Taxonomy of Means and Ends in Aquaculture Production—Part 2: The Technical Solutions of Controlling Solids, Dissolved Gasses and pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scope
2.2. Literature Review
2.3. Synthesis
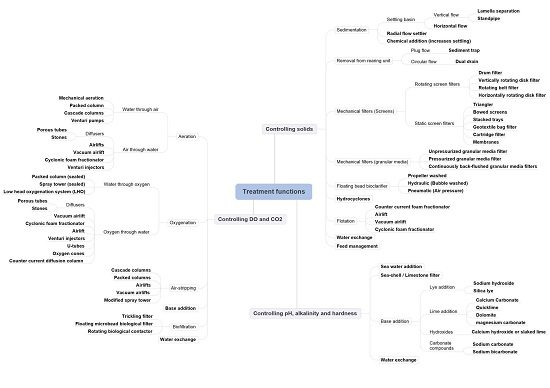
3. Resolving Treatment Functions for Controlling Solids, Controlling Dissolved Gasses and Controlling pH, Alkanity and Hardness
3.1. General Solutions: Feed Management and Water Exchange
3.1.1. Feed Management
3.1.2. Water Exchange
3.2. Controlling Solids
3.2.1. Removal of Solids from the Rearing Unit
3.2.2. Sedimentation Methods
Settling Basin
3.2.3. Mechanical Filters: Screen Filters
Rotating Microscreen Filters
Static Screen Flters
3.2.4. Mechanical Filters: Granular Media
3.2.5. Floating Bead Bioclarifier
3.2.6. Hydrocyclones
3.2.7. Flotation
3.3. Controlling pH, Alkalinity and Hardness
pH and Alkalinity
Hardness
3.3.1. Seawater Addition
3.3.2. A Shell-Sand or Limestone Filter
3.3.3. Base Addition
Hydroxides
Carbonate Compounds
3.4. Controlling DO and CO2
3.4.1. Aeration
Water through Air
Air through Water
3.4.2. Oxygenation
Water through Oxygen
Oxygen through Water
3.4.3. Air Stripping
3.4.4. Base Addition
3.4.5. Biofiltration (DO and CO2 Control)
4. Discussion
Author Contributions
Conflicts of Interest
References
- Martins, C.; Eding, E.; Verdegem, M.; Heinsbroek, L.; Schneider, O.; Blancheton, J.; D’Orbcastel, E.R.; Verreth, J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Björnsdóttir, R.; Oddsson, G.V.; Thorarinsdottir, R.; Unnthorsson, R. Taxonomy of means and ends in aquaculture production—Part 1: The functions. Water 2016, 8, 319. [Google Scholar] [CrossRef]
- Vilbergsson, B.; Oddsson, G.V.; Unnthorsson, R. Taxonomy of means and ends in aquaculture production—Part 3: The technical solutions of controlling N compounds, organic matter, P compounds, metals, temperature and preventing disease. Water 2016, in press. [Google Scholar]
- Vilbergsson, B.; Oddsson, G.V.; Unnthorsson, R. Taxonomy of means and ends in aquaculture production—Part 4: The mapping of technical solutions onto multiple treatment function. Water 2016, in press. [Google Scholar]
- Losordo, T.M.; Masser, M.P.; Rakocy, J. Recirculating Aquaculture Tank Production Systems—A Review of Component Options; Report SRAC Publication No. 453; Southern Regional Aquaculture Center (SRAC): Stoneville, MS, USA, 1999. [Google Scholar]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef]
- Cripps, S.J.; Bergheim, A. Solids management and removal for intensive land-based aquaculture production systems. Aquac. Eng. 2000, 22, 33–56. [Google Scholar] [CrossRef]
- Malone, R.F.; Pfeiffer, T.J. Rating fixed film nitrifying biofilters used in recirculating aquaculture systems. Aquac. Eng. 2006, 34, 389–402. [Google Scholar] [CrossRef]
- Helfrich, L.; Libey, G. Fish Farming in Recirculating Aquaculture Systems (RAS); Department of Fisheries and Wildlife, Virginia Tech: Blacksburg, VA, USA, 1991. [Google Scholar]
- Cho, C.Y.; Bureau, D.P. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac. Res. 2001, 32, 349–360. [Google Scholar] [CrossRef]
- Barrut, B.; Blancheton, J.P.; Callier, M.; Champagne, J.Y.; Grasmick, A. Foam fractionation efficiency of a vacuum airlift—Application to particulate matter removal in recirculating systems. Aquac. Eng. 2013, 54, 16–21. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Larsen, B.K.; Pedersen, P.B. Nitrogen waste from rainbow trout (Oncorhynchus mykiss) with particular focus on urea. Aquac. Eng. 2015, 65, 2–9. [Google Scholar] [CrossRef]
- Koko, G.; Sarker, P. Effects of alternating feeding regimes with varying dietary phosphorus levels on growth, mineralization, phosphorus retention and loading of large rainbow trout. Aquat. Living Resour. 2010, 23, 277–284. [Google Scholar] [CrossRef]
- Zakȩś, Z.; Demska-Zakȩś, K. The effect of feeding on oxygen consumption and ammonia excretion of juvenile tench Tinca tinca (L.) reared in a water recirculating system. Aquac. Int. 2006, 14, 127–140. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Fan, L.; Lu, H.; Liu, L.; Liu, Y. Measuring feeding activity of fish in RAS using computer vision. Aquac. Eng. 2014, 60, 20–27. [Google Scholar] [CrossRef]
- Couturier, M.; Trofimencoff, T.; Buil, J.U.; Conroy, J. Solids removal at a recirculating salmon-smolt farm. Aquac. Eng. 2009, 41, 71–77. [Google Scholar] [CrossRef]
- Lekang, O. Aquaculture Engineering; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Brazil, B.L. Performance and operation of a rotating biological contactor in a tilapia recirculating aquaculture system. Aquac. Eng. 2006, 34, 261–274. [Google Scholar] [CrossRef]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.; Ortiz, I. Kinetics of nitrogen compounds in a commercial marine Recirculating Aquaculture System. Aquac. Eng. 2012, 50, 20–27. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Brazil, B.; Summerfelt, S. Heavy metal and waste metabolite accumulation and their potential effect on rainbow trout performance in a replicated water reuse system operated at low or high system flushing rates. Aquac. Eng. 2009, 41, 136–145. [Google Scholar] [CrossRef]
- Liltved, H.; Cripps, S.J. Removal of particle-associated bacteria by prefiltration and ultraviolet irradiation. Aquac. Res. 1999, 30, 445–450. [Google Scholar] [CrossRef]
- Summerfelt, R.C.; Penne, C.R. Solids removal in a recirculating aquaculture system where the majority of flow bypasses the microscreen filter. Aquac. Eng. 2005, 33, 214–224. [Google Scholar] [CrossRef]
- Tidwell, J. Aquaculture Production Systems; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Losordo, T.M.; Hobbs, A.O.; DeLong, D.P. The design and operational characteristics of the CP&L/EPRI fish barn: A demonstration of recirculating aquaculture technology. Aquac. Eng. 2000, 22, 3–16. [Google Scholar]
- Davidson, J.; Summerfelt, S.T. Solids removal from a coldwater recirculating system—Comparison of a swirl separator and a radial-flow settler. Aquac. Eng. 2005, 33, 47–61. [Google Scholar] [CrossRef]
- Ebeling, J.; Welsh, C.; Rishel, K. Performance evaluation of an inclined belt filter using coagulation/flocculation aids for the removal of suspended solids and phosphorus from microscreen backwash effluent. Aquac. Eng. 2006, 35, 61–77. [Google Scholar] [CrossRef]
- Fischer, G.J.; Held, J.; Hartleb, C.; Malison, J. Evaluation of brook trout production in a coldwater recycle aquaculture system. Aquac. Eng. 2009, 41, 109–113. [Google Scholar] [CrossRef]
- Lekang, O.I.; Bomo, A.M.; Svendsen, I. Biological lamella sedimentation used for wastewater treatment. Aquac. Eng. 2001, 24, 115–127. [Google Scholar] [CrossRef]
- Summerfelt, S. An integrated approach to aquaculture waste management in flowing water systems. In Proceedings of the Second International Conference on Recirculating Aquaculture, Roanoke, VA, USA, 16–19 July 1998; pp. 87–97.
- Fernandes, P.M.; Pedersen, L.F.; Pedersen, P.B. Daily micro particle distribution of an experimental recirculating aquaculture system—A case study. Aquac. Eng. 2014, 60, 28–34. [Google Scholar] [CrossRef]
- Dolan, E.; Murphy, N.; O’Hehir, M. Factors influencing optimal micro-screen drum filter selection for recirculating aquaculture systems. Aquac. Eng. 2013, 56, 42–50. [Google Scholar] [CrossRef]
- Mäkinen, T.; Lindgren, S.; Eskelinen, P. Sieving as an effluent treatment method for aquaculture. Aquac. Eng. 1988, 7, 367–377. [Google Scholar] [CrossRef]
- Fernandes, P.; Pedersen, L.F.; Pedersen, P.B. Microscreen effects on water quality in replicated recirculating aquaculture systems. Aquac. Eng. 2015, 65, 17–26. [Google Scholar] [CrossRef]
- Sharrer, M.; Rishel, K.; Taylor, A.; Vinci, B.J.; Summerfelt, S.T. The cost and effectiveness of solids thickening technologies for treating backwash and recovering nutrients from intensive aquaculture systems. Bioresour. Technol. 2010, 101, 6630–6641. [Google Scholar] [CrossRef] [PubMed]
- Guerdat, T.C.; Losordo, T.M.; DeLong, D.P.; Jones, R.D. An evaluation of solid waste capture from recirculating aquaculture systems using a geotextile bag system with a flocculant-aid. Aquac. Eng. 2013, 54, 1–8. [Google Scholar] [CrossRef]
- Holan, A.; Wold, P.A.; Leiknes, T. Membrane performance and fouling behavior of membrane bioreactors installed in marine recirculating aquaculture systems. Aquac. Eng. 2014, 58, 45–51. [Google Scholar] [CrossRef]
- Holan, A.; Wold, P.A.; Leiknes, T. Intensive rearing of cod larvae (Gadus morhua) in recirculating aquaculture systems (RAS) implementing a membrane bioreactor (MBR) for enhanced colloidal particle and fine suspended solids removal. Aquac. Eng. 2014, 58, 52–58. [Google Scholar] [CrossRef]
- Ebeling, J.J.M. Engineering aspects of recirculating aquaculture systems. Mar. Technol. Soc. J. 2000, 34, 68–78. [Google Scholar] [CrossRef]
- Steicke, C.; Jegatheesan, V.; Zeng, C. Mechanical mode floating medium filters for recirculating systems in aquaculture for higher solids retention and lower freshwater usage. Bioresour. Technol. 2007, 98, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.F.; Beecher, L.E. Use of floating bead filters to recondition recirculating waters in warmwater aquaculture production systems. Aquac. Eng. 2000, 22, 57–73. [Google Scholar] [CrossRef]
- Malone, R.F.; Beecher, L.E.; DeLosReyes, A.A., Jr. Sizing and management of floating bead bioclarifiers. In Proceedings of the Second International Conference on Recirculating Aquaculture, Roanoke, VA, USA, 16–19 July 1998; pp. 16–19.
- Malone, R.F. Air Charged Backwashing Bioclarifier. U.S. Patent 5,770,080 A, 23 June 1998. [Google Scholar]
- Loyless, J.; Malone, R.F. Evaluation of air-lift pump capabilities for water delivery, aeration, and degasification for application to recirculating aquaculture systems. Aquac. Eng. 1998, 18, 117–133. [Google Scholar] [CrossRef]
- Barrut, B.; Blancheton, J.P.; Champagne, J.Y.; Grasmick, A. Water delivery capacity of a vacuum airlift—Application to water recycling in aquaculture systems. Aquac. Eng. 2012, 48, 31–39. [Google Scholar] [CrossRef]
- Brambilla, F.; Antonini, M.; Ceccuzzi, P.; Terova, G.; Saroglia, M. Foam fractionation efficiency in particulate matter and heterotrophic bacteria removal from a recirculating seabass (Dicentrarchus labrax) system. Aquac. Eng. 2008, 39, 37–42. [Google Scholar] [CrossRef]
- Loyless, J.; Malone, R. A sodium bicarbonate dosing methodology for pH management in freshwater-recirculating aquaculture systems. Progress. Fish Cult. 1997, 59, 198–205. [Google Scholar] [CrossRef]
- Boyd, C.; Tucker, C.; Viriyatum, R. Interpretation of pH, acidity, and alkalinity in aquaculture and fisheries. N. Am. J. Aquac. 2011, 73, 403–408. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture, 2nd ed.; Cayuga Aqua Ventures: Ithaca, NY, USA, 2010. [Google Scholar]
- Summerfelt, S.; Vinci, B.; Piedrahita, R. Oxygenation and carbon dioxide control in water reuse systems. Aquac. Eng. 2000, 22, 87–108. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S.; Somridhivej, B. Alkalinity and hardness: Critical but elusive concepts in aquaculture. J. World Aquac. Soc. 2016, 47, 6–41. [Google Scholar] [CrossRef]
- Stickney, R.R. Aquaculture: An Introductory Text; CAB International: Cambridge, MA, USA, 2005. [Google Scholar]
- Rosseland, B.O.; Skogheim, O.K. Neutralization of acidic brook-water using a shell-sand filter or sea-water: Effects on eggs, alevins and smolts of salmonids. Aquaculture 1986, 58, 99–110. [Google Scholar] [CrossRef]
- Teien, H.C.; Kroglund, F.; Atland, A.; Rosseland, B.O.; Salbu, B. Sodium silicate as alternative to liming-reduced aluminium toxicity for Atlantic salmon (Salmo salar L.) in unstable mixing zones. Sci. Total Environ. 2006, 358, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Furtado, P.S.; Gaona, C.A.P.; Poersch, L.H.; Wasielesky, W. Application of different doses of calcium hydroxide in the farming shrimp Litopenaeus vannamei with the biofloc technology (BFT). Aquac. Int. 2013, 22, 1009–1023. [Google Scholar] [CrossRef]
- Sharrer, M.J.; Rishel, K.; Summerfelt, S. Evaluation of geotextile filtration applying coagulant and flocculant amendments for aquaculture biosolids dewatering and phosphorus removal. Aquac. Eng. 2009, 40, 1–10. [Google Scholar] [CrossRef]
- Whangchai, N.; Migo, V.P.; Alfafara, C.G.; Young, H.K.; Nomura, N.; Matsumura, M. Strategies for alkalinity and pH control for ozonated shrimp pond water. Aquac. Eng. 2004, 30, 1–13. [Google Scholar] [CrossRef]
- Das, B.K.; Das, N. Impacts of quicklime (CaO) on the toxicity of copper (CuSO4, 5H2O) to fish and fish food organisms. Chemosphere 2005, 61, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kaviraj, A.; Dutta, T.K. Use of quick lime (CaO) as a means to reduce cadmium toxicity in common carp, Cyprinus carpio. J. Appl. Aquac. 2008, 10, 87–95. [Google Scholar] [CrossRef]
- Hargreaves, J.A.; Sheely, L.D.; To, F.S. A control system to simulate diel pH fluctuation in eutrophic aquaculture ponds. J. World Aquac. Soc. 2007, 31, 390–402. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Zühlke, A.; Kolarevic, J.; Reiten, B.K.M.; Selset, R.; Gutierrez, X.; Terjesen, B.F. Effects of alkalinity on ammonia removal, carbon dioxide stripping, and system pH in semi-commercial scale water recirculating aquaculture systems operated with moving bed bioreactors. Aquac. Eng. 2015, 65, 46–54. [Google Scholar] [CrossRef]
- Colt, J. Water quality requirements for reuse systems. Aquac. Eng. 2006, 34, 143–156. [Google Scholar] [CrossRef]
- Hackney, G.E.; Colt, J.E. The performance and design of packed column aeration systems for aquaculture. Aquac. Eng. 1982, 1, 275–295. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Davidson, J.; Waldrop, T. Evaluation of full-scale carbon dioxide stripping columns in a coldwater recirculating system. Aquac. Eng. 2003, 28, 155–169. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Davidson, J.W.; Waldrop, T.B.; Tsukuda, S.M.; Bebak-Williams, J. A partial-reuse system for coldwater aquaculture. Aquac. Eng. 2004, 31, 157–181. [Google Scholar] [CrossRef]
- Watten, B.J.; Sibrell, P.L.; Montgomery, G.A.; Tsukuda, S.M. Modification of pure oxygen absorption equipment for concurrent stripping of carbon dioxide. Aquac. Eng. 2004, 32, 183–208. [Google Scholar] [CrossRef]
- Vinci, B.J.; Watten, B.J.; Timmons, M.B. Gas-phase axial dispersion in a spray tower. Aquac. Eng. 1996, 15, 1–11. [Google Scholar] [CrossRef]
- Davenport, M.T.; Timmons, M.B.; Vinci, B.J.; Crum, M.K. Experimental evaluation of low head oxygenators. Aquac. Eng. 2001, 24, 245–256. [Google Scholar] [CrossRef]
- Wood, L.G.; Watten, B.J.; Haugh, C.; Libey, G.S.; Dillaha, T.A. Modeling gas transfer and biological respiration in a recirculating aquaculture system. Aquac. Eng. 1996, 15, 359–379. [Google Scholar] [CrossRef]
- Barrut, B.; Blancheton, J.P.; Champagne, J.Y.; Grasmick, A. Mass transfer efficiency of a vacuum airlift—Application to water recycling in aquaculture systems. Aquac. Eng. 2012, 46, 18–26. [Google Scholar] [CrossRef]
- Seginer, I.; Mozes, N. A note on oxygen supply in RAS: The effect of water temperature. Aquac. Eng. 2012, 50, 46–54. [Google Scholar] [CrossRef]
- Timmons, M.B.; Holder, J.L.; Ebeling, J.M. Application of microbead biological filters. Aquac. Eng. 2006, 34, 332–343. [Google Scholar] [CrossRef]













| Article | Description |
|---|---|
| Part 1—The Functions [3] | The transformational view on aquaculture is introduced and functions are divided into input, treatment and output functions. There are 5 input functions, 10 treatment functions and 4 output functions. Key parameters used to control are identified and a nearly exhaustive list of possible methods of technical solutions is provided. The results are presented as a map. |
| Part 2—Technical solutions for controlling solids, dissolved gasses and pH functions (the current article) | The map of aquaculture production is used to find all possible technical solutions for all the methods in 3 treatment functions: the controlling solids, dissolved gasses and pH functions. The result is a partial taxonomy of treatment functions through methods to technical solutions. |
| Part 3—Technical solutions for controlling N compounds, organic matter, P compounds, metals, temperature and disease prevention functions [4] | The map of aquaculture production is used to find all possible technical solutions for all the methods in the 6 treatment functions, the controlling N compounds, organic matter, P compounds, metals, temperature and disease prevention functions. A complete taxonomy of technical solutions is presented. |
| Part 4—The mapping of technical solutions onto multiple treatment functions [5] | The one-on-one relationship between a technical solution and a treatment function relaxed. The technical solutions from Parts 2 and 3 are analysed and all their effects on treatment functions are mapped out. Each relationship is put into one of three categories: intended, positive effect and negative effect. The result is a quality-function-deployment presentation of the interaction between solutions and functions. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilbergsson, B.; Oddsson, G.V.; Unnthorsson, R. Taxonomy of Means and Ends in Aquaculture Production—Part 2: The Technical Solutions of Controlling Solids, Dissolved Gasses and pH. Water 2016, 8, 387. https://doi.org/10.3390/w8090387
Vilbergsson B, Oddsson GV, Unnthorsson R. Taxonomy of Means and Ends in Aquaculture Production—Part 2: The Technical Solutions of Controlling Solids, Dissolved Gasses and pH. Water. 2016; 8(9):387. https://doi.org/10.3390/w8090387
Chicago/Turabian StyleVilbergsson, Bjorgvin, Gudmundur V. Oddsson, and Runar Unnthorsson. 2016. "Taxonomy of Means and Ends in Aquaculture Production—Part 2: The Technical Solutions of Controlling Solids, Dissolved Gasses and pH" Water 8, no. 9: 387. https://doi.org/10.3390/w8090387
APA StyleVilbergsson, B., Oddsson, G. V., & Unnthorsson, R. (2016). Taxonomy of Means and Ends in Aquaculture Production—Part 2: The Technical Solutions of Controlling Solids, Dissolved Gasses and pH. Water, 8(9), 387. https://doi.org/10.3390/w8090387