Prediction of Iron Release during Riverbank Filtration
Abstract
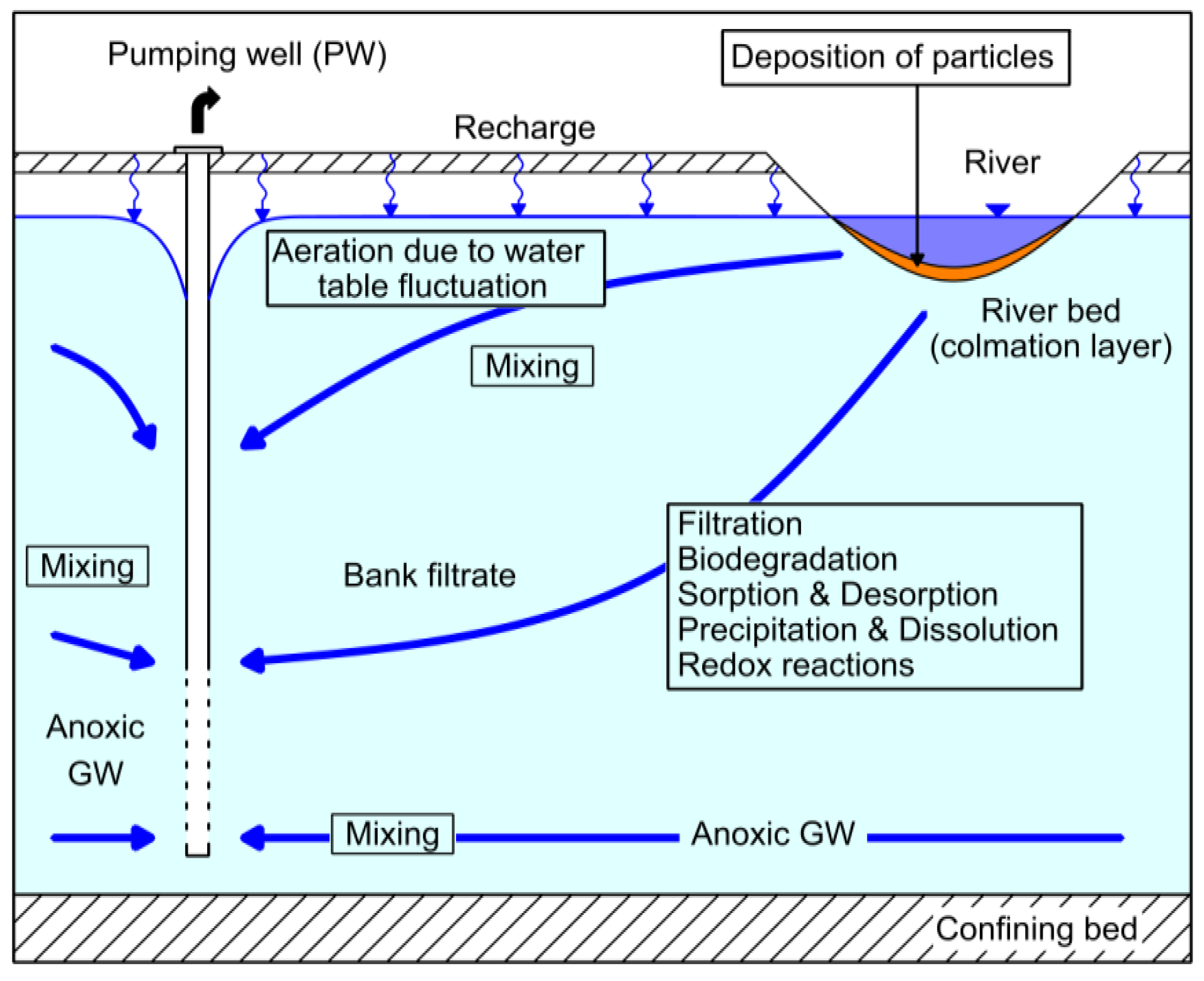
:1. Introduction
- (1)
- the highly biologically active colmation (clogging) zone in the riverbed, in which intensive filtration, degradation, and sorption processes take place over short flow paths and residence times; and
- (2)
- the subsequent subsurface aquifer passage with more moderate degradation and sorption rates, and also the increasing effects of mixing processes.
2. Materials and Methods
2.1. Water Quality Investigation at RBF Site at Torgau
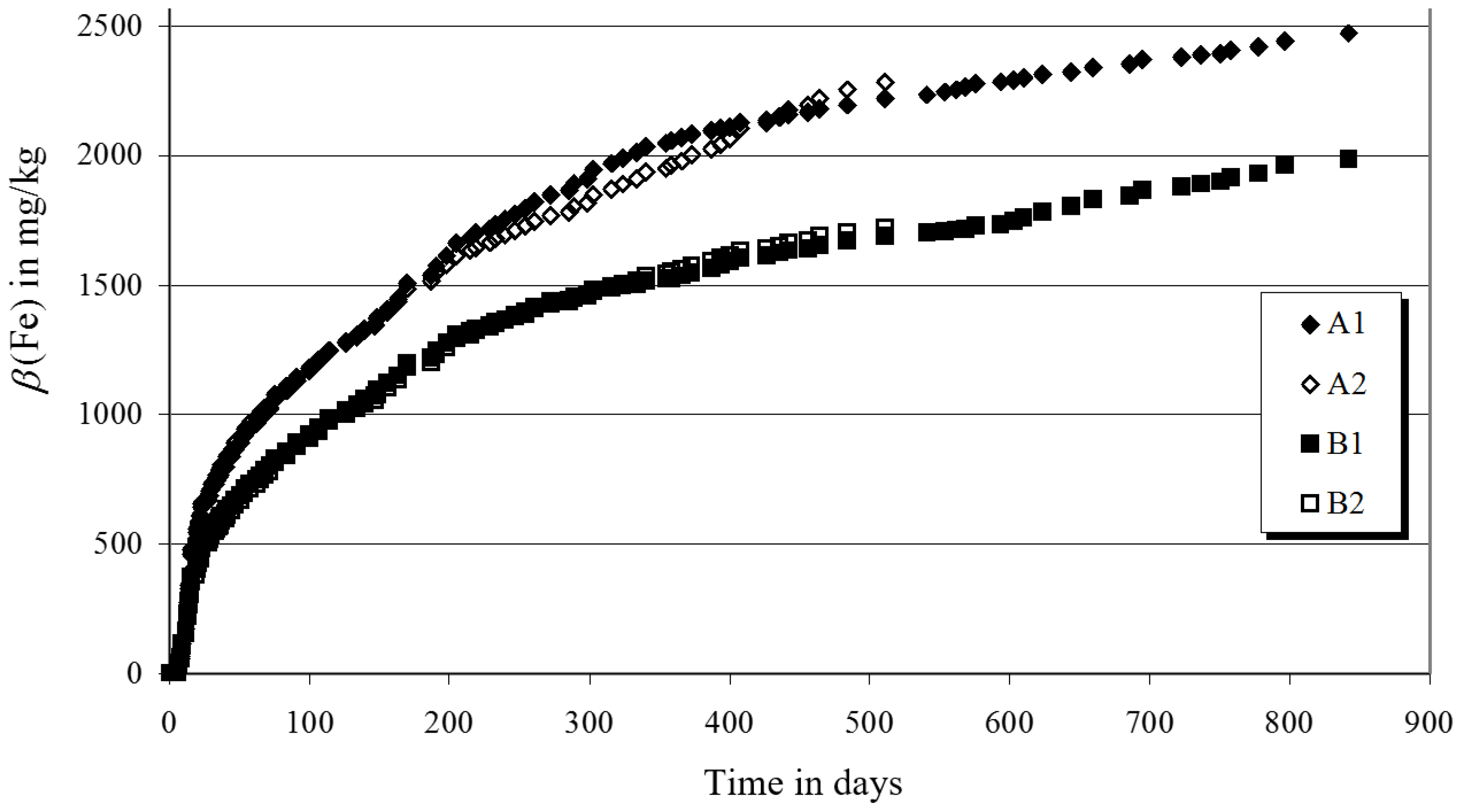
2.2. Laboratory Experiments
3. Results
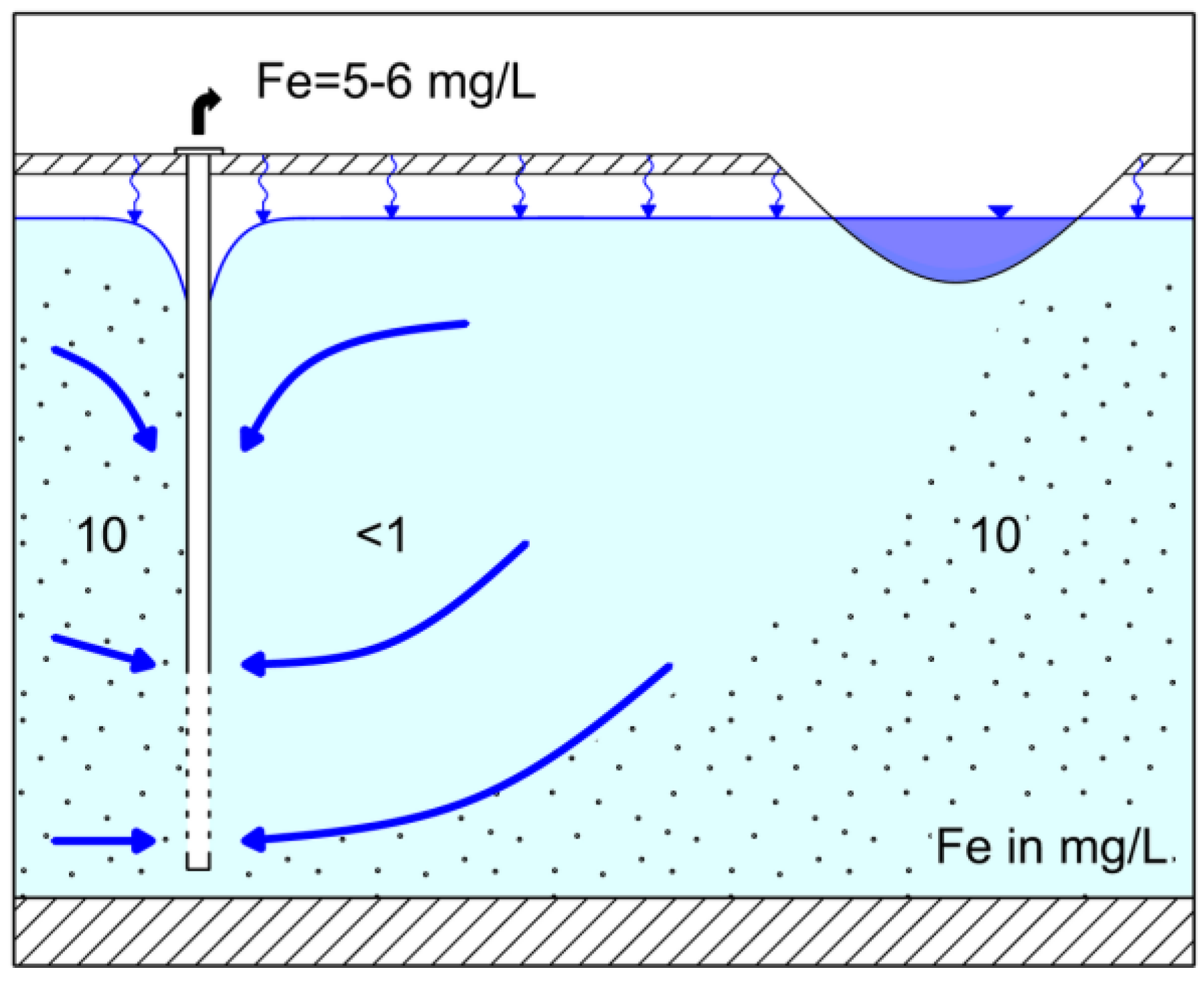
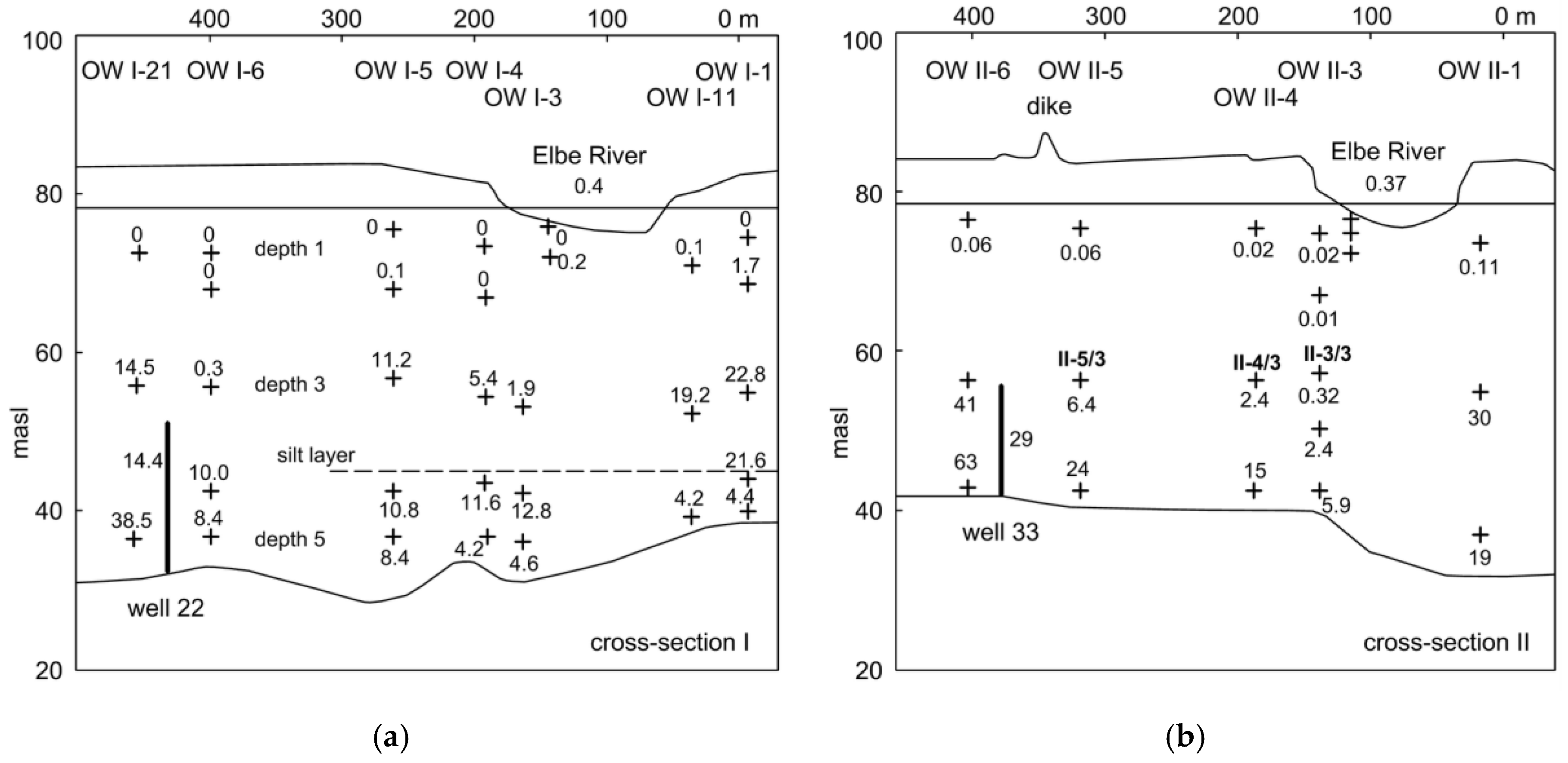
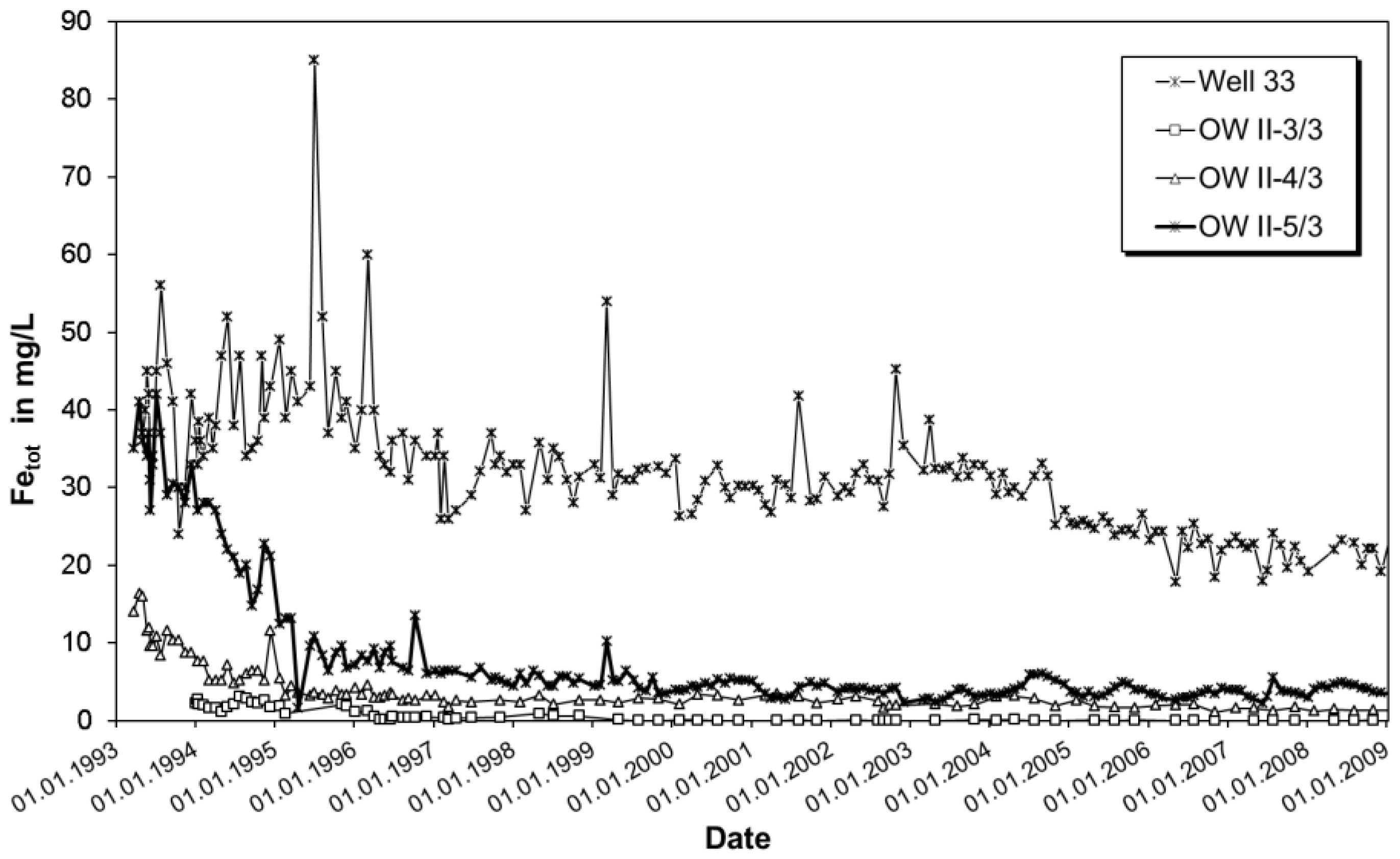
3.1. Field Observations
3.2. Laboratory Experiments
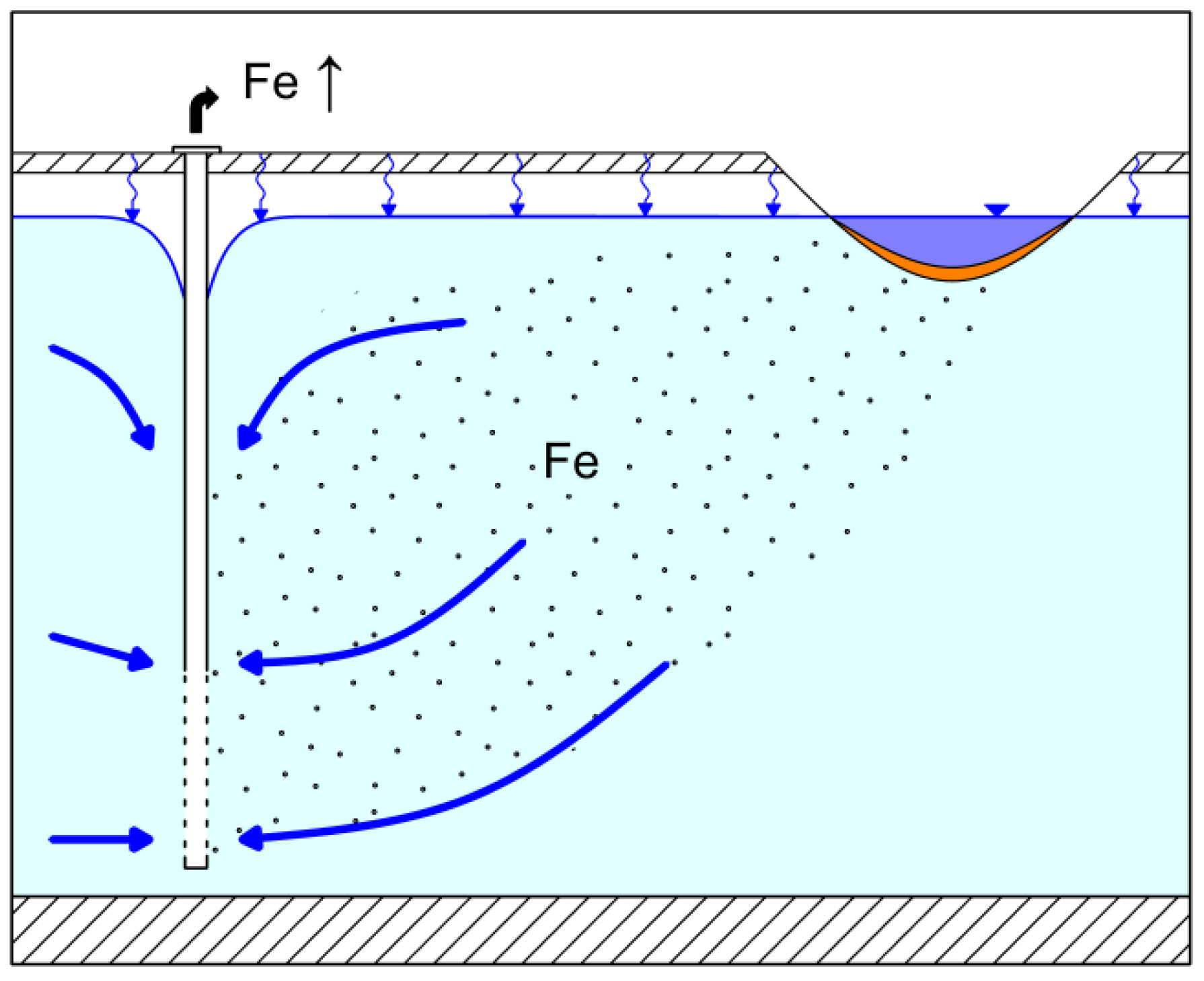
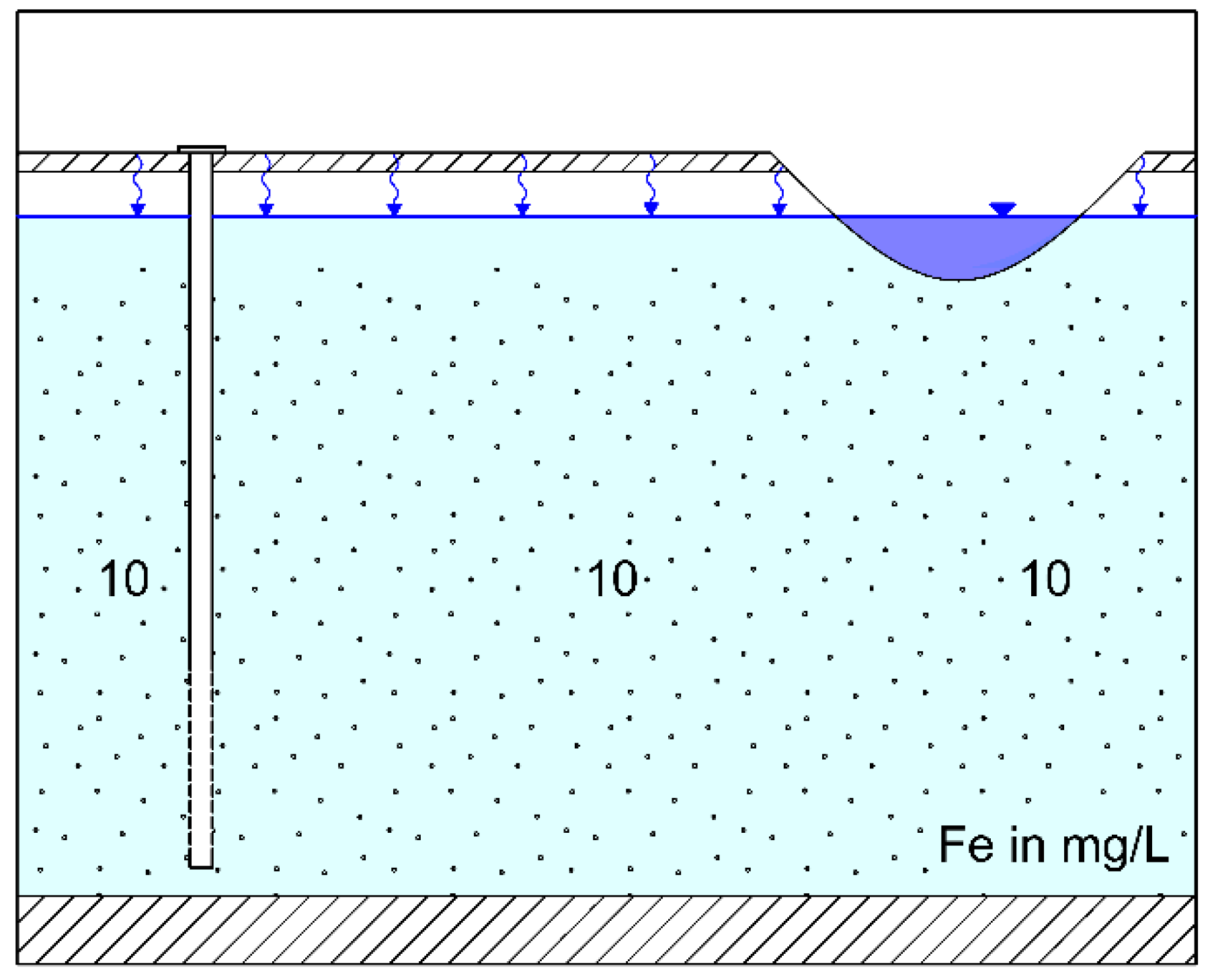
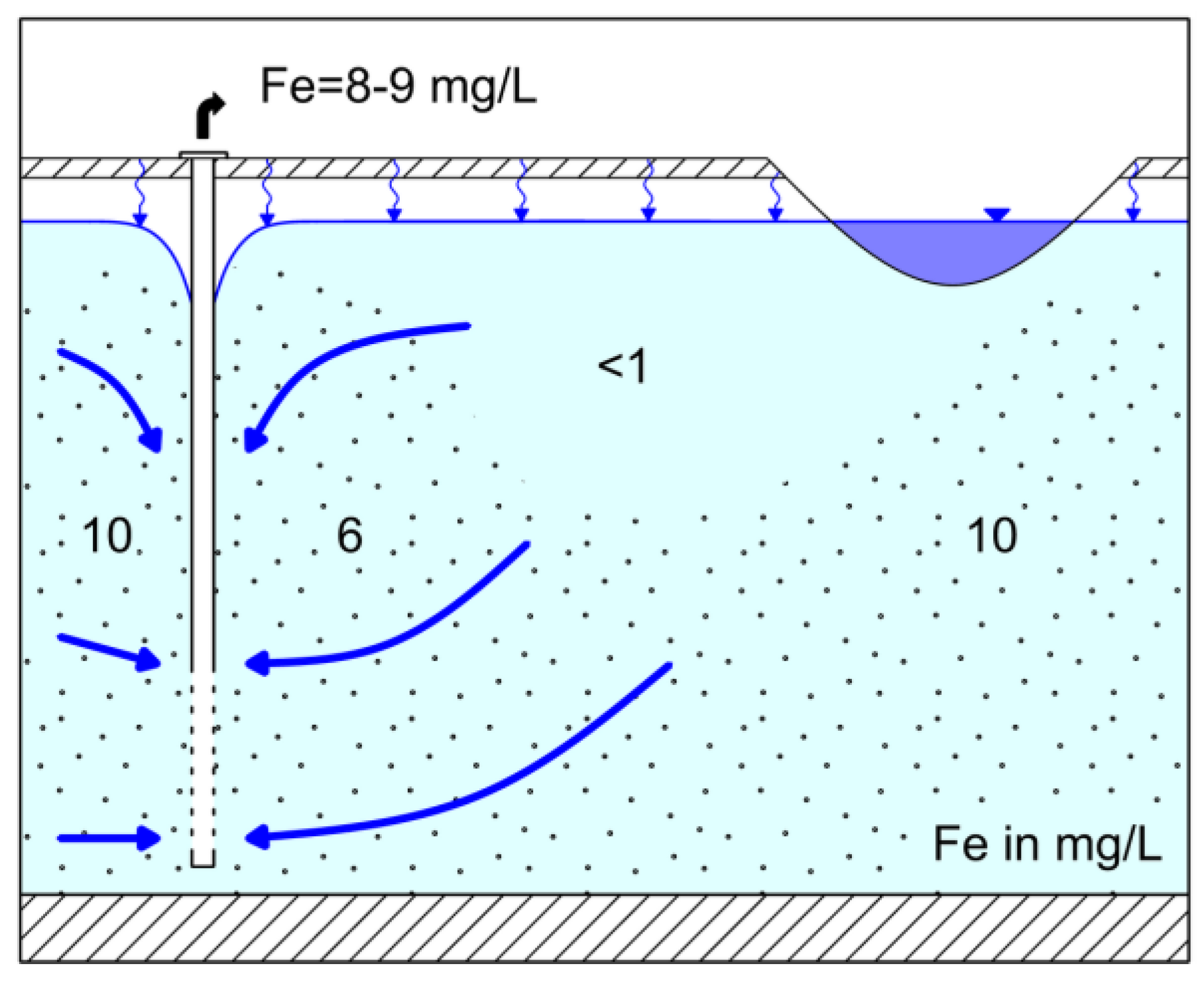
4. Discussion
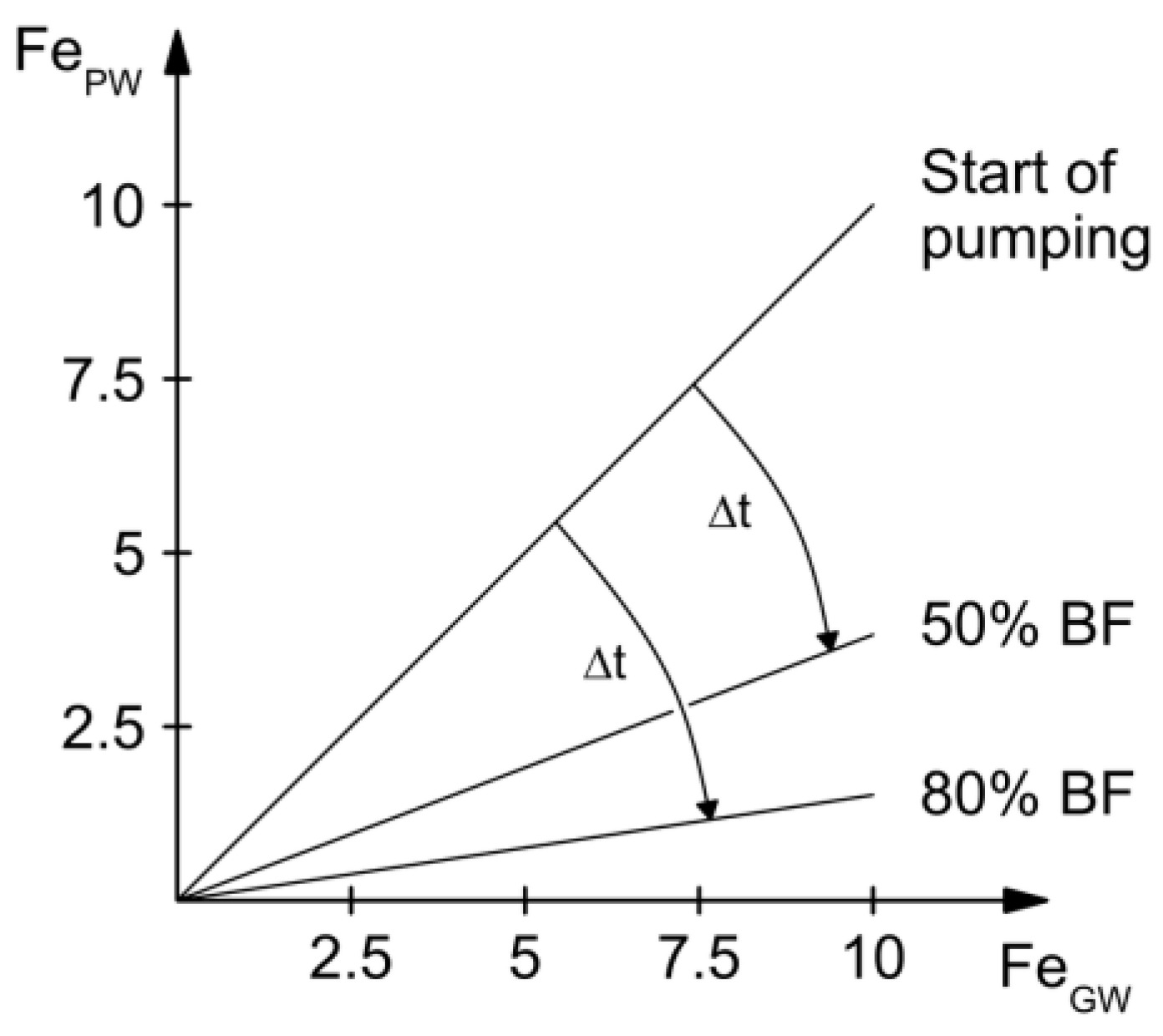
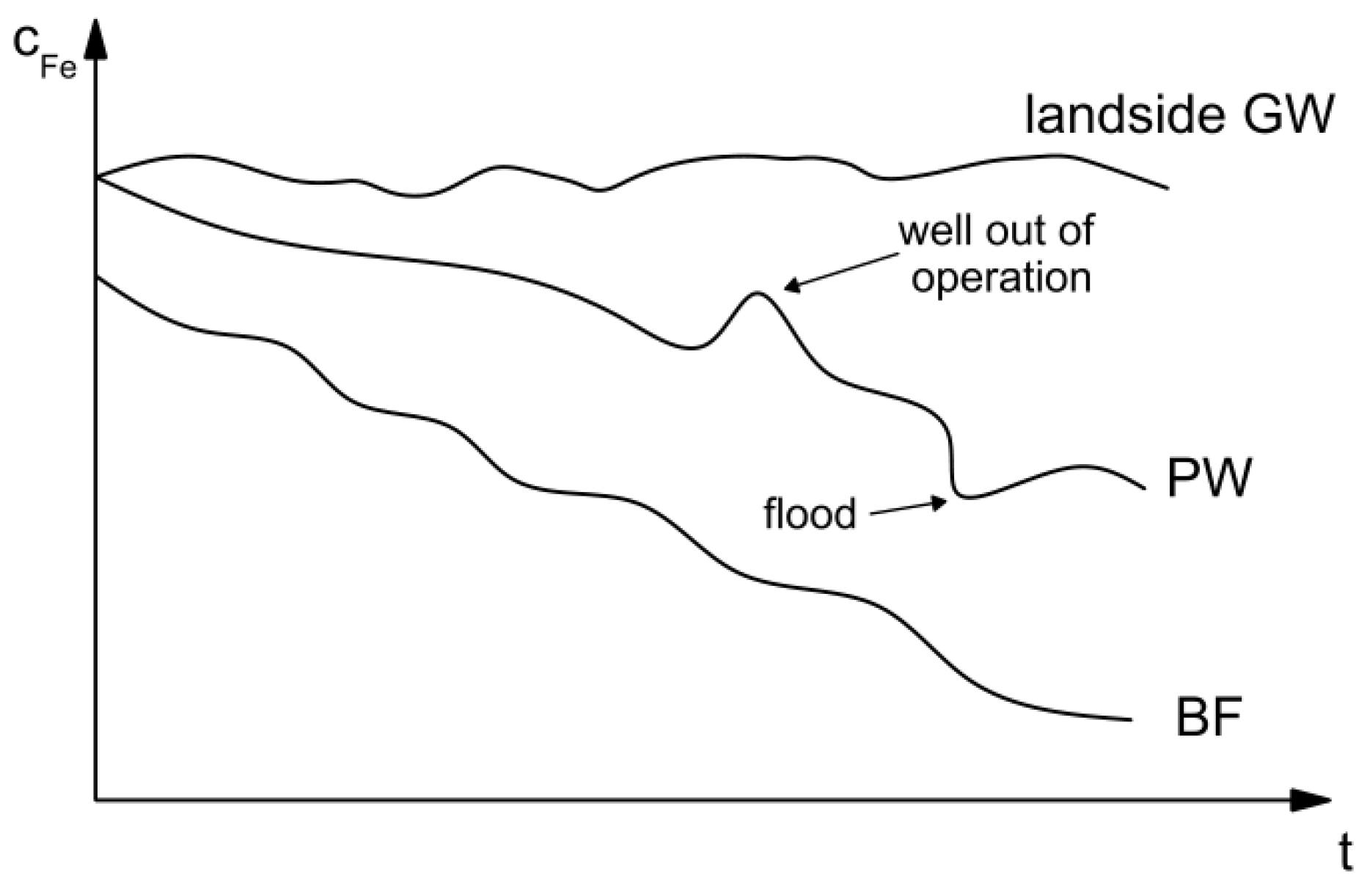
4.1. Control of Iron Concentration at an Existing RBF Site at Torgau
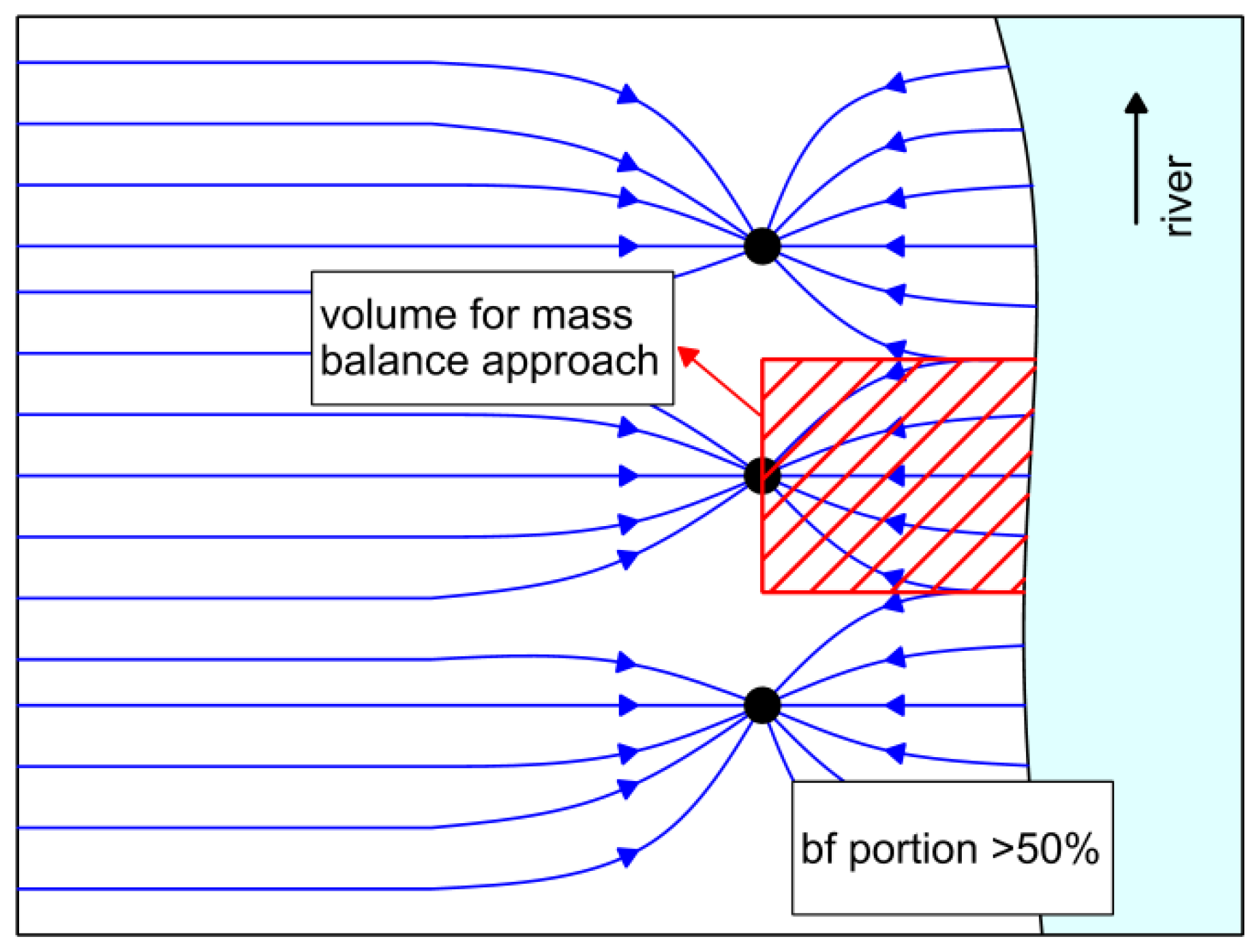
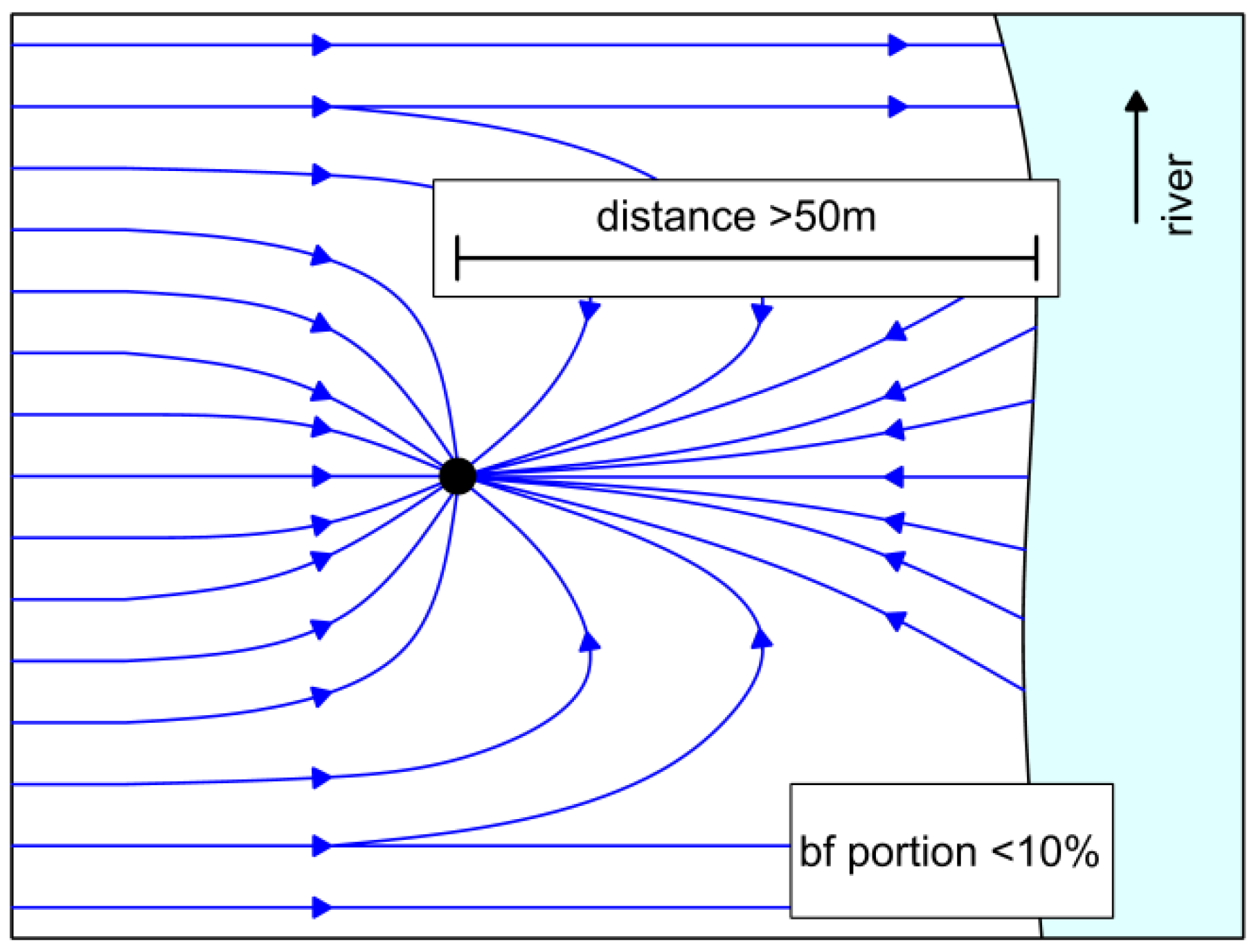
4.2. Control of Iron Concentration as an Aspect of Planning New RBF Sites
4.3. Further Issues Related to Manganese Release
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hiscock, K.; Grischek, T. Attenuation of groundwater pollution by bank filtration. J. Hydrol. 2002, 266, 139–144. [Google Scholar] [CrossRef]
- Kuehn, W.; Mueller, U. River bank filtration—An overview. J. AWWA 2000, 92, 60–69. [Google Scholar]
- Stuyfzand, P.J. Fate of pollutants during artificial recharge and bank filtration in the Netherlands. In Artificial Recharge of Groundwater; Peters, Ed.; Balkema: Rotterdam, The Netherlands, 1998; pp. 119–125. [Google Scholar]
- Ray, C.; Melin, G.; Linsky, R.B. Riverbank Filtration–Improving Source Water Quality; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; p. 364. [Google Scholar]
- Grischek, T.; Hiscock, K.M.; Metschies, T.; Dennis, P.; Nestler, W. Factors affecting denitrification during infiltration of river water into a sand and gravel aquifer in Saxony, Germany. J. Water Res. 1998, 32, 450–460. [Google Scholar] [CrossRef]
- Diem, S.; Rudolf von Rohr, M.; Hering, J.G.; Kohler, H.P.; Schirmer, M.; von Gunten, U. NOM degradation during river infiltration: Effects of the climate variables temperature and discharge. J. Water Res. 2013, 47, 6585–6595. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.; Mai, N.T.; Lan, V.N.; Trang, P.T.; Sø, H.U.; Nhan, P.Q.; Larsen, F.; Viet, P.H.; Jakobsen, R. Fate of arsenic during Red River water infiltration into aquifers beneath Hanoi, Vietnam. Environ. Sci. Technol. 2017, 51, 838–845. [Google Scholar] [CrossRef] [PubMed]
- German Standard Methods for the Examination of Water, Waste Water and Sludge; Cations (Group E); Determination of Iron (E1); DIN 38406-1; Beuth Verlag GmbH: Berlin, Germany, 1983.
- Grischek, T. Management of Bank Filtration Sites along the Elbe River. Ph.D. Thesis, Communication of the Institute for Groundwater Management, TU Dresden, Dresden, Germany, 2003; p. 252. (In German). [Google Scholar]
- Brand, K. Investigation of precipitation and remobilization of heavy metals Pb, Cd, Cu and Zn during bank filtration. In Schriftenreihe Angewandte Geologie Karlsruhe; Angewandte Geologie Karlsruhe: TH Karlsruhe, Germany, 1989; Volume 6, pp. 73–77. (In German) [Google Scholar]
- Nestler, W.; Walther, W.; Jacobs, F.; Trettin, R.; Freyer, K. Water Production in Alluvial Aquifers along the River Elbe; UFZ-Research Report 7; Helmholtz Centre for Environmental Research: Leipzig, Germany, 1998; p. 203. (In German) [Google Scholar]
- Clayton, R.G. Geochemical modelling of an unconfined aquifer adjacent to the River Elbe, Dresden. Master’s Thesis, NRG Fossil Fuels and Environmental Geochemistry, University of Newcastle, Callaghan, NSW, Australia, 1993. [Google Scholar]
- Bartak, R.; Grischek, T.; Ghodeif, K.O.; Wahaab, R.A. Shortcomings of the RBF pilot site in Dishna. Egypt. J. Hydro. Eng. 2015, 20. [Google Scholar] [CrossRef]
- NWRC. National Water Quality Monitoring Component: National Water Quality and Availability Management (NAWQAM) Project; National Water Research Center: Cairo, Egypt, 2003. [Google Scholar]
- El Arabi, N. Problems of groundwater quality related to the urban environment in Greater Cairo. In Impacts of Urban Growth on Surface Water and Groundwater Quality; IAHS Publ. No. 259; IASH Press: Wallingford, UK, 1999; pp. 29–38. [Google Scholar]
- Emara, M.M.; El Sabagh, I.; Kotb, A.; Turkey, A.S.; Husseen, D. Evaluation of drinking groundwater for the rural areas adjacent to the nearby desert of Giza governorate of Greater Cairo, Egypt. In Environmental Security in Harbors and Coastal Areas; Linkov, I., Kiker, G.A., Wenning, R.J., Eds.; Springer: Berlin, Germany, 2007; pp. 379–394. [Google Scholar]
- Ghodeif, K.; Grischek, T.; Bartak, R.; Wahaab, R.; Herlitzius, J. Potential of river bank filtration (RBF) in Egypt. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Paufler, S.; Grischek, T. Sources and behavior of manganese during riverbank filtration. In Proceedings of the 42nd IAH Congress AQUA 2015, Rome, Italy, 13–18 September 2015; p. 246. [Google Scholar]
- Antoniou, E.A.; Stuyfzand, P.J.; van Breukelen, B.M. Reactive transport modeling of an aquifer storage and recovery (ASR) pilot to assess long-term water quality improvements and potential solutions. Appl. Geochem. 2013, 35, 173–186. [Google Scholar] [CrossRef]
- Antoniou, E.A.; van Breukelen, B.M.; Putters, B.; Stuyfzand, P.J. Hydrogeochemical patterns, processes and mass transfers during aquifer storage and recovery (ASR) in an anoxic sandy aquifer. Appl. Geochem. 2012, 27, 2435–2452. [Google Scholar] [CrossRef]
- Henzler, A.; Greskowiak, J.; Massmann, G. Seasonality of temperatures and redox zonations during bank filtration – A modeling approach. J. Hydrol. 2016, 535, 282–292. [Google Scholar] [CrossRef]
- Sandhu, C. A Concept for the Investigation of Riverbank Filtration Sites for Potable Water Supply in India. Ph.D. Thesis, Faculty of Civil Engineering and Architecture, Dresden University of Applied Sciences, and Faculty of Environmental Sciences, Dresden, Germany, 2015. [Google Scholar]
- Herlitzius, J.; Sumpf, H.; Grischek, T. German-Russian cooperation for clean drinking water. Int. J. Water Manag. Bluefacts 2012, 76–81. [Google Scholar]
- Ahrns, J.; Klügel, S.; Schoenheinz, D.; Eichhorn, D.; Grischek, T. Subsurface iron removal at river bank filtration sites. In Proceedings of the IWA Eastern European Regional Young Water Professionals Conference, Minsk, Belarus, 21–22 May 2009; pp. 290–297. [Google Scholar]


| Elbe River | Bank filtrate | Well 22 | Landside GW | Change from River to BF | ||
|---|---|---|---|---|---|---|
| Parameter | OW I-4/2 | OW I-6/3 | OW I-21/3 | OW I-6/3 | ||
| pH | 7.5 | 7.0 | 6.9 | 6.6 | 6.1 | - |
| EC in µS/cm | 479 | 475 | 466 | 581 | 761 | - |
| O2 in mg/L | 10 | <0.1 | <0.1 | <0.1 | <0.1 | down >99% |
| DOC in mg/L | 5.5 | 3.2 | 2.8 | 2.2 | 1.6 | down 49% |
| NH4+ in mg/L | 0.4 | 0.02 | 0.06 | 0.16 | 0.02 | down 85% |
| NO3− in mg/L | 22.1 | 16.1 | <1 | 1.6 | <1 | down >95% |
| Fe2+ in mg/L | <0.1 | <0.1 | 0.3 | 14.4 | 14.5 | up >200% |
| Mn in mg/L | 0.11 | 0.14 | 0.08 | 0.63 | 0.59 | down 27% |
| Cl− in mg/L | 26.5 | 27.2 | 27.0 | 33.4 | 42.9 | - |
| SO42- in mg/L | 85 | 91 | 104 | 162 | 257 | up 22% |
| Ca2+ in mg/L | 56.6 | 49.5 | 54.1 | 65.3 | 76.3 | - |
| Mg2+ in mg/L | 11.0 | 10.1 | 10.3 | 11.9 | 15.2 | - |
| Na+ in mg/L | 22.9 | 23.8 | 22.4 | 22.7 | 25.6 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grischek, T.; Paufler, S. Prediction of Iron Release during Riverbank Filtration. Water 2017, 9, 317. https://doi.org/10.3390/w9050317
Grischek T, Paufler S. Prediction of Iron Release during Riverbank Filtration. Water. 2017; 9(5):317. https://doi.org/10.3390/w9050317
Chicago/Turabian StyleGrischek, Thomas, and Sebastian Paufler. 2017. "Prediction of Iron Release during Riverbank Filtration" Water 9, no. 5: 317. https://doi.org/10.3390/w9050317






