Spatiotemporal Distributions of Sr and Ba along an Estuarine River with a Large Salinity Gradient to the Gulf of Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Water Sample Collection
2.4. Water Sample Analysis
2.5. Statistical Analyses
3. Results
3.1. Hydrology and Ambient Conditions
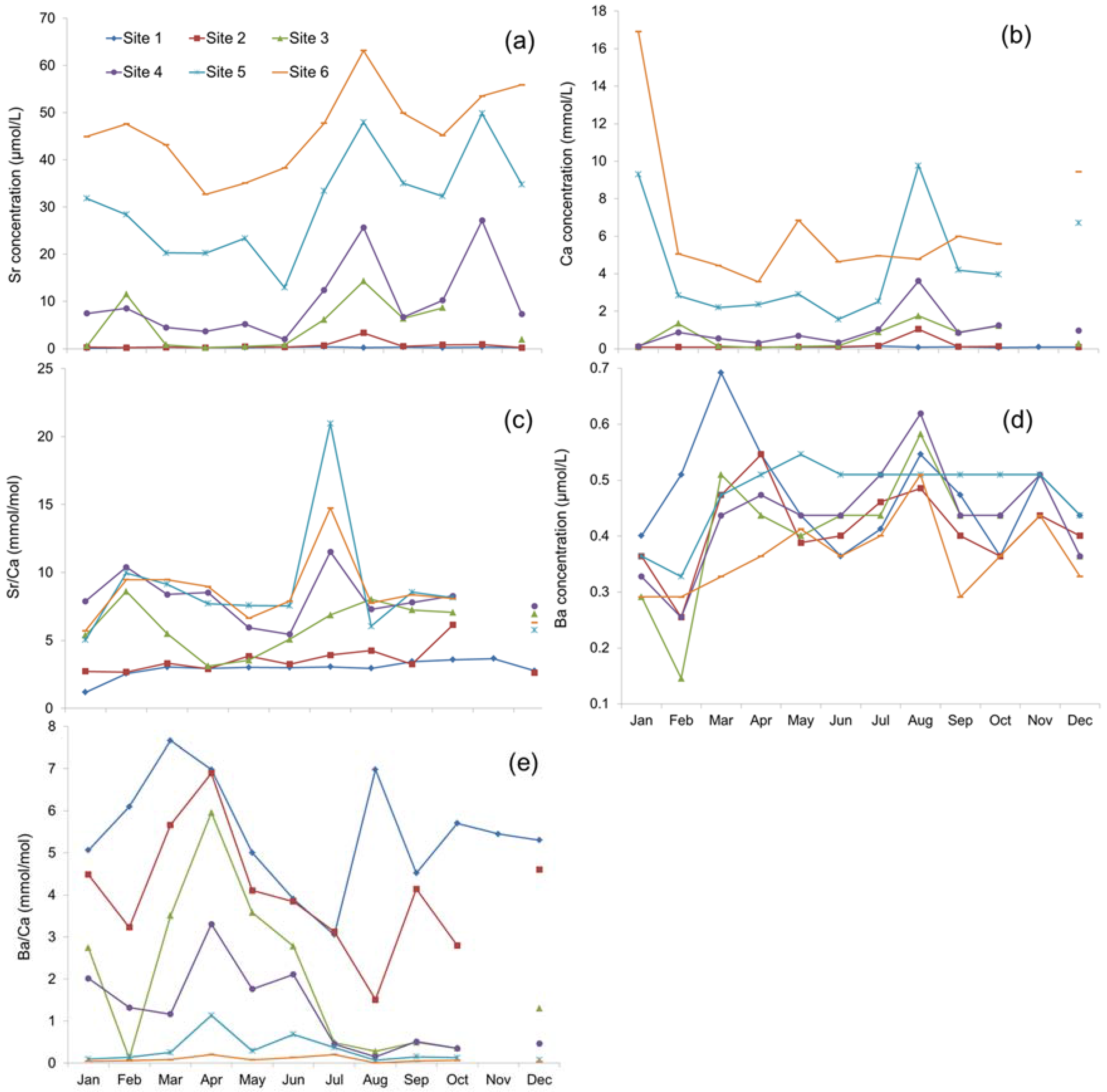
3.2. Strontium, Ca Concentrations and Sr/Ca Ratio
3.3. Barium Concentration and Ba/Ca Ratio
3.4. Relationships between Concentrations and Ratios of Sr, Ba, and Ca and Environmental Parameters
4. Discussion
4.1. Spatial Variation
4.2. Temporal Variation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birch, G.F.; Lean, J.; Gunns, T. Growth and decline of shoreline industry in Sydney estuary (Australia) and influence on adjacent estuarine sediments. Environ. Monit. Assess. 2015, 187, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dorado, S.; Booe, T.; Steichen, J.; McInnes, A.S.; Windham, R.; Shepard, A.; Lucchese, A.E.B.; Preischel, H.; Pinckney, J.L.; Davis, S.E.; et al. Towards an understanding of the interactions between freshwater inflows and phytoplankton communities in a subtropical estuary in the Gulf of Mexico. PLoS ONE 2015, 10, e0130931. [Google Scholar] [CrossRef] [PubMed]
- Scavia, D.; Rabalais, N.N.; Turner, R.E.; Justic, D.; Wiseman, W.J. Predicting the response of Gulf of Mexico hypoxia to variations in Mississippi River nitrogen load. Limnol. Oceanogr. 2003, 48, 951–956. [Google Scholar] [CrossRef]
- Ferreira, V.; Castagneyrol, B.; Koricheva, J.; Gulis, V.; Chauvet, E.; Graca, M.A.S. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol. Rev. 2015, 90, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.B.; Pagnocca, F.C.; Pinheiro, M.A.A.; Fontes, R.F.C.; de Oliveira, A.J.F.C. Heavy metals and TPH effects on microbial abundance and diversity in two estuarine areas of the southern-central coast of São Paulo State, Brazil. Mar. Pollut. Bull. 2015, 96, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Geselbracht, L.L.; Freeman, K.; Birch, A.P.; Brenner, J.; Gordon, D.R. Modeled sea level rise impacts on coastal ecosystems at six major estuaries on Florida’s gulf coast: Implications for adaptation planning. PLoS ONE 2015, 10, e0132079. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Kang, J.C.; Yan, G.D.; Han, G.Q.; Han, Q.C. Spatio-Temporal Variability of Sea Level in the East China Sea. J. Coast. Res. 2015, 73, 40–47. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis. Available online: https://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4_wg1_full_report.pdf (accessed on 7 June 2016).
- Church, J.A.; White, N.J. Sea level rise from the late 19th to the early 21st century. Surv. Geophys. 2011, 32, 585–602. [Google Scholar] [CrossRef]
- Munk, W. Twentieth century sea level: An enigma. Proc. Natl. Acad. Sci. USA 2002, 99, 6550–6555. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Douglas, B.C. Mass and volume contributions to twentieth-century global sea level rise. Nature 2004, 428, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. Available online: https://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WGIAR5_SPM_brochure_en.pdf (accessed on 7 June 2016).
- Turner, A. Trace-metal partitioning in estuaries: Importance of salinity and particle concentration. Mar. Chem. 1996, 54, 27–39. [Google Scholar] [CrossRef]
- Wen, L.S.; Santschi, P.; Gill, G.; Paternostro, C. Estuarine trace metal distributions in Galveston Bay: Importance of colloidal forms in the speciation of the dissolved phase. Mar. Chem. 1999, 63, 185–212. [Google Scholar] [CrossRef]
- Sanudo-Wilhelmy, S.A.; Riviera-Duarte, I.; Flegal, A.R. Distribution of colloidal trace metals in the San Francisco Bay Estuary. Geochim. Cosmochim. Acta 1996, 60, 4933–4944. [Google Scholar] [CrossRef]
- Edmond, J.M.; Spivack, A.; Grant, B.C.; Hu, M.H.; Chen, Z.; Chen, S.; Zeng, X. Chemical dynamics of the Changjiang Estuary. Cont. Shelf Res. 1985, 4, 17–36. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, E.R.; Zhang, J. Modeling on adsorption-desorption of trace metals to suspended particle matter in the Changjiang Estuary. Environ. Geol. 2008, 53, 1751–1766. [Google Scholar] [CrossRef]
- Windom, H.L.; Smith, R.; Rawlinson, C. Trace metal transport in a tropical estuary. Mar. Chem. 1989, 24, 293–305. [Google Scholar] [CrossRef]
- Zwolsman, J.J.G.; van Eck, G.T.M. Geochemistry of major elements and trace metals in suspended matter of the Scheldt estuary, southwest Netherlands. Mar. Chem. 1999, 66, 91–111. [Google Scholar] [CrossRef]
- Zhou, J.L.; Liu, Y.P.; Abrahams, P.W. Trace metal behaviour in the Conwy Estuary, North Wales. Chemosphere 2003, 51, 429–440. [Google Scholar] [CrossRef]
- Spangenberg, J.V.; Cherr, G.N. Developmental effects of barium exposure in a marine bivalve (Mytilus californianus). Environ. Toxicol. Chem. 1996, 15, 1769–1774. [Google Scholar] [CrossRef]
- Lira, V.F.; Santos, G.A.P.; Derycke, S.; Larrazabal, M.E.L.; Fonseca-Genevois, V.G.; Moens, T. Effects of barium and cadmium on the population development of the marine nematode Rhabditis (Pellioditis) marina. Mar. Environ. Res. 2011, 72, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Polonini, H.C.; Brandão, H.M.; Raposo, N.R.; Mouton, L.; Yéprémian, C.; Couté, A.; Brayner, R. Ecotoxicological studies of micro- and nanosized barium titanate on aquatic photosynthetic microorganisms. Aquat. Toxicol. 2014, 154, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.; Siebe, C.; Herre, A.; Roth, B.; Cram, S.; Stahr, K. Contribution of oil industry activities to environmental loads of heavy metals in the Tabasco Lowlands, Mexico. Water Air Soil Pollut. 2009, 197, 35–47. [Google Scholar] [CrossRef]
- Melton, H.R.; Smith, J.P.; Martin, C.R.; Nedwed, T.J.; Mairs, H.L.; Raught, D.L. Offshore Discharge of Drilling Fluids and Cuttings—A Scientific Perspective on Public Policy. Available online: http://www.anp.gov.br/brasil-rounds/round8/round8/guias_r8/perfuracao_r8/Bibliografia/IBP44900.PDF (accessed on 7 June 2016).
- International Association of Oil & Gas Producers (OGP). Environmental Aspects of the Use and Disposal of Non-Aqueous Drilling Fluids Associated with Offshore Oil & Gas Operations. Available online: http://www.ogp.org.uk/pubs/342.pdf (accessed on 7 June 2016).
- Elsdon, T.S.; Gillanders, B.M. Alternative life-history patterns of estuarine fish: Barium in otoliths elucidates freshwater residency. Can. J. Fish. Aquat. Sci. 2005, 62, 1145–1152. [Google Scholar] [CrossRef]
- Nielson, A.E.; Christoffersen, J. The mechanisms of crystal growth and dissolution. In Biological Mineralization and Demineralization; Nancollas, G.H., Ed.; Springer-Verlag: New York, NY, USA, 1982; pp. 37–77. [Google Scholar]
- Elsdon, T.S.; Gillanders, B.M. Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry. Rev. Fish Biol. Fish. 2003, 13, 219–235. [Google Scholar] [CrossRef]
- Bath, G.E.; Thorrold, S.R.; Jones, C.M.; Campana, S.E.; McLaren, J.W.; Lam, J.W.H. Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 2000, 64, 1705–1714. [Google Scholar] [CrossRef]
- Wells, B.K.; Bath, G.E.; Thorrold, S.R.; Jones, C.M. Incorporation of strontium, cadmium, and barium in juvenile spot (Leiostomus xanthurus) scales reflects water chemistry. Can. J. Fish. Aquat. Sci. 2000, 57, 2122–2129. [Google Scholar] [CrossRef]
- Britsch, L.D.; Dunbar, J.B. Land loss rates: Louisiana coastal plain. J. Coast. Res. 1993, 9, 324–338. [Google Scholar]
- Törnqvist, T.E.; Wallace, D.J.; Storms, J.E.A.; Wallinga, J.; Van Dam, R.L.; Blaauw, M.; Derksen, M.S.; Klerks, C.J.W.; Meijneken, C.; Snijders, E.M.A. Mississippi Delta subsidence primarily caused by compaction of Holocene strata. Nat. Geosci. 2008, 1, 173–176. [Google Scholar] [CrossRef]
- Morton, R.A.; Bernier, J.C. Recent subsidence-rate reduction in the Mississippi Delta and their geological implications. J. Coast. Res. 2010, 26, 555–561. [Google Scholar] [CrossRef]
- Dixon, T.H.; Amelung, F.; Ferretti, A.; Novali, F.; Rocca, F.; Dokka, R.; Sella, G.; Kim, S.W.; Wdowinski, S.; Whitman, D. Subsidence and flooding in New Orleans. Nature 2006, 441, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Ivins, E.R.; Dokka, R.K.; Blom, R.G. Post-glacial sediment load and subsidence in coastal Louisiana. Geophys. Res. Lett. 2007, 34, L16303. [Google Scholar] [CrossRef]
- Louisiana Department of Natural Resources. Prepared for the Louisiana Coastal Wetlands Conservation and Restoration Task Force, Hydrologic Investigation of the Lousiana Chenier Plain. Available online: http://www.lacoast.gov/reports/static/HILCP_1.pdf (accessed on 7 June 2016).
- Nichol, S.L.; Boyd, R.; Penland, S. Hydrology of a wave-dominated estuary: Lake Calcasieu, southwest Louisiana. GCAGS Trans. 1992, 42, 835–844. [Google Scholar]
- Louisiana Department of Wildlife and Fisheries (LDWF). Part VI-A: Waterbody Management Plan Series. Available online: http://184.106.97.89/sites/default/files/calcasieu_river_mp-a_12-13-12.pdf (accessed on 7 June 2016).
- United States Army Corps of Engineers (USACE). The Calcasieu Saltwater Barrier. Available online: http://www.mvn.usace.army.mil/Portals/56/docs/PAO/Brochures/CalcasieuSWB.pdf (accessed on 7 June 2016).
- Xu, Y.F.; Marcantonio, F. Strontium isotope variations in the lower Mississippi River and its estuarine mixing zone. Mar. Chem. 2007, 105, 118–128. [Google Scholar] [CrossRef]
- Joung, D.J.; Shiller, A.M. Dissolved barium behavior in Louisiana Shelf waters affected by the Mississippi/Atchafalaya River mixing zone. Geochim. Cosmochim. Acta 2014, 141, 303–313. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Web Soil Survey. 2014. Available online: http://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx (accessed on 7 June 2016). [Google Scholar]
- United States Department of Agriculture (USDA). Soil Survey of Calcasieu Parish, Louisiana, 1988. Available online: http://www.nrcs.usda.gov/Internet/FSE_MANUSCRIPTS/louisiana/LA019/0/calcasieu.pdf (accessed on 7 June 2016).
- United States Department of Agriculture (USDA). Soil Survey of Cameron Parish, Louisiana, 1995. Available online: http://www.nrcs.usda.gov/Internet/FSE_MANUSCRIPTS/louisiana/LA023/0/Cameron.pdf (accessed on 7 June 2016).
- He, S.; Xu, Y.J. Three decadal inputs of total organic carbon from four major coastal river basins to the summer hypoxic zone of the Northern Gulf of Mexico. Mar. Pollut. Bull. 2015, 90, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Viers, J.; Dupré, B. Trace elements in river waters. In Surface and Ground Water, Weathering, and Soils: Treatise on Geochemistry; Drever, J.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 5, pp. 225–272. [Google Scholar]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.Department of the Interior, US Geological Survey: Alexandria, VA, USA, 1985.
- Hamer, P.; Henderson, A.; Hutchison, M.; Kemp, J.; Green, C.; Feutry, P. Atypical correlation of otolith strontium:calcium and barium:calcium across a marine-freshwater life history transition of a diadromous fish. Mar. Freshw. Res. 2015, 66, 411–419. [Google Scholar] [CrossRef]
- Brown, R.J.; Severin, K.P. Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Can. J. Fish. Aquat. Sci. 2009, 66, 1790–1808. [Google Scholar]
- Walther, B.D.; Nims, M.K. Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: I. freshwater endmembers and mixing Curves. Estuar. Coasts 2015, 38, 754–768. [Google Scholar] [CrossRef]
- Moffett, D.; Smith, C.; Stevens, Y.; Ingerman, L.; Swarts, S.; Chappell, L. Toxicological Profile for Barium and Barium Compounds. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp24.pdf (accessed on 7 June 2016).
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ. Geochem. Health 2014, 36, 797–814. [Google Scholar] [CrossRef] [PubMed]
- US Geological Survey (USGS). Mineral Commodity Summary. Available online: http://minerals.usgs.gov/minerals/pubs/mcs/ (accessed on 7 June 2016).
- Chakrabarti, R.; Basu, A.R.; Santo, A.P.; Tedesco, D.; Vaselli, O. Isotopic and geochemical evidence for a heterogeneous mantle plume origin of the Virunga volcanics, Western rift, East African Rift system. Chem. Geol. 2009, 259, 273–289. [Google Scholar] [CrossRef]
- Cuoco, E.; Tedesco, D.; Poreda, R.J.; Williams, J.C.; De Francesco, S.; Balagizi, C.; Darrah, T.H. Impact of volcanic plume emissions on rain water chemistry during the January 2010 Nyamuragira eruptive event: Implications for essential potable water resources. J. Hazard. Mater. 2013, 244, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Moore, W.S.; Kloepfer, J.; Sochaski, M.A. The flux of barium to the coastal waters of the southeastern USA: The importance of submarine groundwater discharge. Geochim. Cosmochim. Acta 1998, 62, 3047–3054. [Google Scholar] [CrossRef]
- Stecher, H.A., III; Kogut, M.B. Rapid barium removal in the Delaware estuary. Geochim. Cosmochim. Acta 1999, 63, 1003–1012. [Google Scholar] [CrossRef]
- Nozaki, Y.; Yamamoto, Y.; Manaka, T.; Amakawa, H.; Snidvongs, A. Dissolved barium and radium isotopes in the Chao Phraya River estuarine mixing zone in Thailand. Cont. Shelf Res. 2001, 21, 1435–1448. [Google Scholar] [CrossRef]
- Secor, D.H.; Rooker, J.R. Is otolith strontium a useful scalar of lifecycles in estuarine fishes? Fish. Res. 2000, 46, 359–371. [Google Scholar] [CrossRef]
- Milton, D.A.; Chenery, S.R. Sources and uptake of trace metals in otoliths of juvenile barramundi Lates calcarifer. J. Exp. Mar. Biol. Ecol. 2001, 264, 47–65. [Google Scholar] [CrossRef]
- Martin, G.B.; Thorrold, S.R. Temperature and salinity effects on magnesium, manganese and barium incorporation in otoliths of larval and early juvenile spot Leiostomus zanthurus. Mar. Ecol. Prog. Ser. 2005, 293, 223–232. [Google Scholar] [CrossRef]
- Shiller, A.M. Dissolved trace elements in the Mississippi River: Seasonal, interannual, and decadal variability. Geochim. Cosmochim. Acta 1997, 61, 4321–4330. [Google Scholar] [CrossRef]
- Andersson, P.S.; Wasserburg, G.J.; Ingri, J. The sources and transport of Sr and Nd isotopes in the Baltic Sea. Earth Planet. Sci Lett. 1992, 113, 459–472. [Google Scholar] [CrossRef]
- Surge, D.M.; Lohmann, K.C. Temporal and spatial differences in salinity and water chemistry in SW Florida estuaries: Effects of human-impacted watersheds. Estuaries 2002, 25, 393–408. [Google Scholar] [CrossRef]
- Wang, R.M.; You, C.F.; Chu, H.Y.; Hung, J.J. Seasonal variability of dissolved major and trace elements in the Gaoping (Kaoping) River Estuary, Southwestern Taiwan. J. Mar. Syst. 2009, 76, 444–456. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Health Advisory—Barium; U.S. Environmental Protection Agency: Washington, DC, USA, 1985.
- Jones, M.T.; Gislason, S.R.; Burton, K.W.; Pearce, C.R.; Mavromatis, V.; von Strandmann, P.A.E.P.; Oelkers, E.H. Quantifying the impact of riverine particulate dissolution in seawater on ocean chemistry. Earth Planet. Sci. Lett. 2014, 395, 91–100. [Google Scholar] [CrossRef]
- Odum, H.T. The stability of the world strontium cycle. Science 1951, 114, 407–411. [Google Scholar] [CrossRef] [PubMed]

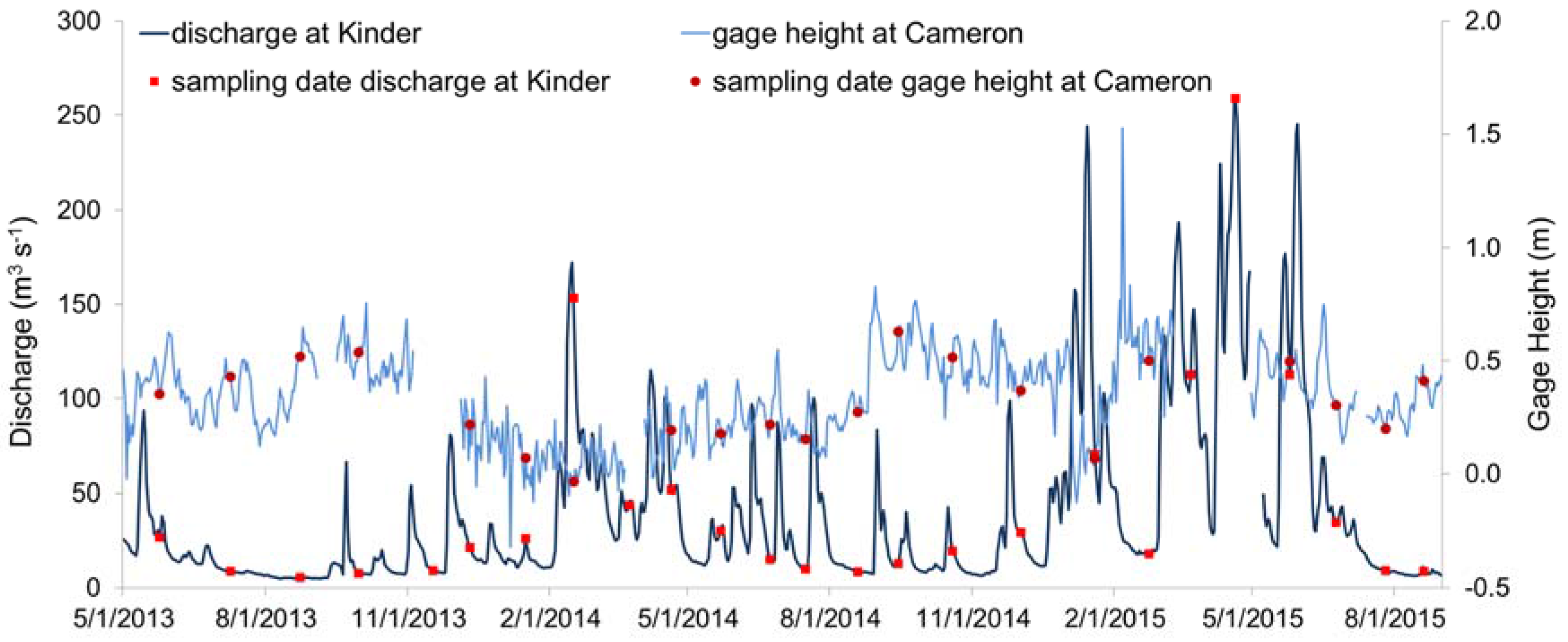



| Site ID | Latitude | Longitude | River Distance from Gulf (km) |
|---|---|---|---|
| Site 1 | 30.297 | −93.118 | 87.9 |
| Site 2 | 30.299 | −93.188 | 73.5 |
| Site 3 | 30.253 | −93.245 | 62.6 |
| Site 4 | 30.219 | −93.250 | 58.1 |
| Site 5 | 30.006 | −93.334 | 27.0 |
| Site 6 | 29.805 | −93.349 | 4.5 |
| Site ID | n | Salinity (ppt) | pH | Temperature (°C) | DO (mg/L) | TSS (mg/L) |
|---|---|---|---|---|---|---|
| Site 1 | 25 | 0.07 ± 0.18 | 6.30 ± 0.96 | 21.61 ± 7.09 | 5.40 ± 2.52 | 16.36 ± 7.66 |
| Site 2 | 25 | 0.40 ± 1.24 | 6.08 ± 1.06 | 21.90 ± 7.45 | 4.78 ± 2.64 | 14.28 ± 13.71 |
| Site 3 | 13 | 3.05 ± 5.29 | 6.45 ± 0.64 | 23.01 ± 6.97 | 4.64 ± 2.91 | 16.48 ± 18.43 |
| Site 4 | 25 | 5.89 ± 5.67 | 6.59 ± 0.92 | 22.17 ± 7.53 | 5.20 ± 2.69 | 12.37 ± 7.97 |
| Site 5 | 24 | 14.15 ± 6.67 | 7.51 ± 0.57 | 22.55 ± 7.67 | 6.75 ± 2.70 | 28.53 ± 23.63 |
| Site 6 | 25 | 19.91 ± 5.73 | 8.18 ± 0.64 | 22.89 ± 7.31 | 7.77 ± 2.56 | 54.79 ± 33.78 |
| Effects | Salinity | pH | Temperature | DO | TSS |
|---|---|---|---|---|---|
| Sr | 0.98 | 0.67 | 0.05 | 0.29 | 0.42 |
| Ca | 0.83 | 0.53 | 0.02 | 0.25 | 0.47 |
| Sr/Ca | 0.58 | 0.48 | 0.03 | 0.16 | 0.23 |
| Ba | −0.05 | 0.02 | 0.33 | −0.42 | −0.17 |
| Ba/Ca | −0.69 | −0.63 | −0.12 | −0.17 | −0.24 |
| Salinity | 1.00 | 0.70 | 0.07 | 0.27 | 0.37 |
| pH | - | 1.00 | 0.34 | 0.04 | 0.26 |
| Temperature | - | - | 1.00 | −0.79 | −0.02 |
| DO | - | - | - | 1.00 | 0.15 |
| TSS | - | - | - | - | 1.00 |
| Site ID | Sr (µmol/L) | Ca (mmol/L) | Sr/Ca (mmol/mol) | Ba (µmol/L) | Ba/Ca (mmol/mol) |
|---|---|---|---|---|---|
| Site 1 | 0.35 ± 0.31 | 0.13 ± 0.17 | 2.89 ± 0.80 | 0.46 ± 0.11 | 4.82 ± 1.75 |
| Site 2 | 0.77 ± 1.42 | 0.23 ± 0.53 | 3.49 ± 1.51 | 0.42 ± 0.09 | 3.94 ± 1.74 |
| Site 3 | 5.13 ± 5.72 | 0.68 ± 0.67 | 6.06 ± 1.93 | 0.41 ± 0.11 | 2.10 ± 1.93 |
| Site 4 | 9.91 ± 9.58 | 1.10 ± 1.24 | 8.11 ± 3.38 | 0.43 ± 0.11 | 1.22 ± 1.41 |
| Site 5 | 30.85 ± 15.24 | 4.66 ± 4.56 | 8.67 ± 6.01 | 0.47 ± 0.09 | 0.30 ± 0.49 |
| Site 6 | 46.21 ± 14.94 | 8.19 ± 9.89 | 8.41 ± 5.07 | 0.36 ± 0.07 | 0.09 ± 0.10 |
| Effects | Value | Num DF | Den DF | F | p |
|---|---|---|---|---|---|
| Site | 0.02 | 25 | 153.81 | 10.76 | <0.0001 |
| Month | 0.08 | 55 | 193.37 | 2.49 | <0.0001 |
| Site × Month | 0.05 | 240 | 209.57 | 0.73 | 0.9910 |
| Effects | Effects | Num DF | Den DF | F | p |
|---|---|---|---|---|---|
| Sr | Site | 5 | 54 | 76.40 | <0.0001 |
| Month | 11 | 54 | 3.14 | 0.0024 | |
| Site × Month | 54 | 54 | 0.60 | 0.9701 | |
| Ca | Site | 5 | 51 | 22.06 | <0.0001 |
| Month | 11 | 51 | 1.81 | 0.0767 | |
| Site × Month | 50 | 51 | 0.65 | 0.9374 | |
| Sr/Ca | Site | 5 | 51 | 38.42 | <0.0001 |
| Month | 11 | 51 | 1.91 | 0.0594 | |
| Site × Month | 50 | 51 | 0.79 | 0.8012 | |
| Ba | Site | 5 | 42 | 4.83 | 0.0014 |
| Month | 11 | 42 | 4.61 | 0.0001 | |
| Site × Month | 52 | 42 | 1.00 | 0.5055 | |
| Ba/Ca | Site | 5 | 40 | 70.98 | <0.0001 |
| Month | 11 | 40 | 7.58 | <0.0001 | |
| Site × Month | 47 | 40 | 1.34 | 0.1710 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Xu, Y.J. Spatiotemporal Distributions of Sr and Ba along an Estuarine River with a Large Salinity Gradient to the Gulf of Mexico. Water 2016, 8, 323. https://doi.org/10.3390/w8080323
He S, Xu YJ. Spatiotemporal Distributions of Sr and Ba along an Estuarine River with a Large Salinity Gradient to the Gulf of Mexico. Water. 2016; 8(8):323. https://doi.org/10.3390/w8080323
Chicago/Turabian StyleHe, Songjie, and Y. Jun Xu. 2016. "Spatiotemporal Distributions of Sr and Ba along an Estuarine River with a Large Salinity Gradient to the Gulf of Mexico" Water 8, no. 8: 323. https://doi.org/10.3390/w8080323







