Spatio-Temporal Variation and Controlling Factors of Water Quality in Yongding River Replenished by Reclaimed Water in Beijing, North China
Abstract
:1. Introduction
2. Materials and Methods
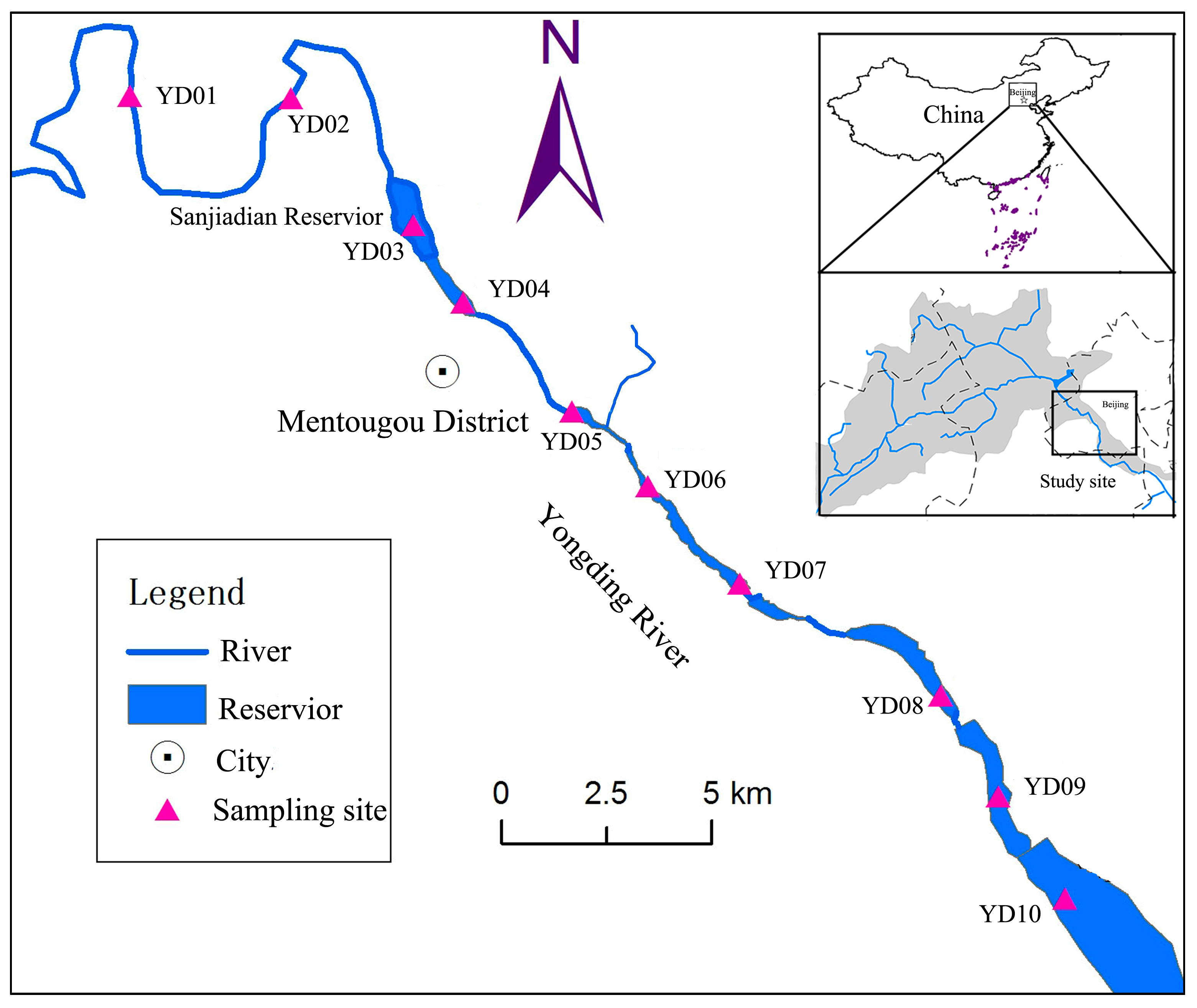
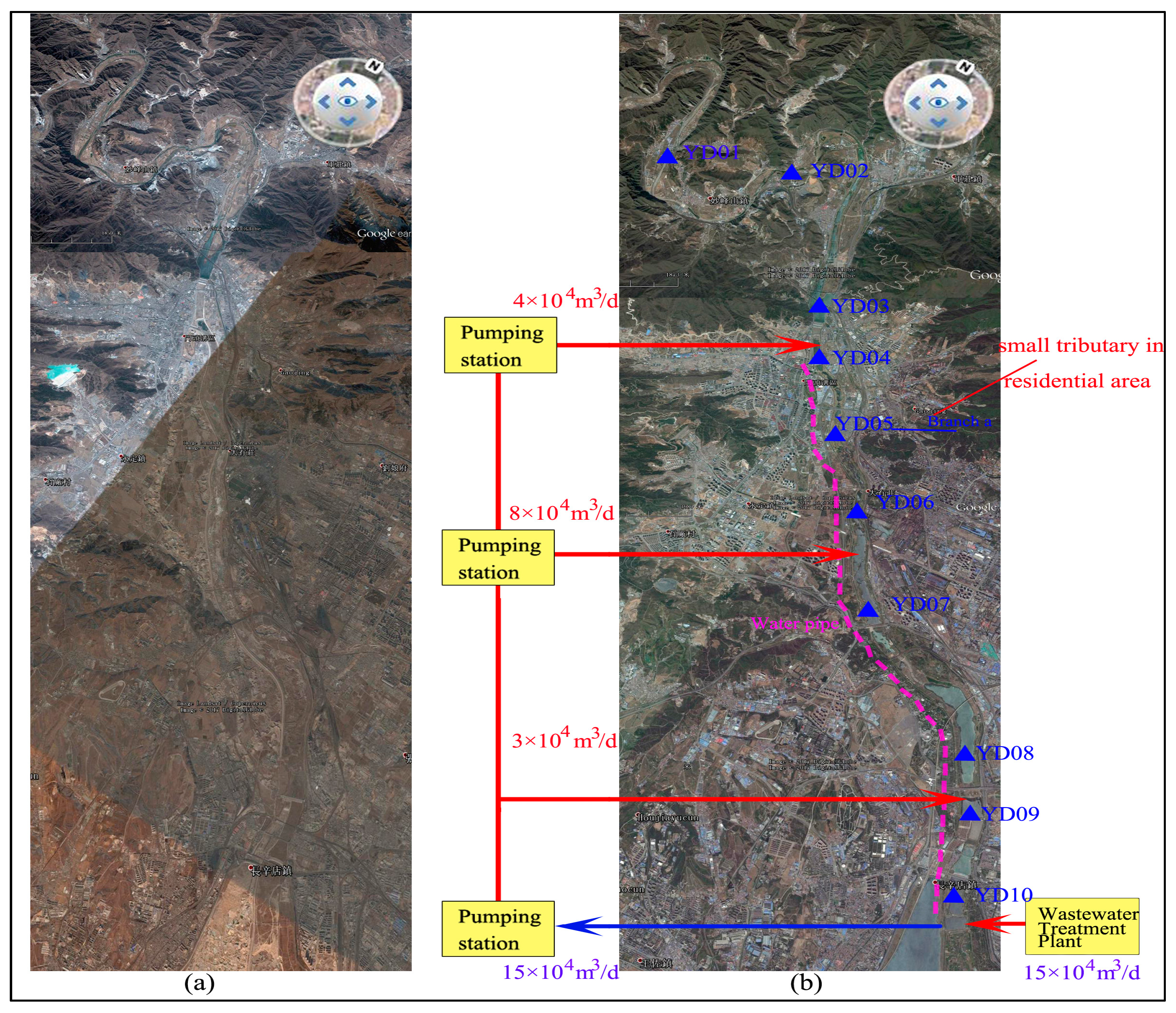
2.1. Study Site
2.2. Methods
2.2.1. Water Sampling
2.2.2. Analytical Techniques
2.2.3. Data Analysis
3. Results and Discussion
3.1. Physical and Chemical Compositions
3.2. Spatial and Temporal Variation of Water Quality
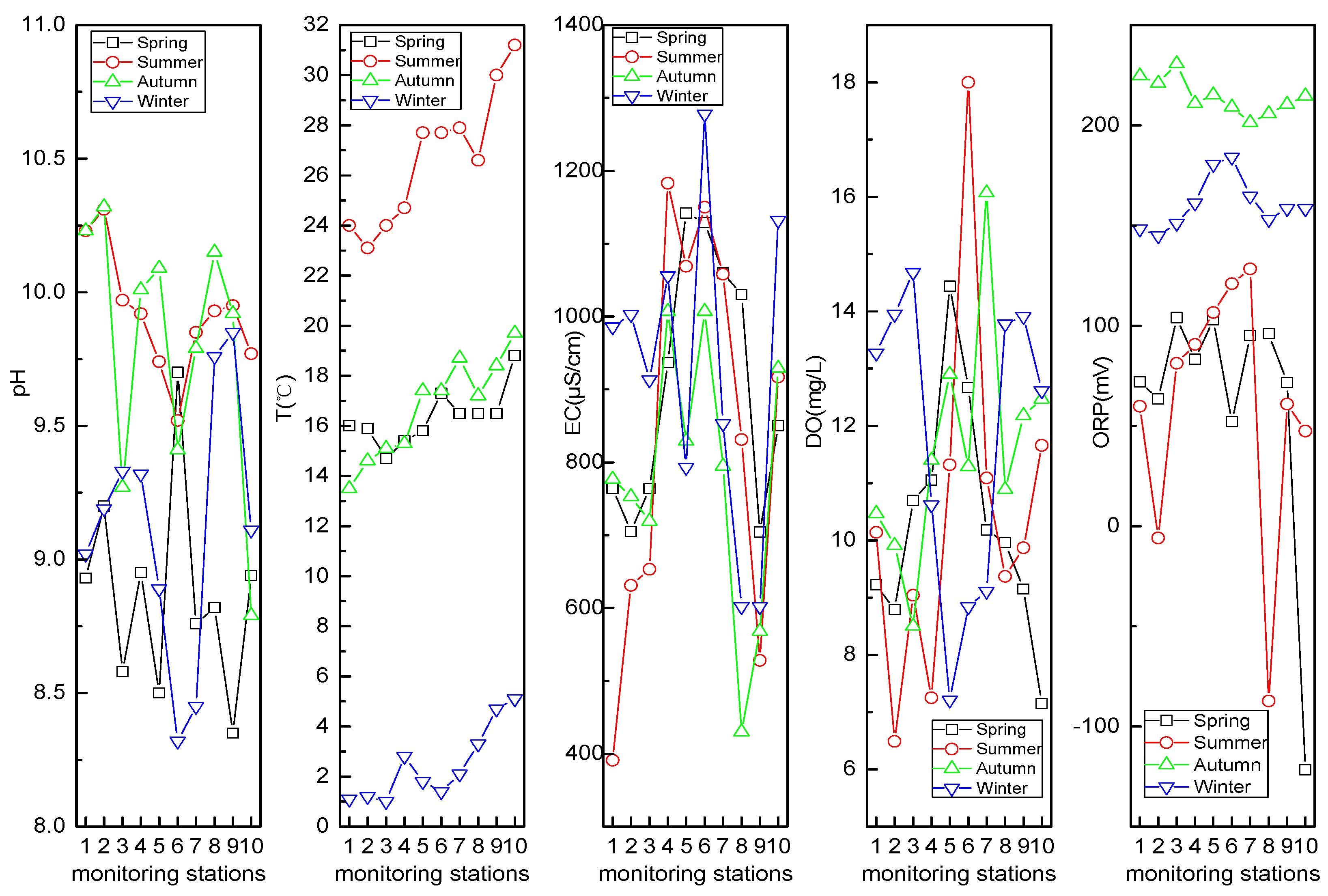
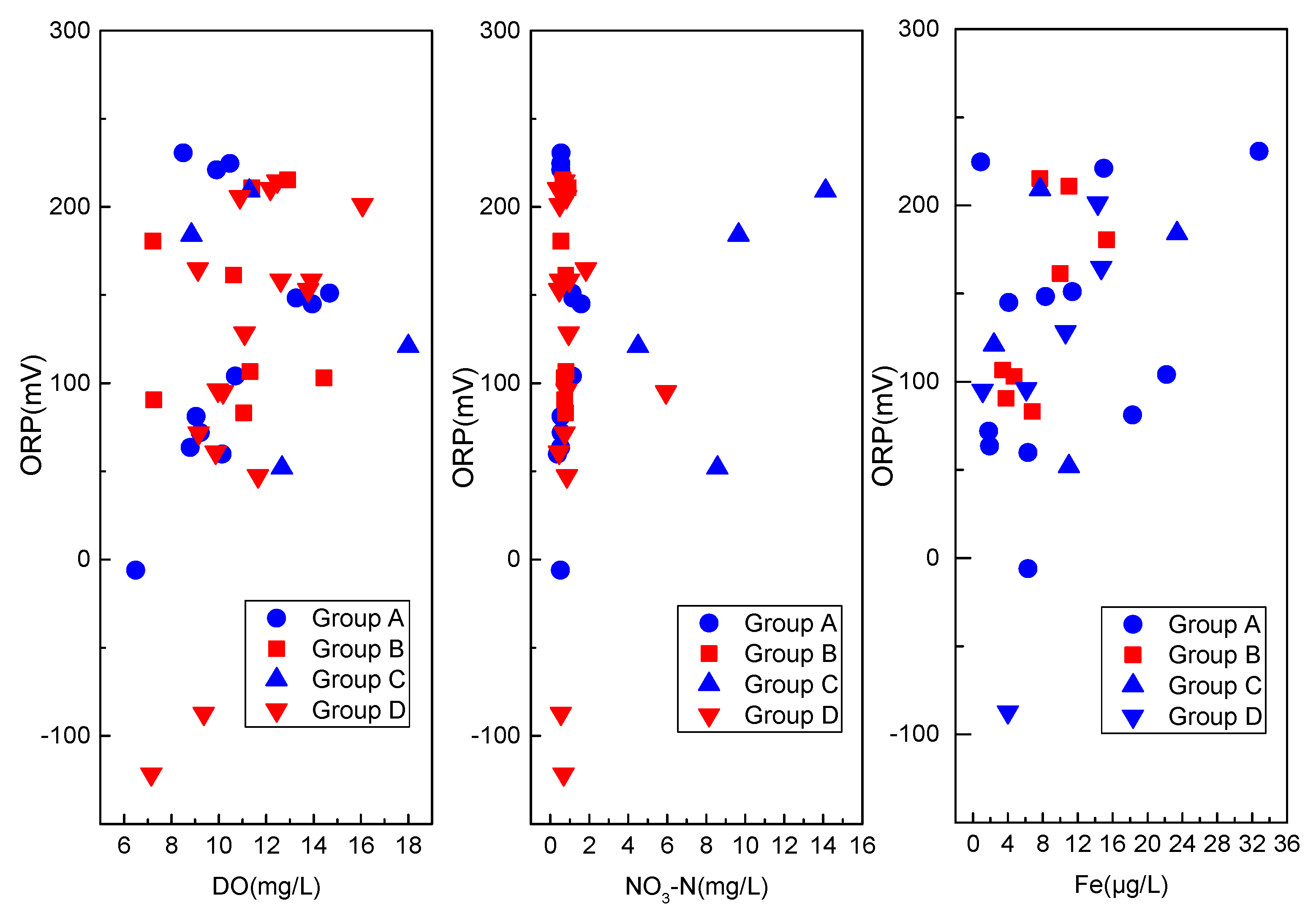
3.2.1. pH, T, EC, DO, and ORP
3.2.2. Nitrogen and Phosphorus
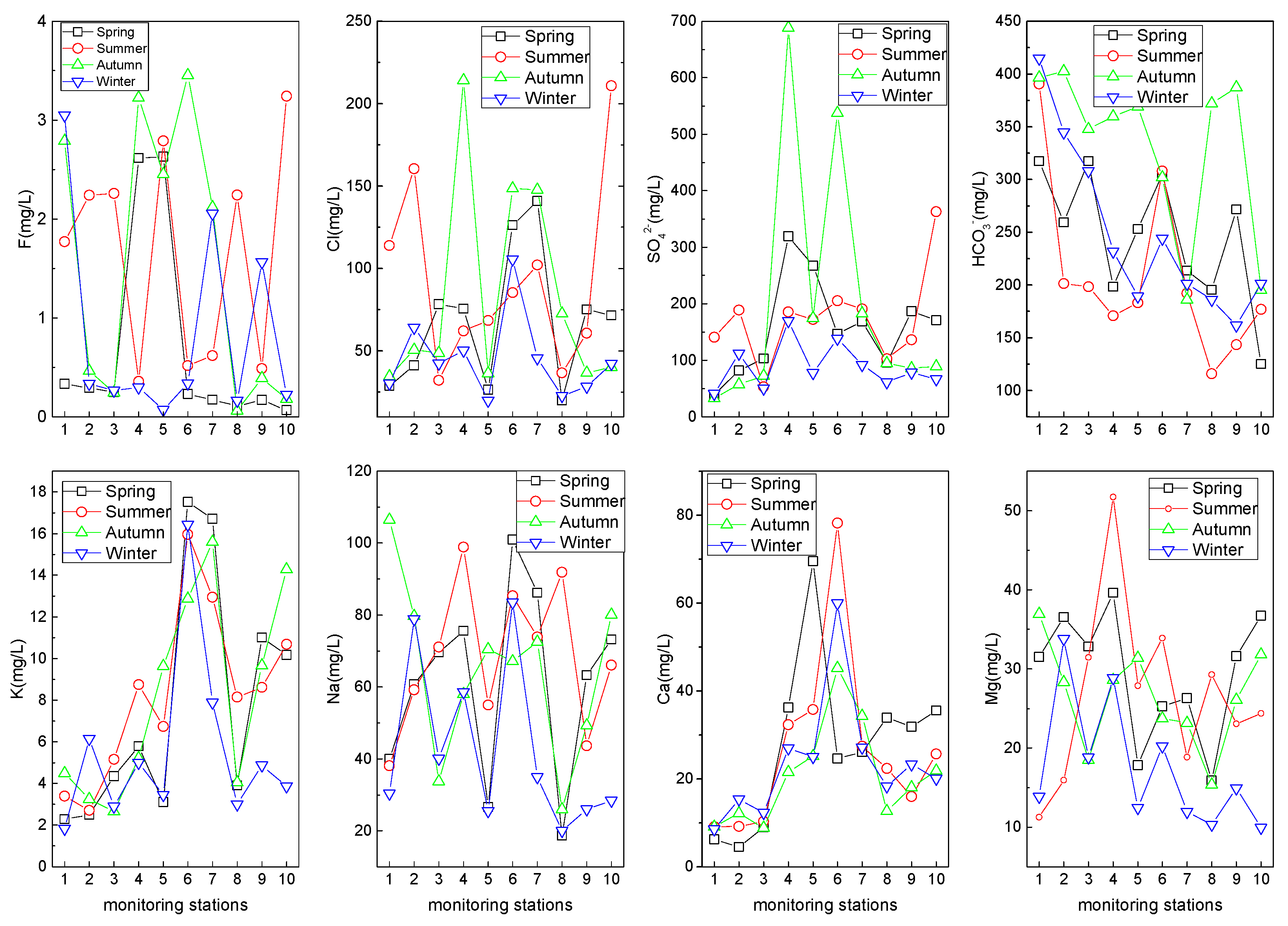
3.2.3. Major Cations and Anions
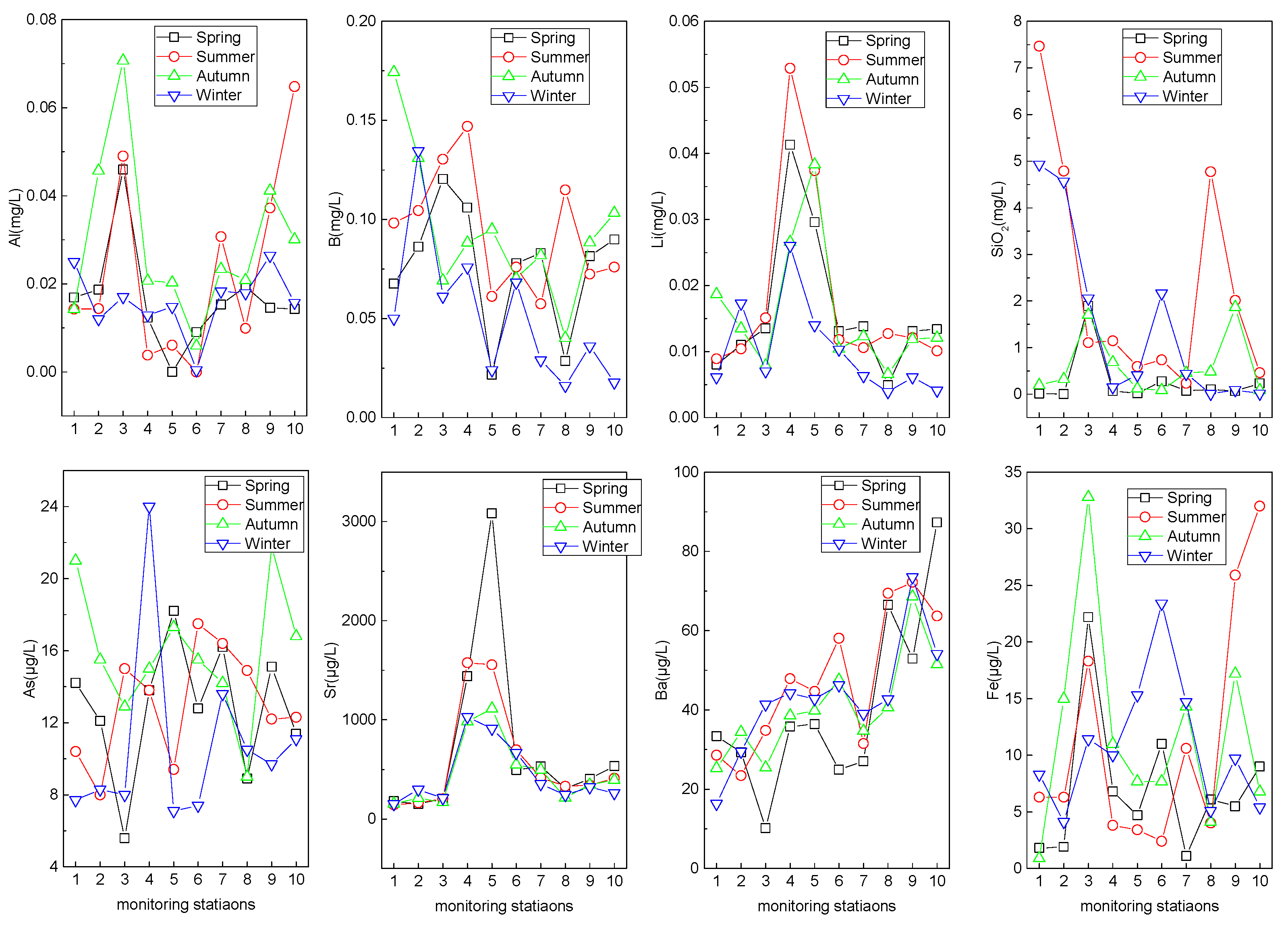
3.2.4. Trace Metallic Elements
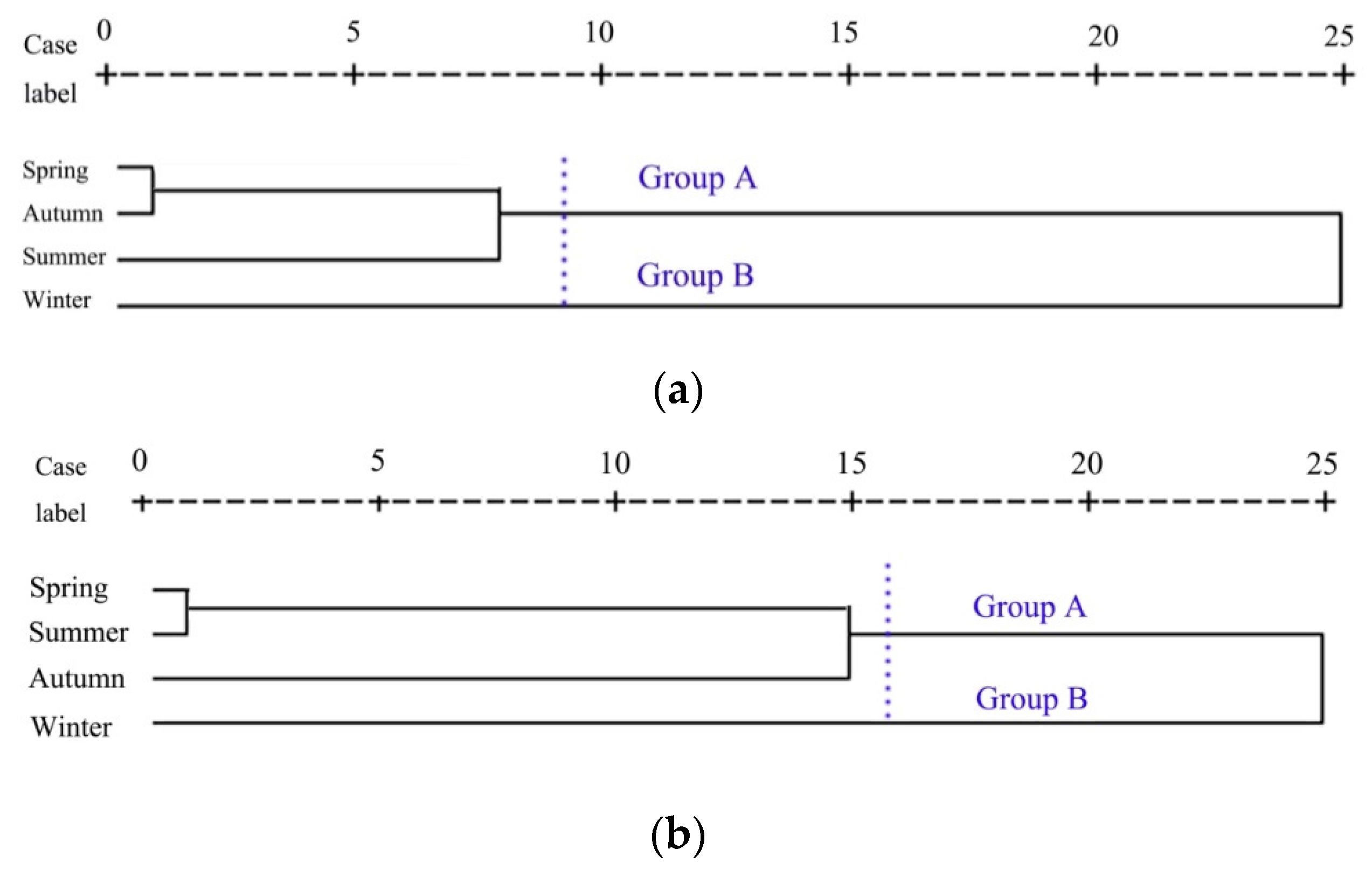
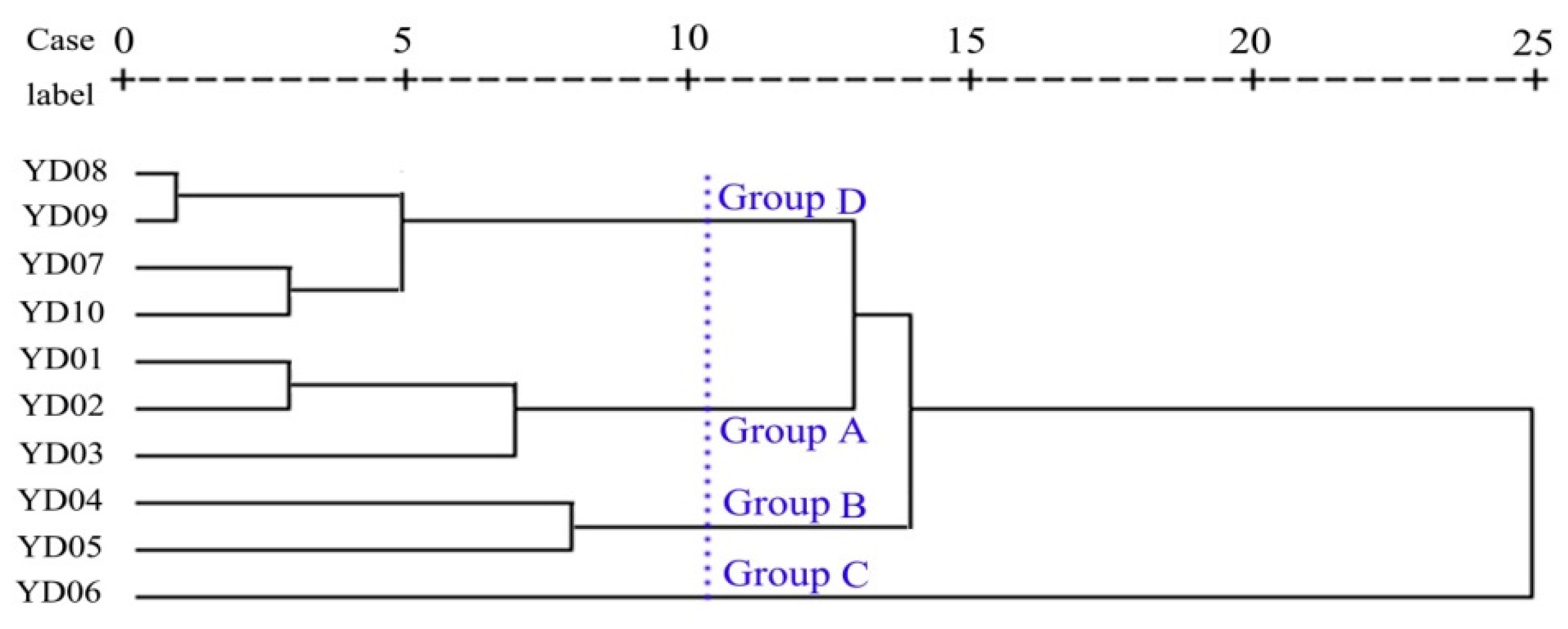
3.3. Cluster Analysis and Spatio-Temporal Similarity
3.4. Principal Components and Controlling Factors
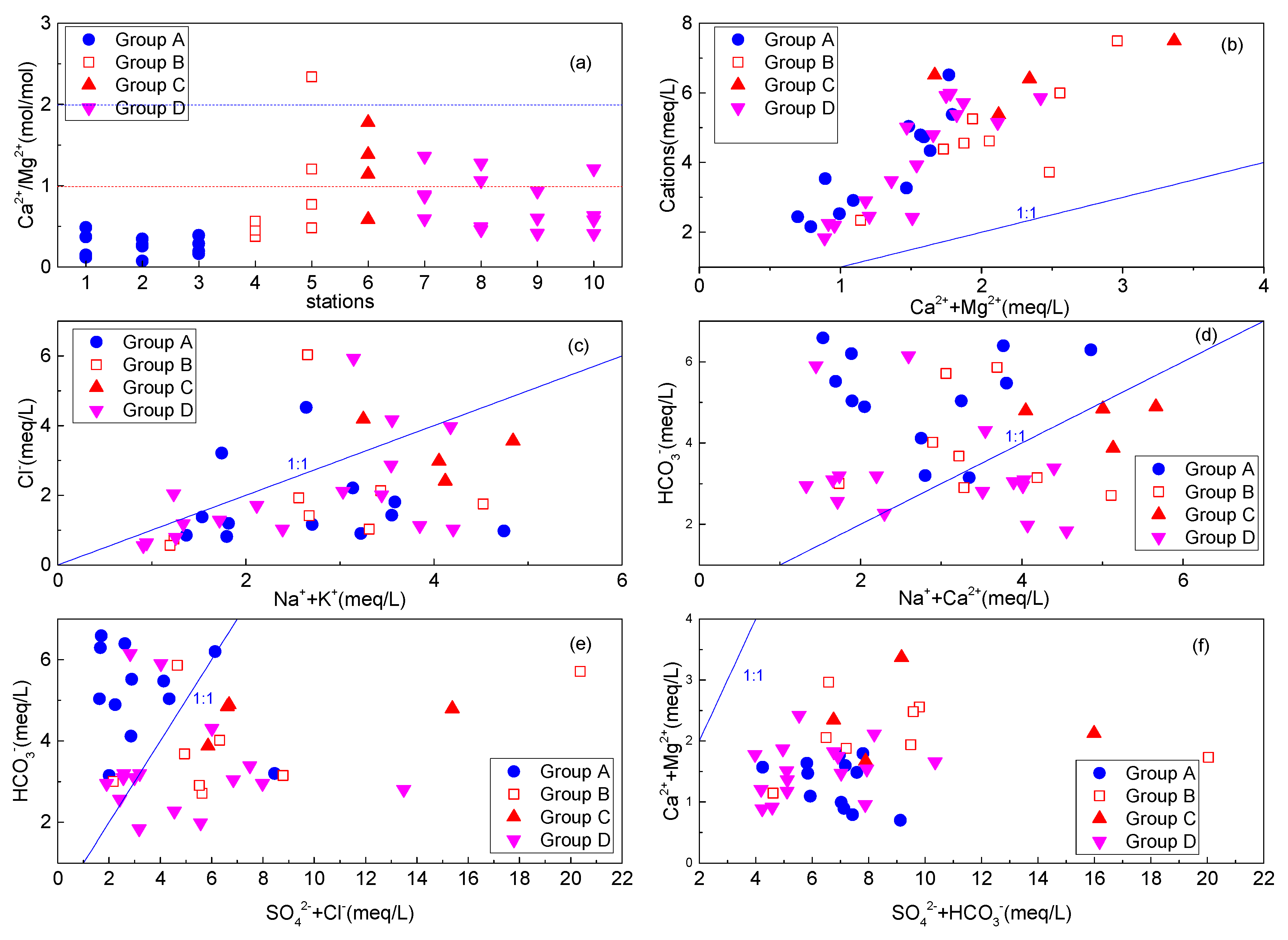
3.5. Ionic Relationship and Hydro Geochemical Process
3.5.1. Gibbs Plot and Controlling Mechanism
3.5.2. Bivariate Plot and Dissolution Reaction
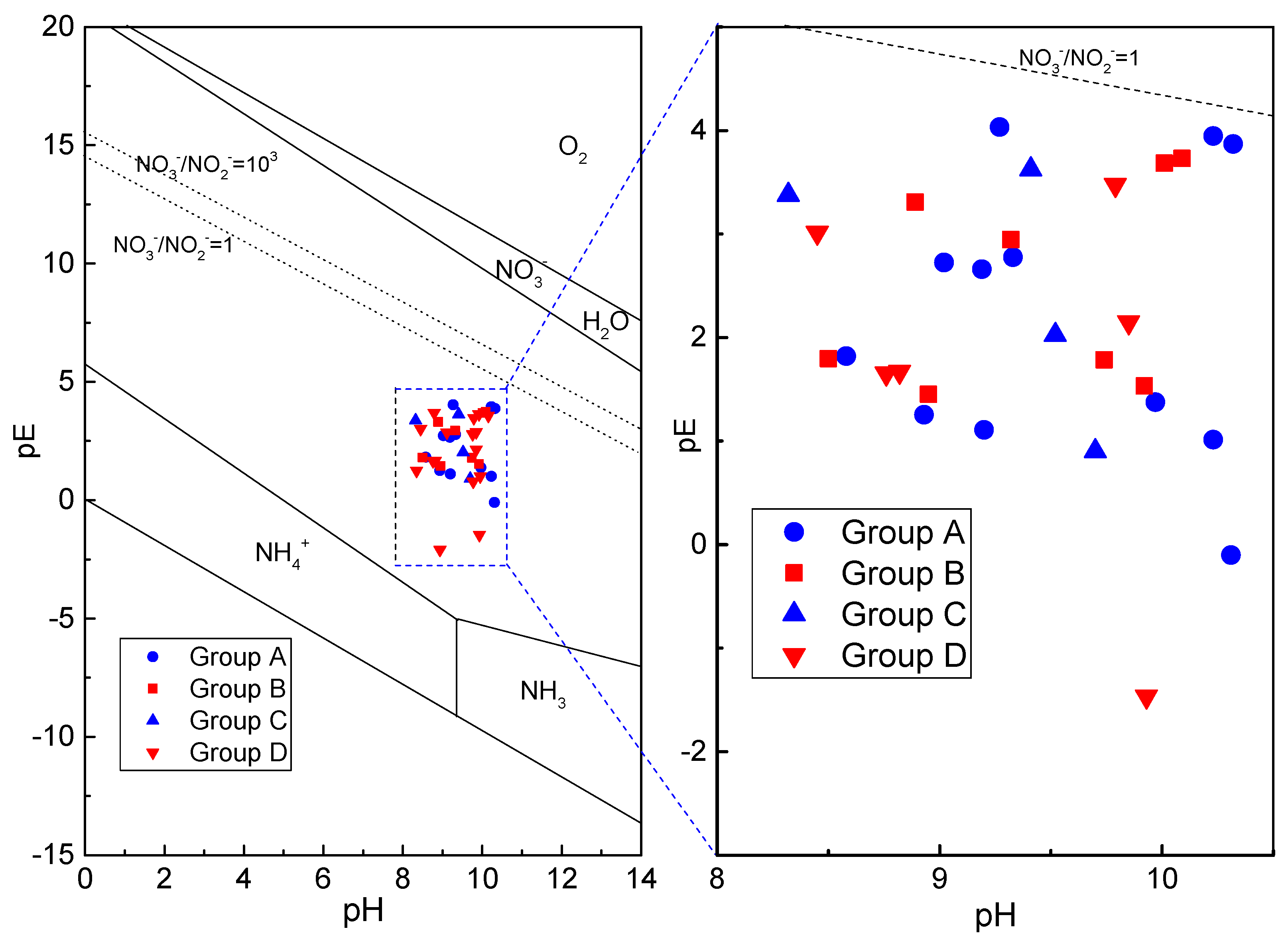
3.5.3. Nitrogen Forms and Redox Environment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jun, X. Water cycle and water resources security in North China: Problems and challenges. Adv. Geogr. 2002, 21, 517–526. [Google Scholar]
- Zhou, Z.; Li, M.; Liu, W.; Jiao, Z.; Sun, F. Beijing Water Resoureces Bulletin; B.W. Authority: Beijing, China, 2015.
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.-Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Yang, H.; Abbaspour, K.C. Analysis of wastewater reuse potential in Beijing. Desalination 2007, 212, 238–250. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, Y. Using reclaimed water for agricultural and landscape irrigation in China: A review. Irrig. Drain. 2017. [Google Scholar] [CrossRef]
- Furumai, H. Rainwater and reclaimed wastewater for sustainable urban water use. Phys. Chem. Earth Parts A/B/C 2008, 33, 340–346. [Google Scholar] [CrossRef]
- Li, H.; Gong, X.; Gu, B.; Li, J.; Zhu, X. Study on water quality control measures of reclaimed water used in Yongding River landscape water. Beijing Water Aff. 2012, 3, 41–43. (In Chinese) [Google Scholar]
- Qin, C.; Liu, H.; Liu, L.; Smith, S.; Sedlak, D.L.; Gu, A.Z. Bioavailability and characterization of dissolved organic nitrogen and dissolved organic phosphorus in wastewater effluents. Sci. Total Environ. 2015, 511, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, X.C.; Zhang, Q.; Duan, R.; Wang, N. Characteristics of a landscape water with high salinity in a coastal city of China and measures for eutrophication control. Ecol. Indic. 2016, 61, 268–273. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; McCarthy, M.J.; Peierls, B.L.; Wilhelm, S.W. Algal blooms: Noteworthy nitrogen. Science 2014, 346, 175. [Google Scholar] [CrossRef] [PubMed]
- Asano, T. Artificial Recharge of Groundwater; Elsevier: Boston, MA, USA, 2016. [Google Scholar]
- Chen, W.; Lu, S.; Jiao, W.; Wang, M.; Chang, A.C. Reclaimed water: A safe irrigation water source? Environ. Dev. 2013, 8, 74–83. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, H.; Li, S.; Ma, J.; Peng, Y.; Li, F. Study on effects of reclaimed water irrigation on the soil and shallow groundwater salinity. J. Tarim Univ. 2016, 28, 72–79. (In Chinese) [Google Scholar]
- Duan, W.; Takara, K.; He, B.; Luo, P.; Nover, D.; Yamashiki, Y. Spatial and temporal trends in estimates of nutrient and suspended sediment loads in the Ishikari River, Japan, 1985 to 2010. Sci. Total Environ. 2013, 461–462, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.X.; Li, S.M.; Li, J.B.; Zhang, Q.K.; Liu, F. Water quality assessment for wastewater reclamation using principal component analysis. J. Environ. Inf. 2013, 21, 45–54. [Google Scholar] [CrossRef]
- Subba, R.N.; Prakasa, R.J.; John, D.D.; Srinivasa, R.K.; Krishna, C. Multivariate analysis for identifying the governing factors of groundwater quality. J. Environ. Hydrol. 2001, 9, 1–9. [Google Scholar]
- Haag, I.; Westrich, B. Processes governing river water quality identified by principal component analysis. Hydrol. Process. 2002, 16, 3113–3130. [Google Scholar] [CrossRef]
- Petersen, W.; Bertino, L.; Callies, U.; Zorita, E. Process identification by principal component analysis of river water-quality data. Ecol. Model. 2001, 138, 193–213. [Google Scholar] [CrossRef]
- Duan, W.; He, B.; Nover, D.; Yang, G.; Chen, W.; Meng, H.; Zou, S.; Liu, C. Water Quality Assessment and Pollution Source Identification of the Eastern Poyang Lake Basin Using Multivariate Statistical Methods. Sustainability 2016, 8, 133. [Google Scholar] [CrossRef]
- Costa, M.; Gonçalves, A.M. Clustering and forecasting of dissolved oxygen concentration on a river basin. Stoch. Environ. Res. Risk Assess. 2010, 25, 151–163. [Google Scholar] [CrossRef]
- Oketola, A.A.; Adekolurejo, S.M.; Osibanjo, O. Water quality assessment of River Ogun using multivariate statistical techniques. J. Environ. Prot. 2013, 4, 466–479. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Hudson-Edwards, K.A.; Velde, G.V.D.; Roelofs, J.G.M. Controls on water chemistry of the Pilcomayo river (Bolivia, South-America). Appl. Geochem. 2004, 19, 1745–1758. [Google Scholar] [CrossRef]
- Stallard, R.F.; Edmond, J.M. Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. J. Geophys. Res. 1983, 88, 9671–9688. [Google Scholar] [CrossRef]
- Liu, B.; Liu, C.Q.; Zhang, G.; Zhao, Z.Q.; Li, S.L.; Hu, J.; Ding, H.; Lang, Y.C.; Li, X.D. Chemical weathering under mid–to cool temperate and monsoon-controlled climate: A study on water geochemistry of the Songhuajiang River system, northeast China. Appl. Geochem. 2013, 31, 265–278. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, J.; Qin, X.; Rioual, P.; Zhang, Y.; Liu, Z.; Yan, M.; Li, H.; Ren, X.; Xiong, H. Identification of rock weathering and environmental control in arid catchments (northern Xinjiang) of Central Asia. J. Asian Earth Sci. 2013, 66, 277–294. [Google Scholar] [CrossRef]
- Jiang, B.; Wong, C.P.; Lu, F.; Ouyang, Z.; Wang, Y. Drivers of drying on the Yongding River in Beijing. J. Hydrol. 2014, 519, 69–79. [Google Scholar] [CrossRef]
- Liang, S.; Fan, H.; Wang, L.; Yang, J.; Gao, J.; Feng, M. Study on suitability observation of ecological revetment model of Yongding River. Soil Water Conserv. Res. 2012, 19, 153–158. [Google Scholar]
- Mujica, L.E.; Vehí, J.; Ruiz, M.; Verleysen, M.; Staszewski, W.; Worden, K. Multivariate statistics process control for dimensionality reduction in structural assessment. Mech. Syst. Signal Process. 2008, 22, 155–171. [Google Scholar] [CrossRef]
- Reimann, C.; Filzmoser, P. Normal and lognormal data distribution in geochemistry death of a myth. Consequences for the statistical treatment of geochemical and environmental data. Environ. Geol. 2000, 39, 1001–1014. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, C.; Song, X.; Liu, C.; Zhang, Y. Characteristics of nitrate in major rivers and aquifers of the Sanjiang Plain, China. J. Environ. Monit. 2012, 14, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Surface Water Environmental Quality Standard (gb3838-2002); China Environmental Science Press: Beijing, China, 2002.
- Chen, L.; Yang, K.; Song, X.; He, Y.; Fan, J. Urban Wastewater Recycling Landscape Water Quality (GB/T 18921-2002); General Administration of Quality Supervision, Inspection and Quarantine of the People ‘s Republic of China: Beijing, China, 2002.
- Truesdale, G.; Downing, A.; Lowden, G. The solubility of oxygen in pure water and sea-water. J. Chem. Technol. Biotechnol. 1955, 5, 53–62. [Google Scholar] [CrossRef]
- Tromans, D. Temperature and pressure dependent solubility of oxygen in water: A thermodynamic analysis. Hydrometallurgy 1998, 48, 327–342. [Google Scholar] [CrossRef]
- Zhou, W.; Yuan, X.; Long, A.; Huang, H.; Yue, W. Different hydrodynamic processes regulated on water quality (nutrients, dissolved oxygen, and phytoplankton biomass) in three contrasting waters of Hong Kong. Environ. Monit. Assess. 2014, 186, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.; Willems, P.; El-Sadek, A.; Berlamont, J. Modelling of dissolved oxygen and biochemical oxygen demand in river water using a detailed and a simplified model. Int. J. River Basin Manag. 2003, 1, 97–103. [Google Scholar] [CrossRef]
- Gelda, R.K.; Effler, S.W. A river water quality model for chlorophyll and dissolved oxygen that accommodates zebra mussel metabolism. Water Qual. Ecosyst. Model. 2000, 1, 271–309. [Google Scholar] [CrossRef]
- Abowei, J.F.N. Salinity, dissolved oxygen, pH and surface water temperature conditions in Nkoro River, Niger Delta, Nigeria. Adv. J. Food Sci. Technol. 2010, 2, 67–71. [Google Scholar]
- Kaiser, H.F. The varimax criteria for analytical rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Mazlum, N.; Ozer, A.; Mazlum, S.U. Interpretation of water quality data by principal components analysis. J. Eng. Environ. Sci. 1999, 23, 19–26. [Google Scholar]
- Kim, J.-H.; Kim, R.-H.; Lee, J.; Cheong, T.-J.; Yum, B.-W.; Chang, H.-W. Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol. Process. 2005, 19, 1261–1276. [Google Scholar] [CrossRef]
- Strauss, E.A.; Richardson, W.B.; Bartsch, L.A.; Cavanaugh, J.C.; Bruesewitz, D.A.; Imker, H.; Heinz, J.A.; Soballe, D.M. Nitrification in the Upper Mississippi River: Patterns, controls, and contribution to the NO3− budget. J. N. Am. Benthol. Soc. 2004, 61, 1102–1112. [Google Scholar] [CrossRef]
- Zhao, L.; Delatolla, R.; Mohammadian, A. Nitrification kinetics & modified model for the Rideau River, Canada. Water Qual. Res. J. Can. 2013, 48, 192–201. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Huh, Y.; Qin, J.; Pho, N.V. Chemical weathering in the Hong (Red) River basin: Rates of silicate weathering and their controlling factors. Geochim. Cosmochim. Acta 2007, 71, 1411–1430. [Google Scholar] [CrossRef]
- Fan, B.L.; Zhao, Z.Q.; Tao, F.X.; Liu, B.J.; Tao, Z.H.; Gao, S.; Zhang, L.H. Characteristics of carbonate, evaporite and silicate weathering in huanghe river basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Li, J.; Yuan, G.L.; Deng, X.R.; Jing, X.M.; Sun, T.H.; Lang, X.X.; Wang, G.H. Major ion geochemistry of the Nansihu Lake basin rivers, North China: Chemical weathering and anthropogenic load under intensive industrialization. Environ. Earth Sci. 2016, 75, 453. [Google Scholar] [CrossRef]
- Yan, W.; Yang, L.; Wang, F.; Wang, J.; Ma, P. Riverine N2O concentrations, exports to estuary and emissions to atmosphere from the Changjiang River in response to increasing nitrogen loads. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Khalil, M.I.; Johnston, P.; Cardenas, L.M.; Hatch, D.J.; Butler, M.; Barrett, M.; O’flaherty, V.; Richards, K.G. Denitrification potential in subsoils: A mechanism to reduce nitrate leaching to groundwater. Agric. Ecosyst. Environ. 2012, 147, 13–23. [Google Scholar] [CrossRef]
- Gomez-Velez, J.D.; Harvey, J.W.; Cardenas, M.B.; Kiel, B. Denitrification in the Mississippi River network controlled by flow through river bedforms. Nat. Geosci. 2015, 8, 941–945. [Google Scholar] [CrossRef]


| Parameters | Natural River Water | River Replenished by Reclaimed Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | C.V. (%) | Min | Max | Mean | SD | C.V. (%) | |
| pH | 8.58 | 10.32 | 9.55 | 0.62 | 6.52 | 8.32 | 10.15 | 9.38 | 0.59 | 6.30 |
| T (°C) | 1.0 | 24.0 | 13.7 | 8.48 | 61.96 | 1.4 | 31.2 | 16.4 | 9.15 | 55.95 |
| EC (µS/cm) | 391 | 1003 | 755 | 165.85 | 21.97 | 430 | 1278 | 910 | 220.77 | 24.27 |
| DO (mg/L) | 6.49 | 14.68 | 10.43 | 2.42 | 23.18 | 7.15 | 18.00 | 11.30 | 2.54 | 22.48 |
| ORP (mv) | −6.00 | 230.70 | 124.59 | 75.25 | 60.40 | −121.70 | 215.20 | 124.08 | 84.43 | 68.05 |
| NH3-N (mg/L) | 0.10 | 0.42 | 0.26 | 0.10 | 39.80 | 0.04 | 1.70 | 0.36 | 0.30 | 81.68 |
| NO2-N (mg/L) | 0.00 | 0.03 | 0.01 | 0.01 | 95.35 | 0.00 | 0.35 | 0.05 | 0.09 | 168.52 |
| NO3-N (mg/L) | 0.37 | 1.59 | 0.76 | 0.38 | 50.67 | 0.39 | 14.11 | 2.14 | 3.38 | 157.64 |
| F− (mg/L) | 0.24 | 3.05 | 1.19 | 1.13 | 94.83 | 0.07 | 3.45 | 1.17 | 1.21 | 103.5 |
| Cl− (mg/L) | 28.78 | 160.55 | 60.46 | 39.92 | 66.02 | 19.92 | 214.18 | 77.60 | 54.12 | 69.74 |
| SO42− (mg/L) | 32.86 | 188.91 | 80.99 | 47.35 | 58.47 | 61.21 | 688.27 | 187.47 | 141.94 | 75.71 |
| HCO3− (mg/L) | 198 | 415 | 325 | 74.15 | 22.83 | 116 | 387 | 230 | 76.39 | 33.22 |
| K+ (mg/L) | 1.83 | 6.15 | 3.47 | 1.30 | 37.39 | 3.01 | 17.52 | 9.14 | 4.67 | 51.13 |
| Na+ (mg/L) | 30.37 | 106.50 | 59.00 | 23.26 | 39.42 | 18.63 | 101.00 | 59.28 | 25.41 | 42.87 |
| Ca2+ (mg/L) | 4.50 | 15.37 | 9.55 | 2.82 | 29.50 | 12.70 | 78.20 | 31.26 | 15.35 | 49.12 |
| Mg2+ (mg/L) | 11.26 | 36.97 | 25.83 | 9.43 | 36.53 | 9.95 | 51.73 | 24.69 | 9.62 | 38.95 |
| Al (mg/L) | 0.01 | 0.07 | 0.03 | 0.02 | 66.95 | 0.00 | 0.06 | 0.02 | 0.01 | 76.92 |
| B (mg/L) | 0.05 | 0.17 | 0.10 | 0.04 | 36.32 | 0.02 | 0.15 | 0.07 | 0.03 | 47.14 |
| Sr (µg/L) | 140.4 | 296.4 | 185.2 | 43.3 | 23.37 | 214.6 | 3083.0 | 714.2 | 611.2 | 85.57 |
| Ba (µg/L) | 10.2 | 41.4 | 27.7 | 8.5 | 30.57 | 24.9 | 87.3 | 49.4 | 15.5 | 31.31 |
| Fe (µg/L) | 0.9 | 32.8 | 10.8 | 9.7 | 90.2 | 1.1 | 32.0 | 10.0 | 7.4 | 74.1 |
| Li (µg/L) | 6.1 | 18.7 | 11.5 | 4.1 | 36.2 | 3.9 | 52.9 | 16.6 | 12.6 | 75.7 |
| Se (µg/L) | 0.0 | 16.2 | 7.8 | 4.1 | 52.1 | 2.4 | 17.6 | 9.8 | 4.0 | 41.0 |
| SiO2 (mg/L) | 0.01 | 7.47 | 2.42 | 2.44 | 100.83 | 0.01 | 4.77 | 0.64 | 1.01 | 157.82 |
| T | EC | DO | NO3-N | As | ||||
|---|---|---|---|---|---|---|---|---|
| NR | Group A | 17.88 a | 684.11 a | 9.25 a | 0.59 a | 12.74 a | ||
| Group B | 1.10 b | 967.33 b | 13.97 b | 1.29 b | 8.00 b | |||
| T | ORP | Cl− | SO42− | Na | Mg | B | ||
| RR | Group A | 20.80 a | 110.18 a | 88.50 a | 217.42 a | 65.85 a | 27.75 a | 0.08 a |
| Group B | 3.03 b | 165.79 b | 44.89 b | 97.62 b | 39.56 b | 15.53 b | 0.04 b |
| Groups | EC | K+ | Na+ | Ca2+ | Cl− | SO42− | HCO3− | NH3-N | NO2-N | NO3-N | SiO2 | B | Li | Sr | Ba |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | 755 a | 3.47 a | 59.00 | 9.55 a | 60.46 a | 80.99 a | 325 a | 0.26 a | 0.01 a | 0.77 a | 2.42 a | 0.10 a | 11.45 b | 185.20 a | 27.68 a |
| Group B | 1002 b | 5.96 a | 58.58 | 34.06 b | 69.15 a | 256.88 b | 244 b | 0.27 a | 0.01 a | 0.75 a | 0.40 b | 0.08 ab | 33.26 a | 1461.55 ab | 41.28 b |
| Group C | 1141 b | 15.70 ab | 84.26 a | 52.00 ab | 116.45 b | 256.97 b | 290 b | 0.69 b | 0.21 b | 9.21 b | 0.82 b | 0.07 ab | 11.40 b | 601.15 b | 44.20 b |
| Group D | 806 | 9.09 b | 53.38 b | 24.67 abc | 67.90 a | 135.39 ab | 208 ab | 0.33 a | 0.03 a | 1.07 a | 0.71 b | 0.06 b | 9.63 b | 368.77 abc | 54.71 ab |
| Parameters | Group A | Group B | Group C | Group D | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| pH | −0.59 | 0.33 | 0.25 | −0.02 | 0.13 | 0.38 | 0.50 | 0.58 | 0.42 | −0.17 | 0.26 | 0.08 | 0.62 | 0.94 | 0.20 | −0.29 | −0.05 | 0.15 | 0.30 | 0.19 | −0.09 | 0.23 |
| T | −0.89 | 0.12 | 0.20 | 0.12 | −0.33 | −0.03 | −0.03 | 0.59 | −0.45 | 0.15 | 0.17 | −0.35 | 0.52 | 0.97 | 0.07 | 0.22 | 0.50 | −0.30 | 0.52 | −0.40 | 0.04 | 0.26 |
| EC | 0.88 | 0.05 | −0.35 | −0.04 | 0.09 | 0.05 | −0.08 | 0.19 | −0.83 | −0.28 | 0.33 | 0.24 | 0.15 | 0.25 | 0.48 | 0.48 | 0.01 | 0.65 | 0.06 | 0.06 | 0.42 | 0.31 |
| DO | 0.82 | −0.03 | −0.05 | −0.15 | 0.41 | 0.07 | 0.17 | −0.10 | −0.26 | 0.87 | 0.13 | 0.31 | 0.18 | 0.81 | 0.26 | 0.53 | −0.08 | −0.11 | 0.19 | 0.85 | −0.14 | −0.16 |
| ORP | 0.21 | 0.20 | −0.45 | 0.43 | 0.68 | 0.02 | 0.11 | −0.28 | 0.82 | 0.10 | 0.11 | 0.33 | 0.32 | −0.36 | −0.92 | 0.15 | −0.14 | −0.20 | −0.03 | 0.69 | 0.14 | −0.63 |
| NH3-N | −0.27 | −0.01 | −0.02 | 0.77 | 0.01 | 0.43 | 0.26 | 0.32 | 0.64 | −0.17 | 0.01 | −0.29 | 0.60 | 0.37 | 0.07 | 0.93 | −0.24 | 0.12 | −0.19 | 0.87 | 0.09 | 0.22 |
| NO2-N | 0.78 | −0.25 | 0.13 | 0.00 | −0.21 | −0.34 | 0.01 | −0.58 | −0.14 | −0.09 | −0.34 | −0.56 | −0.12 | −0.14 | 0.99 | −0.08 | 0.05 | −0.05 | 0.11 | −0.11 | 0.88 | −0.04 |
| NO3-N | 0.96 | 0.14 | 0.13 | −0.04 | 0.08 | −0.08 | −0.16 | 0.27 | −0.18 | 0.10 | 0.85 | 0.16 | 0.03 | −0.24 | −0.68 | −0.69 | 0.16 | 0.28 | −0.01 | −0.09 | 0.71 | −0.09 |
| F− | −0.21 | 0.09 | 0.09 | −0.14 | 0.03 | 0.94 | −0.14 | 0.11 | −0.10 | 0.80 | 0.49 | −0.20 | 0.23 | 0.13 | 0.01 | 0.51 | 0.40 | −0.18 | 0.57 | |||
| Cl− | −0.28 | 0.02 | 0.90 | −0.07 | −0.18 | −0.01 | −0.06 | 0.13 | 0.26 | 0.01 | 0.95 | −0.03 | 0.09 | 0.28 | −0.89 | −0.36 | 0.29 | 0.17 | 0.84 | 0.05 | 0.29 | 0.05 |
| SO42− | −0.19 | 0.06 | 0.90 | −0.10 | −0.27 | −0.12 | −0.10 | 0.04 | 0.12 | 0.26 | 0.94 | 0.09 | −0.03 | 0.06 | −0.45 | −0.89 | 0.26 | 0.21 | 0.88 | −0.19 | 0.08 | 0.13 |
| HCO3− | 0.11 | −0.07 | −0.09 | −0.06 | 0.97 | 0.04 | −0.11 | −0.01 | 0.48 | 0.54 | 0.32 | 0.47 | 0.34 | 0.98 | 0.07 | −0.18 | 0.08 | −0.39 | −0.10 | 0.09 | 0.12 | −0.73 |
| K+ | 0.25 | 0.89 | 0.15 | 0.06 | −0.04 | −0.04 | 0.09 | 0.86 | 0.20 | −0.01 | −0.19 | 0.04 | 0.43 | −0.24 | 0.96 | 0.11 | 0.82 | 0.30 | 0.30 | 0.06 | 0.30 | −0.07 |
| Na+ | −0.10 | 0.92 | −0.17 | −0.11 | 0.05 | 0.02 | −0.05 | 0.97 | 0.04 | −0.22 | 0.10 | 0.08 | −0.01 | −0.01 | 1.00 | −0.09 | 0.86 | 0.21 | 0.15 | −0.12 | 0.21 | 0.32 |
| Ca2+ | 0.55 | 0.47 | 0.35 | 0.22 | 0.26 | 0.02 | 0.47 | −0.26 | −0.86 | 0.38 | −0.12 | 0.06 | −0.08 | 0.00 | −0.25 | 0.97 | 0.16 | 0.84 | 0.15 | −0.14 | −0.07 | 0.12 |
| Mg2+ | 0.04 | 0.66 | −0.57 | −0.20 | −0.19 | −0.36 | −0.18 | 0.95 | −0.14 | −0.24 | 0.14 | 0.06 | −0.08 | 0.80 | 0.22 | 0.57 | 0.82 | 0.19 | 0.08 | −0.46 | −0.23 | 0.03 |
| SiO2 | 0.14 | −0.19 | 0.85 | −0.14 | 0.18 | 0.32 | −0.02 | 0.33 | −0.06 | −0.71 | 0.34 | −0.25 | 0.38 | −0.87 | 0.10 | 0.49 | 0.39 | −0.54 | −0.24 | −0.10 | −0.17 | 0.63 |
| Al | −0.06 | −0.07 | −0.26 | 0.90 | 0.02 | −0.19 | −0.16 | 0.04 | 0.85 | 0.36 | 0.26 | 0.02 | 0.21 | 0.37 | −0.10 | −0.93 | 0.03 | −0.37 | 0.72 | 0.16 | −0.13 | −0.13 |
| B | −0.26 | 0.95 | 0.00 | −0.04 | 0.11 | 0.04 | −0.03 | 0.94 | 0.11 | −0.22 | 0.19 | 0.14 | −0.05 | 0.41 | −0.02 | 0.24 | 0.92 | −0.02 | 0.13 | −0.22 | −0.16 | 0.18 |
| Li | 0.18 | 0.82 | −0.19 | −0.07 | −0.09 | 0.35 | 0.16 | 0.90 | −0.36 | 0.08 | 0.08 | 0.06 | 0.21 | 0.62 | 0.77 | 0.16 | 0.72 | −0.19 | 0.29 | −0.13 | 0.05 | −0.03 |
| Se | 0.70 | 0.46 | −0.20 | −0.01 | −0.22 | −0.16 | −0.03 | −0.79 | −0.16 | −0.18 | −0.33 | 0.36 | −0.28 | 0.97 | −0.19 | 0.16 | −0.04 | 0.78 | −0.05 | 0.28 | 0.12 | 0.25 |
| As | −0.54 | 0.39 | −0.63 | −0.03 | 0.20 | 0.17 | 0.25 | 0.12 | −0.13 | 0.17 | 0.06 | 0.93 | −0.14 | −0.22 | −0.13 | 0.97 | 0.83 | −0.10 | −0.11 | 0.18 | 0.17 | −0.16 |
| Sr | 0.67 | 0.51 | 0.11 | 0.10 | −0.05 | −0.38 | 0.27 | −0.17 | −0.89 | 0.35 | −0.07 | 0.08 | 0.03 | 0.12 | −0.56 | 0.82 | 0.64 | 0.56 | 0.32 | −0.17 | 0.16 | 0.03 |
| Ba | −0.04 | −0.02 | −0.25 | −0.12 | −0.11 | −0.15 | 0.94 | 0.19 | −0.02 | −0.85 | −0.24 | −0.02 | 0.39 | 0.22 | 0.08 | −0.15 | −0.02 | −0.08 | −0.03 | −0.47 | −0.76 | 0.22 |
| Fe | 0.02 | −0.09 | 0.04 | 0.97 | 0.00 | −0.15 | −0.09 | −0.55 | 0.75 | −0.20 | 0.03 | 0.06 | −0.29 | 0.50 | 0.84 | −0.22 | ||||||
| Eigenvalues | 6.74 | 5.13 | 3.83 | 2.61 | 2.50 | 1.59 | 1.09 | 8.24 | 5.83 | 4.87 | 2.09 | 2.08 | 1.14 | 9.54 | 7.33 | 7.12 | 5.27 | 3.76 | 3.13 | 3.09 | 2.58 | 2.28 |
| Variance | 24.54 | 19.17 | 15.71 | 11.03 | 8.48 | 7.95 | 7.06 | 27.10 | 22.56 | 15.30 | 14.74 | 8.57 | 39.76 | 30.55 | 29.68 | 21.96 | 15.65 | 13.03 | 12.86 | 10.73 | 9.49 | |
| Cumulative | 24.54 | 43.71 | 59.41 | 70.44 | 78.92 | 86.87 | 93.93 | 27.10 | 49.67 | 64.96 | 79.70 | 88.43 | 97.01 | 39.76 | 70.32 | 100.00 | 21.96 | 37.61 | 50.65 | 63.50 | 74.23 | 83.72 |
| Rock | YD01 | YD02 | YD03 | YD04 | YD05 | YD06 | YD07 | YD08 | YD09 | YD10 |
|---|---|---|---|---|---|---|---|---|---|---|
| CaSO4 | −3.09 | −2.49 | −2.87 | −2.07 | −2.31 | −1.79 | −2.21 | −2.62 | −2.43 | −2.49 |
| CaCO3 | 0.72 | 0.99 | 1.00 | 1.19 | 0.78 | 0.66 | 0.43 | 1.31 | 1.40 | 0.95 |
| CaMg(CO3)2 | 1.62 | 2.30 | 2.16 | 2.42 | 1.23 | 0.80 | 0.47 | 2.40 | 2.67 | 1.64 |
| CaSO4·2H2O | −2.84 | −2.24 | −2.62 | −1.82 | −2.05 | −1.53 | −1.96 | −2.36 | −2.18 | −2.24 |
| NaCl | −7.55 | −6.83 | −7.28 | −7.06 | −7.80 | −6.59 | −7.31 | −7.85 | −7.65 | −7.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Ma, M.; Zheng, F.; Liu, L.; Zhao, N.; Li, X.; Yang, Y.; Guo, J. Spatio-Temporal Variation and Controlling Factors of Water Quality in Yongding River Replenished by Reclaimed Water in Beijing, North China. Water 2017, 9, 453. https://doi.org/10.3390/w9070453
Yu Y, Ma M, Zheng F, Liu L, Zhao N, Li X, Yang Y, Guo J. Spatio-Temporal Variation and Controlling Factors of Water Quality in Yongding River Replenished by Reclaimed Water in Beijing, North China. Water. 2017; 9(7):453. https://doi.org/10.3390/w9070453
Chicago/Turabian StyleYu, Yilei, Muyuan Ma, Fandong Zheng, Licai Liu, Nana Zhao, Xiaoxia Li, Yongmin Yang, and Jia Guo. 2017. "Spatio-Temporal Variation and Controlling Factors of Water Quality in Yongding River Replenished by Reclaimed Water in Beijing, North China" Water 9, no. 7: 453. https://doi.org/10.3390/w9070453




