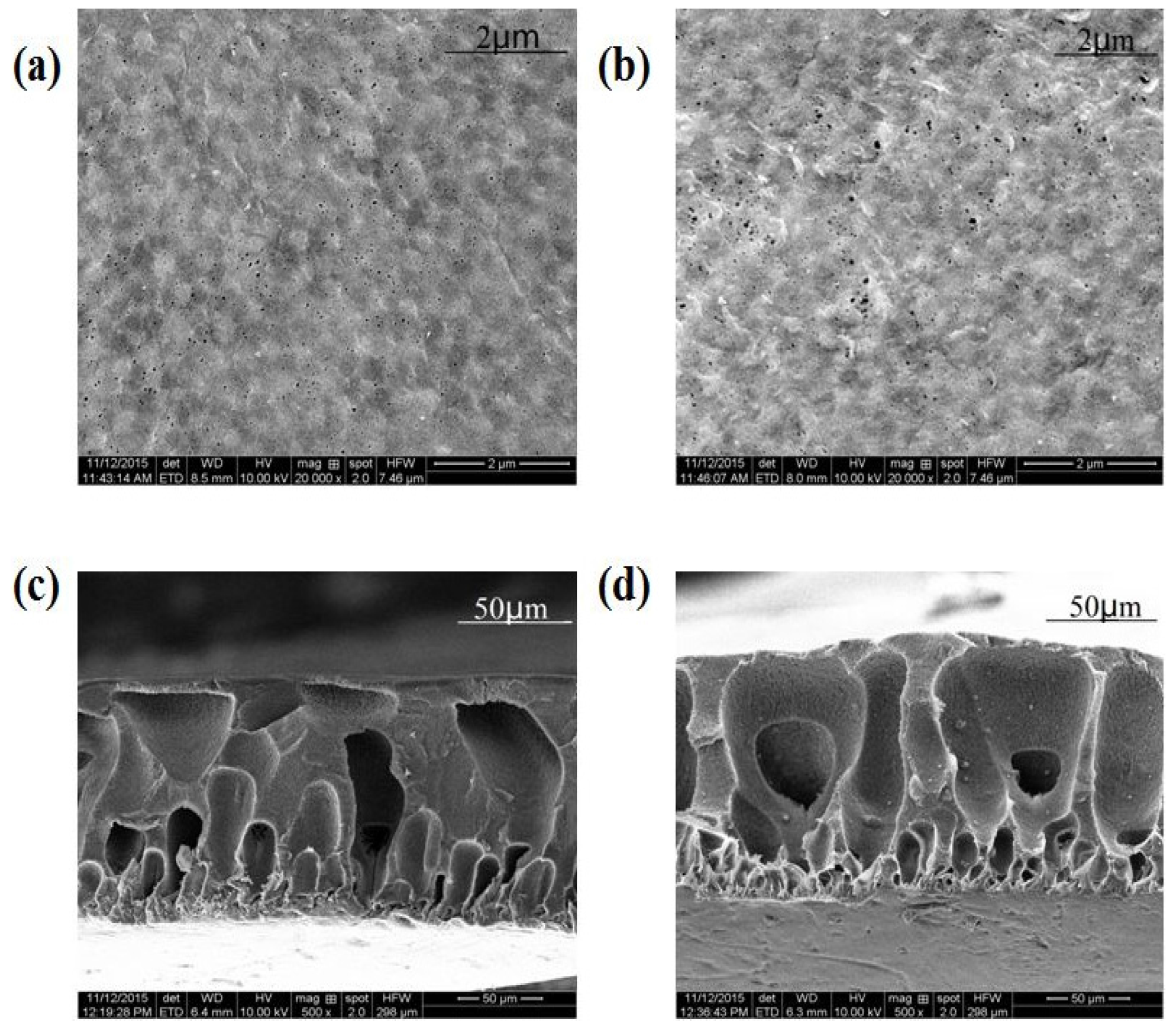
3.1. Characterization of Composite PVDF Membranes
3.2. Removal Efficiencies of E1 and E2 Under UV Photolysis
3.3. Removal Efficiency of E1 and E2 Using the PVDF-PVP Ultrafiltration Membrane
3.4. Removal Efficiencies of E1 and E2 Using the PVDF-PVP-TiO2 Ultrafiltration Membrane
3.5. Photocatalytic Degradation Kinetics Analysis
3.6. Identification of E1 Photoproducts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.H.; Tian, Q.; Fang, J.; Sung, S. Removal of 17-β estradiol in water by sonolysis. Int. Biodeterior. Biodegrad. 2015, 102, 11–14. [Google Scholar] [CrossRef]
- Hanselman, T.A.; Graetz, D.A.; Wilkie, A.C. Manure-Borne estrogens as potential environmental contaminants: A review. Environ. Sci. Technol. 2003, 37, 5471–5478. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yakou, Y.; Takahashi, A.; Higashitani, T.; Komori, K. Comparison between estrogenicities estimated from DNA recombinant yeast assay and from chemical analyses of endocrine disrupters during sewage treatment. Environ. Sci. Technol. 2001, 43, 125–132. [Google Scholar]
- Campbell, C.G.; Borglin, S.E.; Green, F.B.; Grayson, A.; Wozei, E.; Stringfellow, W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere 2006, 65, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000, a national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Ra, J.S.; Cho, J.; Kim, S.D.; Choi, H.K.; Park, J.H.; Kim, K.W.; Inam, E.; Kim, S.D. Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere 2010, 78, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Ran, Y.; Chen, D.Y.; Yang, Y.; Ma, X.X. Occurrence and environmental risk of endocrine-disrupting chemicals in surface waters of the Pearl River,South China. Environ. Monit. Assess. 2009, 156, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, P. Determination and Analysis of Environmental Endocrine Disrupting Chemicals in Trunk Stream and Tributaries of Wei-He River in Xi’an. Master’s Thesis, Chang’an University, Xi’an, China, 2012. [Google Scholar]
- Silva, C.P.; Otero, M.; Esteves, V. Processes for the elimination of estrogenic steroid hormones from water: A review. Environ. Pollut. 2012, 165, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; Carolis, J.D.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalin. 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Ohko, Y.; Iuchi, K.I.; Niwa, C.; Tatsuma, T.; Nakashima, T.; Iguchi, T.; Kubota, Y.; Fujishima, A. 17β-Estrodial Degradation by TiO2 Photocatalysis as Means of Reducing Estrogenic Activity. Environ. Sci. Technol. 2002, 36, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Song, H.C.; Shao, J.H.; He, Y.L.; Liu, B.; Zhong, X.Q. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Song, H.C.; Shao, J.H.; Wang, J.M.; Zhong, Z.Q. The removal of natural organic matter with LiCl-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Li, J.S.; Liang, Y.; Wang, H.; Sun, X.; Wang, L. Preparation and characterization of TiO2/PVDF composite hollow fiber membrane. Acta Polym. Sin. 2004, 37, 709–712. [Google Scholar]
- Coleman, H.M.; Routledge, E.J.; Sumpter, J.P.; Eqqins, B.R.; Byrne, J.A. Rapid loss of estrogenicity of steroid estrogens by UVA photolysis and photocatalysis over an immobilised titanium dioxide catalyst. Water Res. 2004, 38, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, X.L. Direct photolysis of estrogens in aqueous solutions. Sci. Total Environ. 2004, 320, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Ni, M.T.; Ni, Y.J. Competitive degradation and transformation trend of three steroid estrogens in UV system. China Environ. Sci. 2014, 34, 904–911. (In Chinese) [Google Scholar]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Coleman, H.M.; Abdullah, M.I.; Eggins, B.R.; Palmer, F.L. Photocatalytic degradation of 17β-estradiol, oestriol and 17α-ethynylestradiol in water monitored using fluorescence spectroscopy. Appl. Catal. B Environ. 2005, 55, 23–30. [Google Scholar] [CrossRef]
- Appavoo, I.A.; Hu, J.Y.; Huang, Y.; Li, S.F.Y.; Ong, S.L. Response surface modeling of Carbamazepine (CBZ) removal by Graphene-P25 nanocomposites/UVA process using central composite design. Water Res. 2014, 57, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Hu, J.Y. Degradation of acetaminophen by UVA/LED/TiO2 process. Sep. Purif. Technol. 2012, 91, 89–95. [Google Scholar] [CrossRef]
- Coleman, H.M.; Eggins, B.R.; Byrne, J.A.; Palmer, F.L.; King, E. Photocatalytic Degradation of 17-β-estradiol on Immobilized TiO2. Appl. Catal. B Environ. 2000, 24, L1–L5. [Google Scholar] [CrossRef]
- Caupos, E.; Mazellier, P.; Croue, J.P. Photodegradation of estrone enhanced by dissolved organic matter under simulated sunlight. Water Res. 2011, 45, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.L.; Heyne, B.; Blais, J.M.; Temussi, F.; Atkinson, S.K.; Pakdel, F.; Popesku, J.T.; Marlatt, V.L.; Scaiano, J.C.; Previtera, L.; et al. Lumiestrone is photochemically derived from estrone and may be released to the environment without detection. Front. Neuroendocr. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]




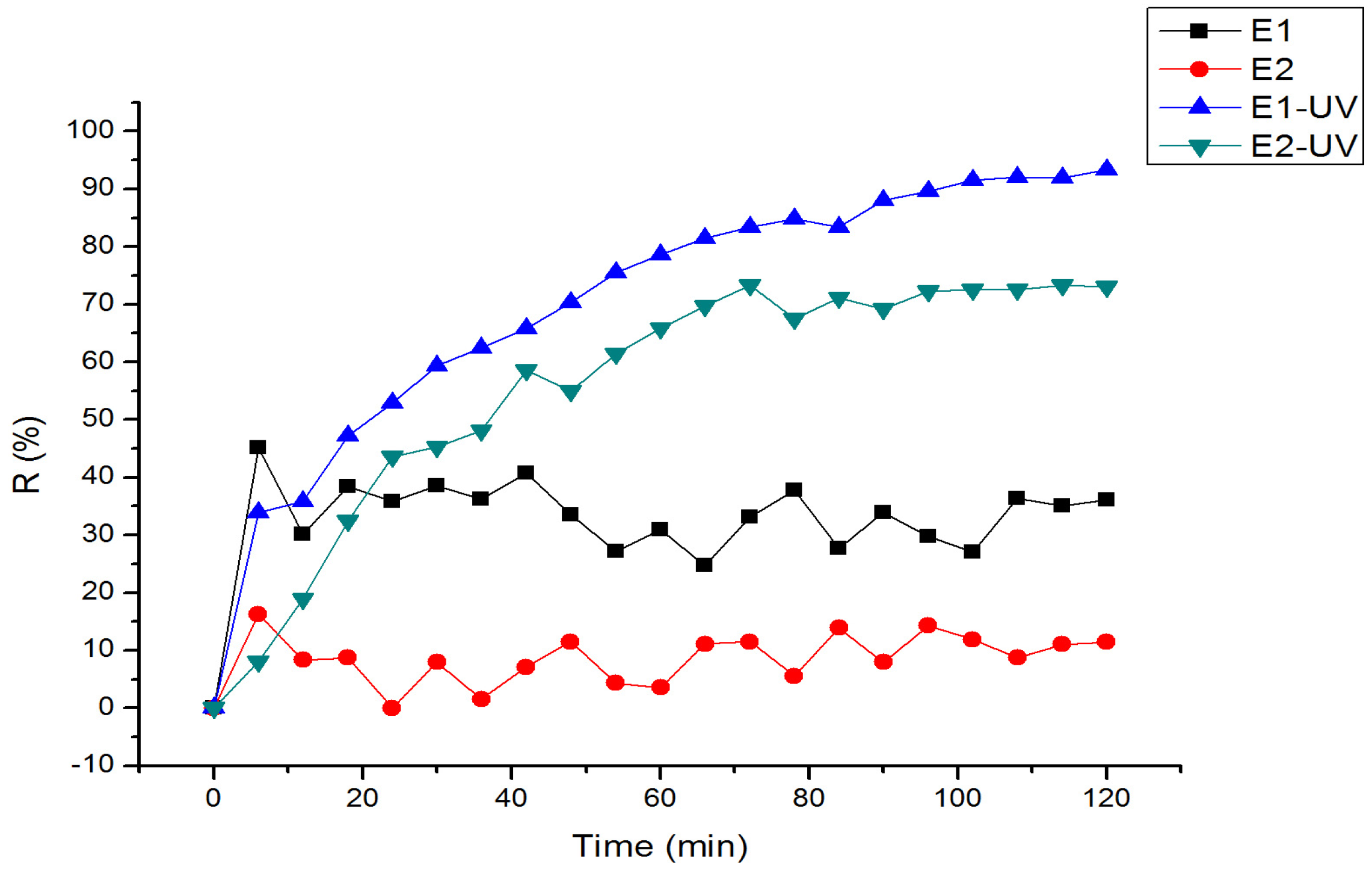



| Condition | Substance | First-Order Kinetics | Second-Order Kinetics | |||
|---|---|---|---|---|---|---|
| Equation | K/min−1 | R2 | Equation | R2 | ||
| PVDF-PVP-TiO2 membrane under UV photocatalysis | E1 | ln(C0/C) = 0.021t + 0.204 | 0.021 | 0.975 | 1/C = 0.337t + 3.258 | 0.954 |
| E2 | ln(C0/C) = 0.016t + 0.089 | 0.016 | 0.943 | 1/C = 0.162t + 4.568 | 0.940 | |
| UV photolysis | E1 | ln(C0/C) = 0.021t − 0.052 | 0.021 | 0.989 | 1/C = 0.360t − 0.055 | 0.943 |
| E2 | ln(C0/C) = 0.0035t − 0.019 | 0.0035 | 0.908 | 1/C = 0.020t + 4.850 | 0.906 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Qu, F.; Jia, R.; Sun, S.; Li, G.; Liang, H. Preliminary Study on the Removal of Steroidal Estrogens Using TiO2-Doped PVDF Ultrafiltration Membranes. Water 2016, 8, 134. https://doi.org/10.3390/w8040134
Wang M, Qu F, Jia R, Sun S, Li G, Liang H. Preliminary Study on the Removal of Steroidal Estrogens Using TiO2-Doped PVDF Ultrafiltration Membranes. Water. 2016; 8(4):134. https://doi.org/10.3390/w8040134
Chicago/Turabian StyleWang, Mingquan, Fangshu Qu, Ruibao Jia, Shaohua Sun, Guibai Li, and Heng Liang. 2016. "Preliminary Study on the Removal of Steroidal Estrogens Using TiO2-Doped PVDF Ultrafiltration Membranes" Water 8, no. 4: 134. https://doi.org/10.3390/w8040134






