Analysis of Forest Fragmentation and Connectivity Using Fractal Dimension and Succolarity
Abstract
1. Introduction
2. Materials and Methods
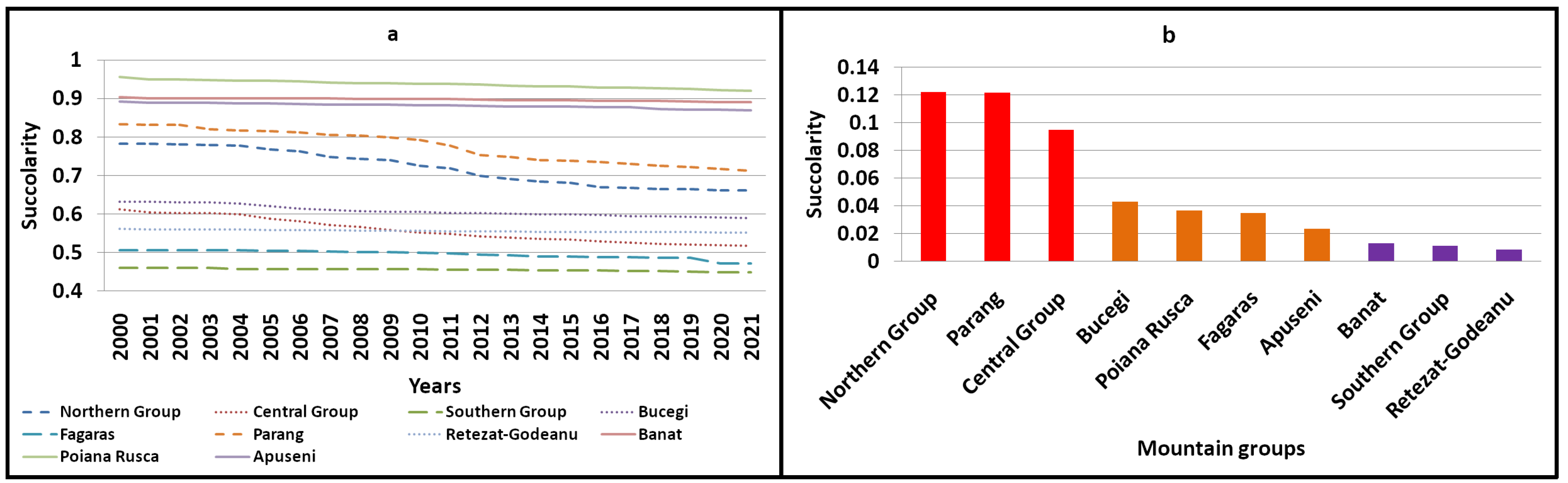
2.1. Succolarity
2.2. Potential Succolarity
2.3. Delta (Δ) Succolarity
2.4. Fractal Fragmentation Index (FFI)
- FFI = 0: This situation occurs when forest areas are very small, typically 1–4 pixels for an object. In such cases, the perimeter image using the outline function appears to be similar to the area image, with DA = DP. In practice, when analysing very small fractal objects or entities, it is important to recognise the limitations associated with the accurate extraction of their contours. The accuracy of contour extraction can be compromised when dealing with diminutive fractal entities, potentially affecting the reliability and interpretability of the fractal fragmentation index (FFI) calculations for these specific entities. Careful consideration of these limitations is essential to ensure the meaningful interpretation of FFI results, particularly in cases involving small fractal structures.
- 0 < FFI < 1: In scenarios where the areas occupied by objects are large and compact, the FFI value is high and close to 1, indicating that the objects are densely packed. Conversely, if the objects are more scattered, smaller, or fewer in number, or if they have a tentacular, irregular, and sprawling pattern, the FFI value decreases and approaches 0.
- FFI = 1: This situation is recorded when the area is geometrically perfect and 100% compact, without any discontinuity (DA = 2 and DP = 1) [54].
2.5. Fractal Fragmentation and Disorder Index (FFDI)
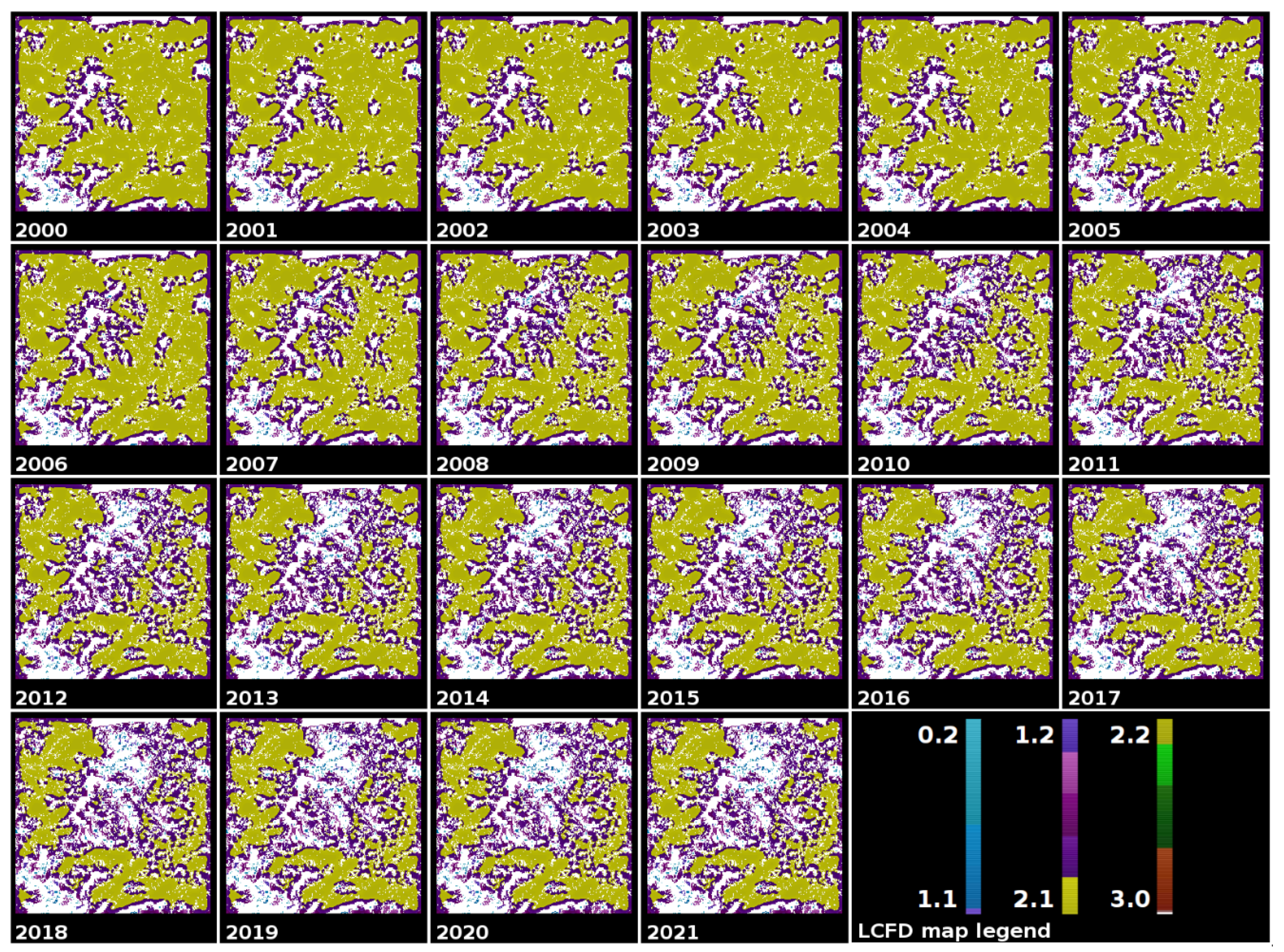
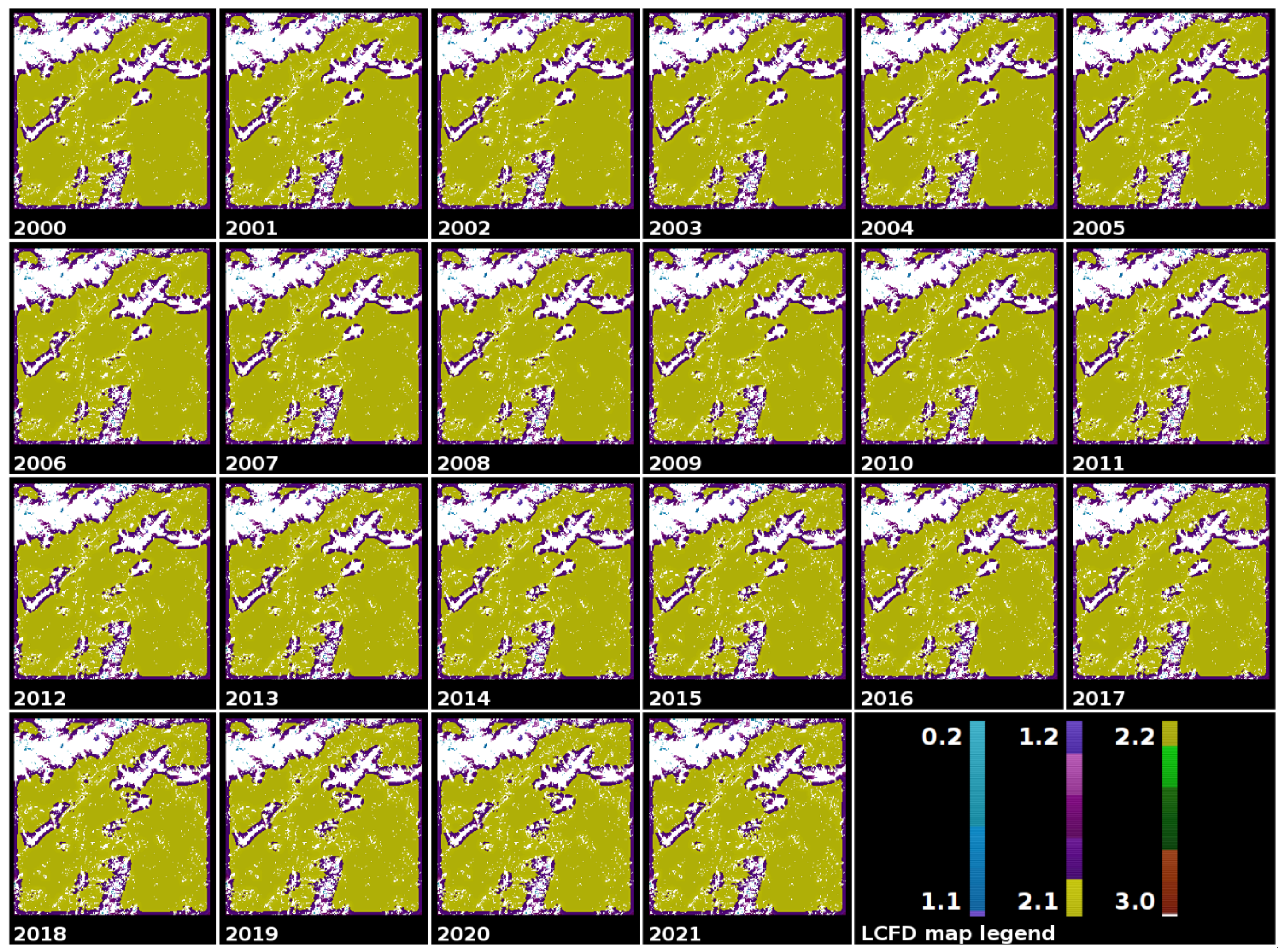
2.6. Local Connected Fractal Dimension (LCFD)
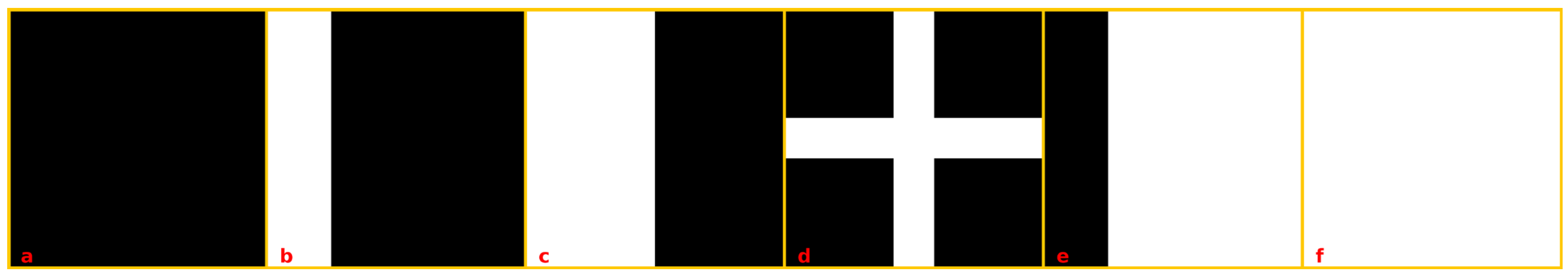
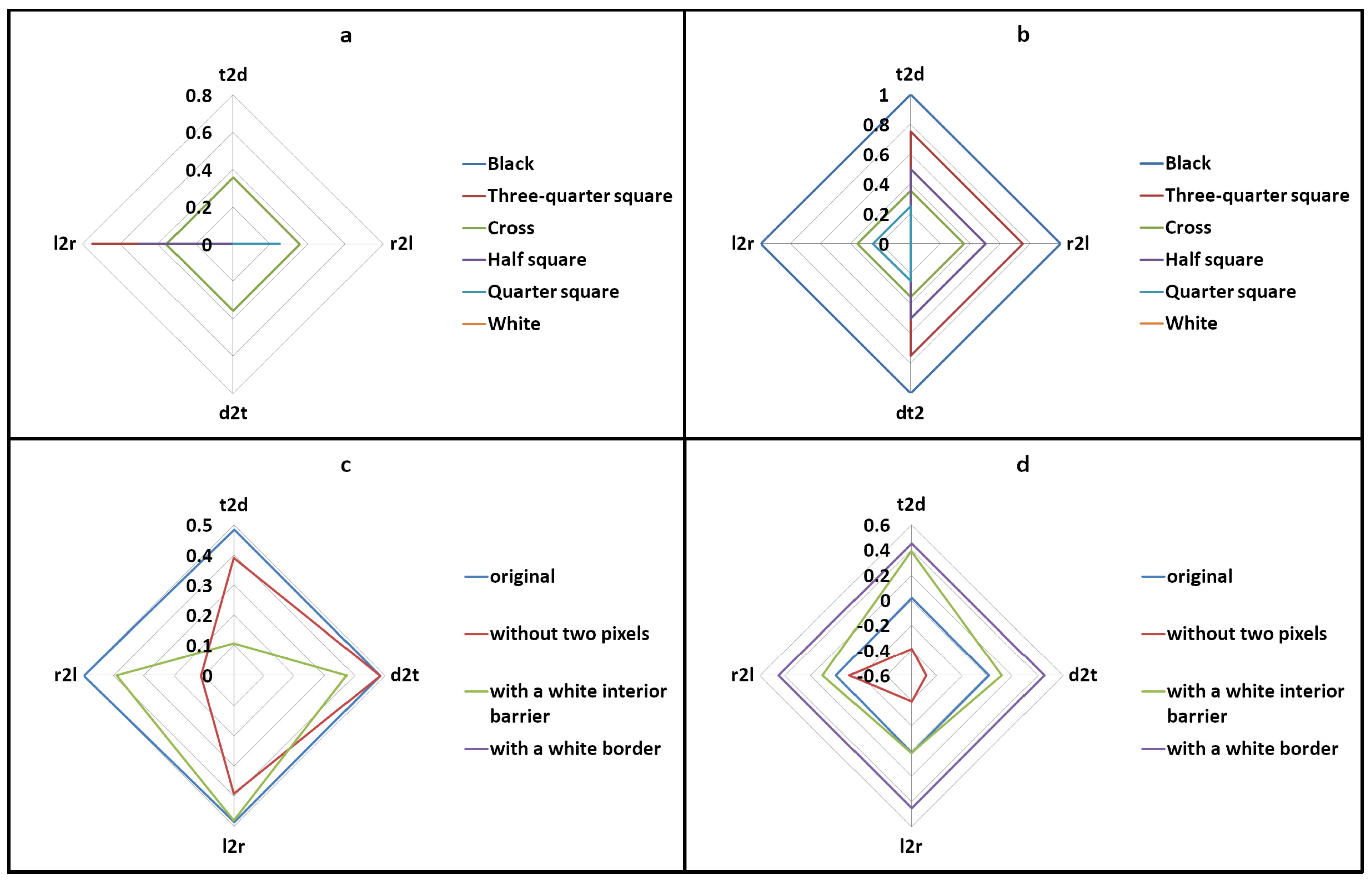
2.7. Image Generation for Testing and Validating Succolarity
- -
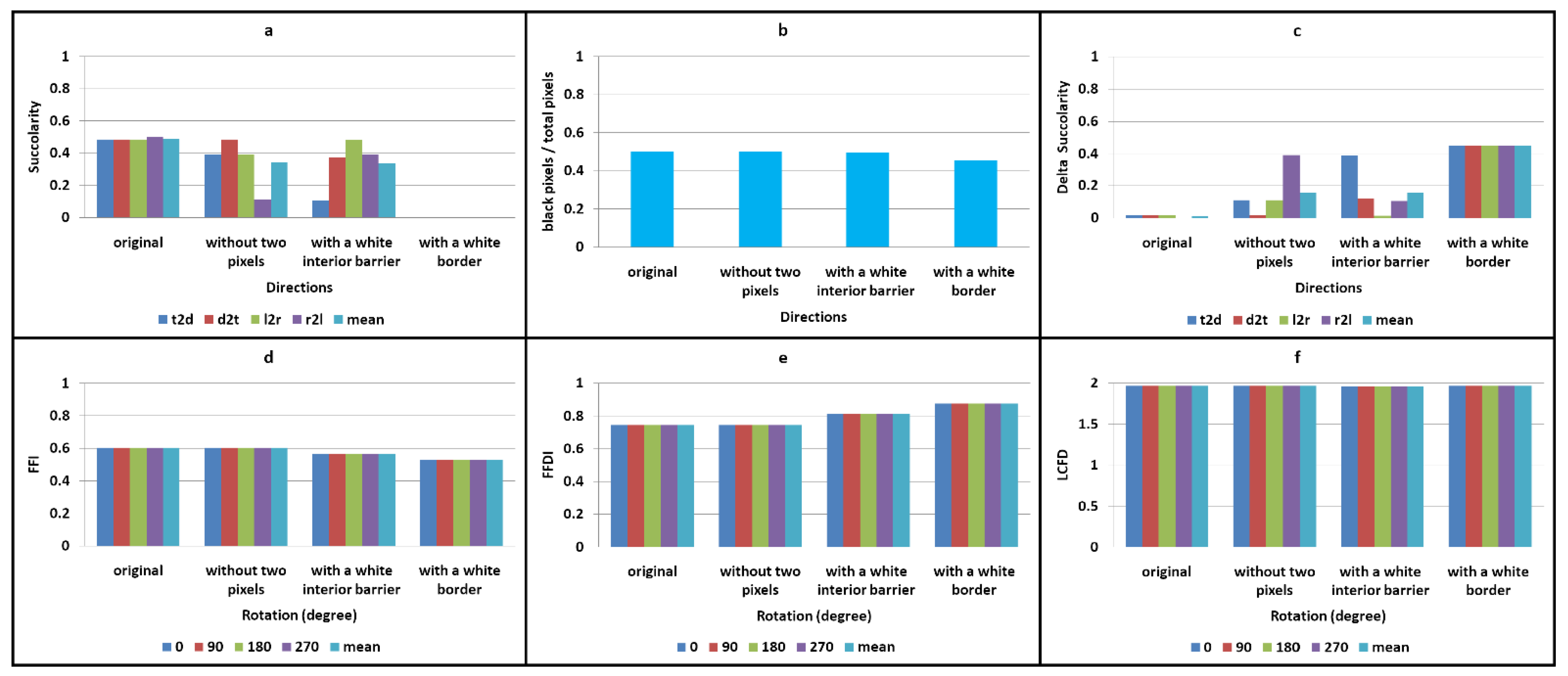
- The first edit included adding a horizontal white line, four pixels thick, in the initial optimum of the image (Figure 3g; for better visualisation, the line is shown in yellow in the figure). When a barrier is completely impenetrable, fluid flow stops, and the black pixels beyond the barrier are not measured. This is because the barrier acts as an impermeable layer, blocking the virtual liquid above. A new concept, Δ succolarity, was introduced to quantify these black pixels located beyond the barrier. Together with the quantifiable pixels located in front of the barrier, they formed the potential succolarity, another proposed concept. The aim of this approach was to measure the effect of a continuous obstacle on the flow of virtual water. The evaluation focused on the four directions of analysis (t2d, d2t, l2r, and r2l) (Figure 3h–k), with a particular emphasis on t2d and d2t (Figure 3h,i). Figure 3h–k depict the four percolations limited by the presence of the barrier, corresponding to the four analysed directions. To aid visualization, the black pixels located beyond the barrier and thus not quantified are represented in grey.
- -
- In the second edit, the HRM image was framed by a white border that was 12 pixels wide (Figure 3f). The purpose of this edit was to measure succolarity when the image was completely impermeable to water (Figure 3l). In the presence of a white border, which acts as a continuous external obstacle in all four directions, all black pixels that could potentially allow percolation are considered “inactive”. This is because the border prevents virtual liquid from penetrating the image. In this scenario, these “inactive” black pixels represent both the potential and Δ succolarity, as their succolarity is equal to 0. These “inactive” black pixels are located beyond the image border (see Figure 3f) and are displayed in grey for clarity (see Figure 3l).
- -
- In the third edit, two black pixels that connected two patches in the original image were removed (as shown by the red line in Figure 3m and by the white in Figure 3n). Figure 3o–r show the four percolations corresponding to the four analysed directions. Two black pixels were removed, creating a small white barrier between the two patches. It is evident that breaking a connection between two patches deactivates some black pixels beyond the rupture of that connection. These pixels no longer facilitate “flow” and become “inactive” due to the obstacle created. To improve visualization, grey pixels located beyond the connection rupture and, therefore, not quantified represent these black pixels. The aim was to identify changes in succolarity when interconnections became obstacles (Figure 3o–r).
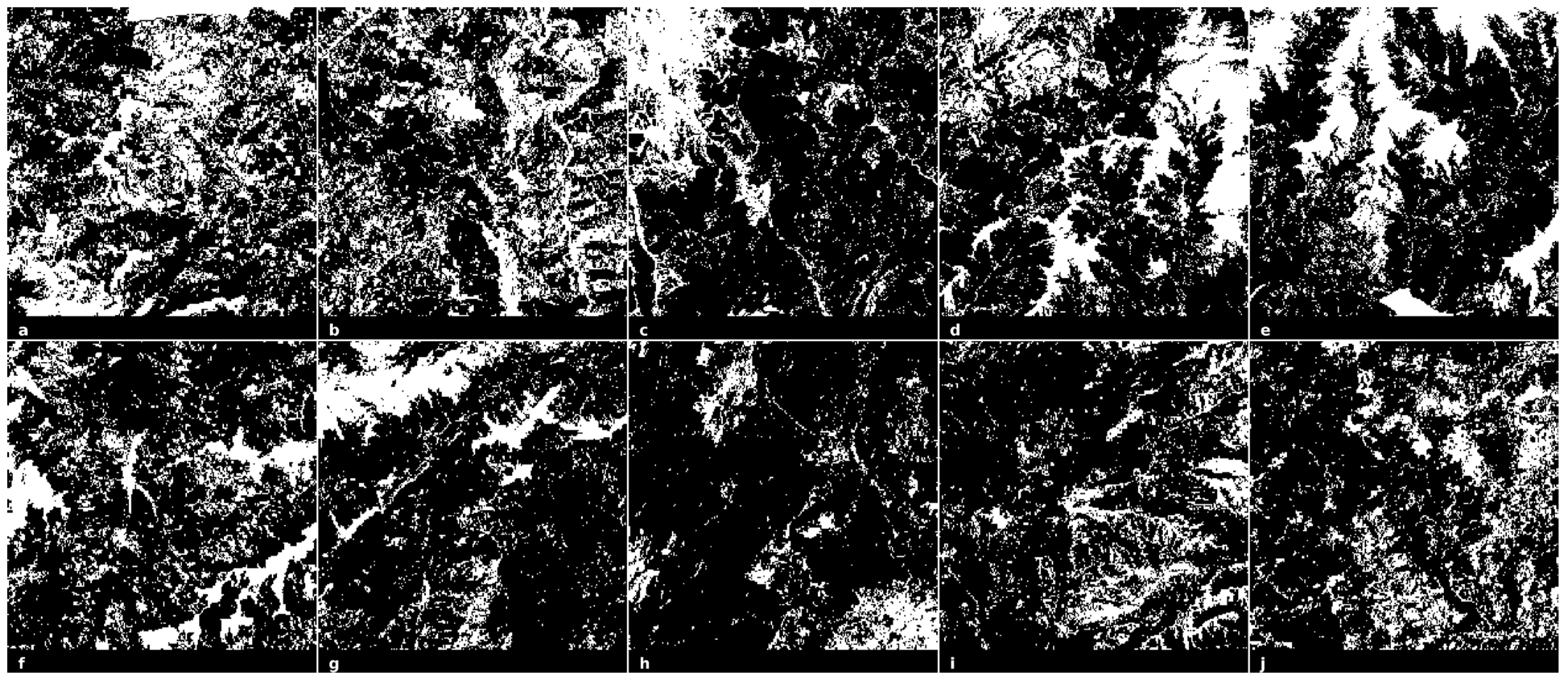
2.8. Tree Cover Images of the Romanian Carpathians Mountains
3. Results
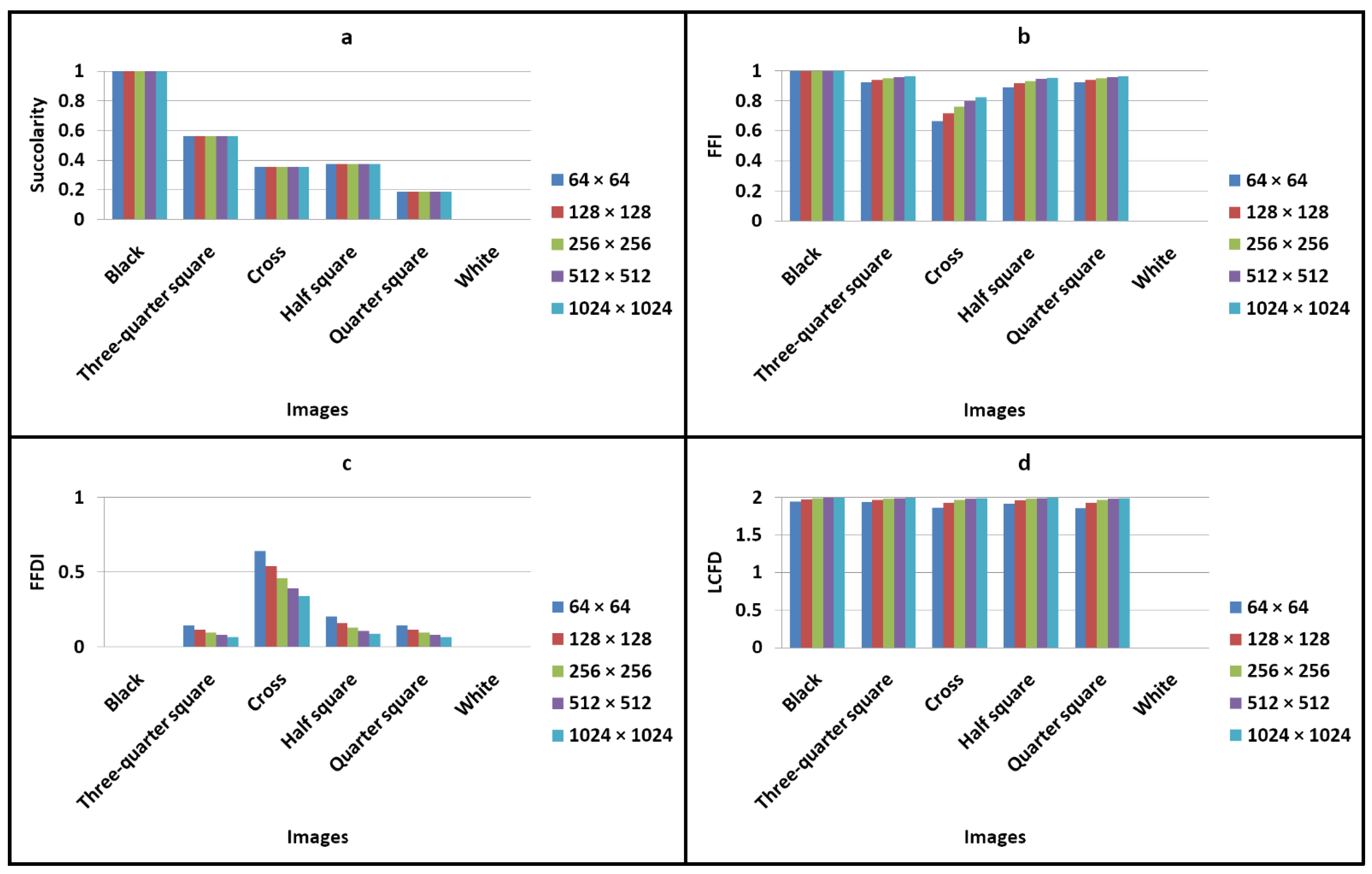
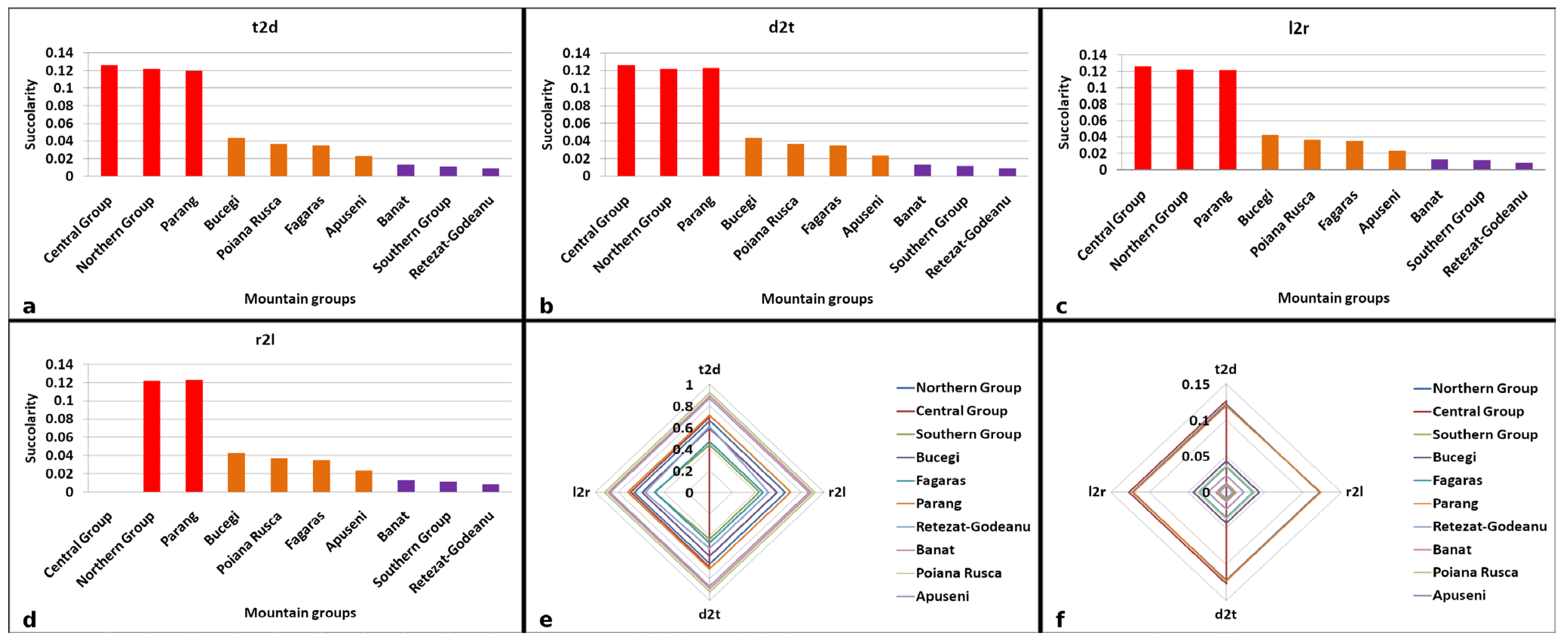
3.1. Invariance of Succolarity to Image Scale
3.2. Examination of Succolarity and FFI, FFDI, and LCFD Metrics for the Generated Images
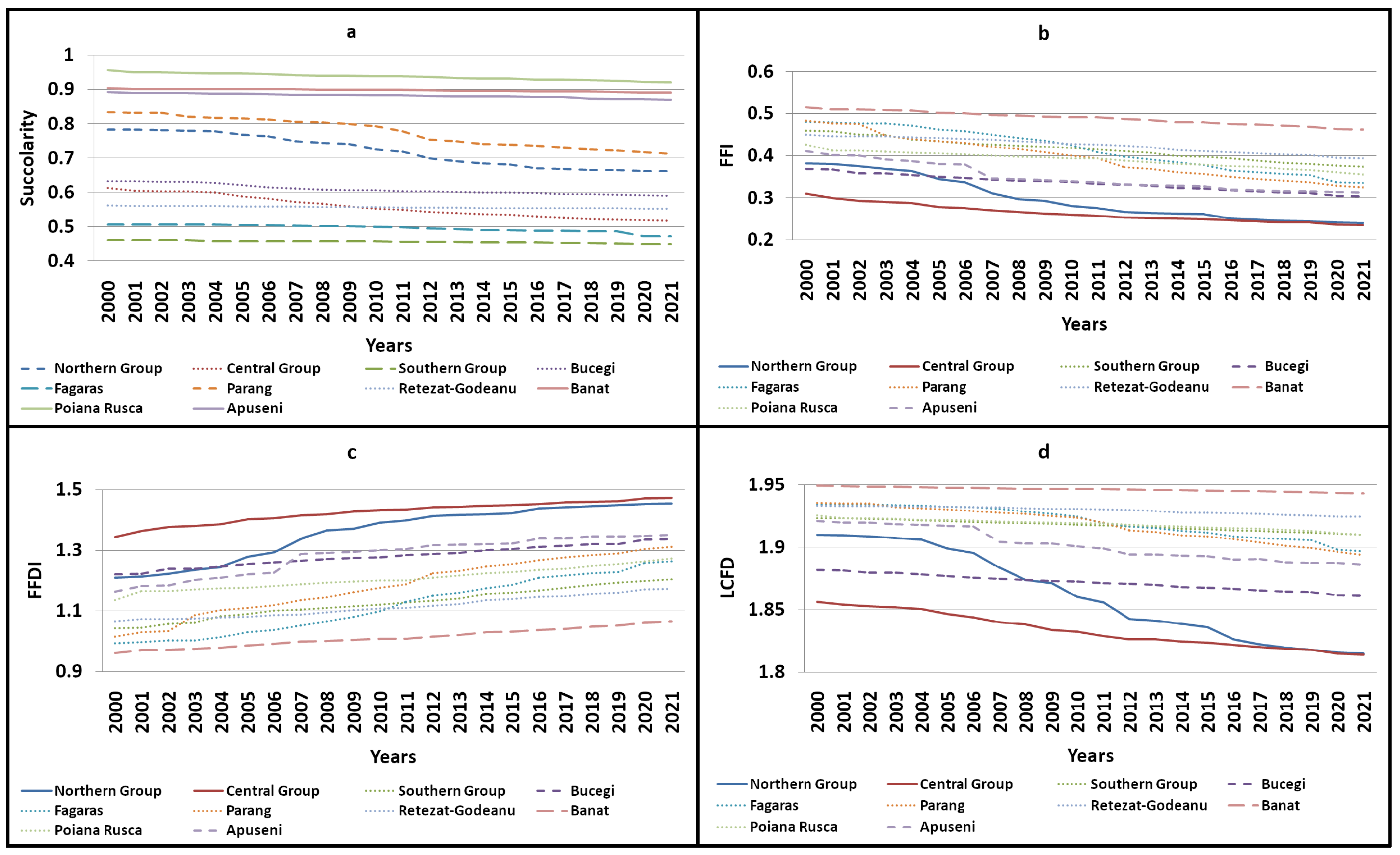
3.3. A Comprehensive Analysis of Forest Succolarity, Connectivity, and Fractality in the Romanian Carpathians
- -
- The Western Carpathians (the Banat, Poiana Ruscă, and Apuseni mountain groups) exhibited high compactness and connectivity with minimal deforestation, avoiding significant fragmentation or isolation of forest patches.
- -
- The Parang and northern mountain groups displayed moderate compactness and connectivity with high dynamicity. Deforestation in Parang resulted in the creation of isolated forest patches, while the northern group experienced extensive deforestation, leading to both continuous fragmentation and reduced connectivity. The Bucegi and Retezat mountain groups were identified as having moderate compactness and connectivity, with reduced dynamicity. Despite being the second-most impacted group by deforestation, the central group had low connectivity and compactness before 2001.
- -
- Low compactness and connectivity (reduced dynamicity) were observed in the Fagaras and southern mountain groups, with minimal deforestation affecting only a few connectivity areas.
4. Discussion
4.1. Succolarity and Fractal Indices
4.2. The Limits of Succolarity
4.3. Analysis of Succolarity and Fractal Index in a Forest Analysis
4.4. Future Work
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grebner, D.L.; Bettinger, P.; Siry, J.P.; Boston, K. Introduction to Forestry and Natural Resources, 2nd ed.; Academic Press: London, UK, 2022; ISBN 978-0-12-819076-0. [Google Scholar]
- Jackson, H.B.; Fahrig, L. Habitat Loss and Fragmentation. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2013; pp. 50–58. ISBN 978-0-12-384720-1. [Google Scholar]
- Dennis, R.L.H. A Decade of the Resource-Based Habitat Paradigm: The Semantics of Habitat Loss. In Encyclopedia of the Anthropocene; Elsevier: Amsterdam, The Netherlands, 2018; pp. 85–96. ISBN 978-0-12-813576-1. [Google Scholar]
- Rogan, J.E.; Lacher, T.E. Impacts of Habitat Loss and Fragmentation on Terrestrial Biodiversity. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018; p. B9780124095489109133. ISBN 978-0-12-409548-9. [Google Scholar]
- FAO (Ed.) Forest, Biodiversity and People; State of the World’s Forests; FAO: Rome, Italy, 2020; ISBN 978-92-5-132419-6. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Girona, M.M.; Aakala, T.; Aquilué, N.; Bélisle, A.-C.; Chaste, E.; Danneyrolles, V.; Díaz-Yáñez, O.; D’Orangeville, L.; Grosbois, G.; Hester, A.; et al. Challenges for the Sustainable Management of the Boreal Forest Under Climate Change. In Boreal Forests in the Face of Climate Change; Girona, M.M., Morin, H., Gauthier, S., Bergeron, Y., Eds.; Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2023; Volume 74, pp. 773–837. ISBN 978-3-031-15987-9. [Google Scholar]
- Marsh, L.K. The Nature of Fragmentation. In Primates in Fragments; Marsh, L.K., Ed.; Springer: Boston, MA, USA, 2003; pp. 1–10. ISBN 978-1-4757-3772-1. [Google Scholar]
- Broadbent, E.N.; Asner, G.P.; Keller, M.; Knapp, D.E.; Oliveira, P.J.C.; Silva, J.N. Forest Fragmentation and Edge Effects from Deforestation and Selective Logging in the Brazilian Amazon. Biol. Conserv. 2008, 141, 1745–1757. [Google Scholar] [CrossRef]
- Jha, C.S.; Goparaju, L.; Tripathi, A.; Gharai, B.; Raghubanshi, A.S.; Singh, J.S. Forest Fragmentation and Its Impact on Species Diversity: An Analysis Using Remote Sensing and GIS. Biodivers. Conserv. 2005, 14, 1681–1698. [Google Scholar] [CrossRef]
- Liu, J.; Coomes, D.A.; Gibson, L.; Hu, G.; Liu, J.; Luo, Y.; Wu, C.; Yu, M. Forest Fragmentation in China and Its Effect on Biodiversity. Biol. Rev. 2019, 94, 1636–1657. [Google Scholar] [CrossRef]
- Quinn, J.F.; Harrison, S.P. Effects of Habitat Fragmentation and Isolation on Species Richness: Evidence from Biogeographic Patterns. Oecologia 1988, 75, 132–140. [Google Scholar] [CrossRef]
- Alerstam, T.; Hedenström, A.; Åkesson, S. Long-distance Migration: Evolution and Determinants. Oikos 2003, 103, 247–260. [Google Scholar] [CrossRef]
- Laurance, W.F. Habitat Destruction: Death by a Thousand Cuts. In Conservation Biology for All; Sodhi, N.S., Ehrlich, P.R., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 73–87. ISBN 978-0-19-955423-2. [Google Scholar]
- Hanski, I. Habitat Loss, the Dynamics of Biodiversity, and a Perspective on Conservation. AMBIO 2011, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, J.; Garzon-Lopez, C.X.; Yeager, L.; Fortunel, C.; Cordeiro, N.J.; Beckman, N.G. The Effects of Habitat Loss and Fragmentation on Plant Functional Traits and Functional Diversity: What Do We Know so Far? Oecologia 2019, 191, 505–518. [Google Scholar] [CrossRef]
- Harris, L.D.; Silva-Lopez, G. Forest Fragmentation and the Conservation of Biological Diversity. In Conservation Biology; Fiedler, P.L., Jain, S.K., Eds.; Springer: Boston, MA, USA, 1992; pp. 197–237. ISBN 978-1-4684-6428-3. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat Fragmentation and Its Lasting Impact on Earth’s Ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Mortelliti, A.; Amori, G.; Boitani, L. The Role of Habitat Quality in Fragmented Landscapes: A Conceptual Overview and Prospectus for Future Research. Oecologia 2010, 163, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Chen, X.-Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jiang, L.; et al. Habitat Fragmentation and Biodiversity Conservation: Key Findings and Future Challenges. Landsc. Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Rudnick, D.; Beier, P.; Cushman, S.; Dieffenbach, F.; Epps, C.W.; Gerber, L.; Hartter, J.; Jenness, J.; Kintsch, J.; Merenlender, A.M.; et al. The Role of Landscape Connectivity in Planning and Implementing Conservation and Restoration Priorities; Issues in Ecology; Ecological Society of America: Washington, DC, USA, 2012; Volume 16. [Google Scholar]
- Zanella, L.; Borém, R.A.T.; Souza, C.G.; Alves, H.M.R.; Borém, F.M. Atlantic Forest Fragmentation Analysis and Landscape Restoration Management Scenarios. Nat. Conserv. 2012, 10, 57–63. [Google Scholar] [CrossRef]
- Chen, S.; Wu, S.; Ma, M. Ecological Restoration Programs Reduced Forest Fragmentation by Stimulating Forest Expansion. Ecol. Indic. 2023, 154, 110855. [Google Scholar] [CrossRef]
- Krummel, J.R.; Gardner, R.H.; Sugihara, G.; O’Neill, R.V.; Coleman, P.R. Landscape Patterns in a Disturbed Environment. Oikos 1987, 48, 321. [Google Scholar] [CrossRef]
- Milne, B.T. Spatial Aggregation and Neutral Models in Fractal Landscapes. Am. Nat. 1992, 139, 32–57. [Google Scholar] [CrossRef]
- Gardner, R.H.; O’Neill, R.V. Pattern, Process, and Predictability: The Use of Neutral Models for Landscape Analysis. In Quantitative Methods in Landscape Ecology: The Analysis and Interpretation of Landscape Heterogeneity; Springer: New York, NY, USA, 1991; pp. 289–307. [Google Scholar]
- Plotnick, R.E.; Gardner, R.H.; O’Neill, R.V. Lacunarity Indices as Measures of Landscape Texture. Landsc. Ecol. 1993, 8, 201–211. [Google Scholar] [CrossRef]
- Zuidema, P.A.; Sayer, J.A.; Dijkman, W. Forest Fragmentation and Biodiversity: The Case for Intermediate-Sized Conservation Areas. Environ. Conserv. 1996, 23, 290–297. [Google Scholar] [CrossRef]
- With, K.A.; King, A.W. Extinction Thresholds for Species in Fractal Landscapes. Conserv. Biol. 1999, 13, 314–326. [Google Scholar] [CrossRef]
- Heilman, G.E.; Strittholt, J.R.; Slosser, N.C.; Dellasala, D.A. Forest Fragmentation of the Conterminous United States: Assessing Forest Intactness through Road Density and Spatial Characteristics. BioScience 2002, 52, 411. [Google Scholar] [CrossRef]
- Demetriou, D.; Stillwell, J.; See, L. A New Methodology for Measuring Land Fragmentation. Comput. Environ. Urban Syst. 2013, 39, 71–80. [Google Scholar] [CrossRef]
- Li, B.-L.; Archer, S. Weighted Mean Patch Size: A Robust Index for Quantifying Landscape Structure. Ecol. Model. 1997, 102, 353–361. [Google Scholar] [CrossRef]
- Arellano-Rivas, A.; De-Nova, J.A.; Munguía-Rosas, M.A. Patch Isolation and Shape Predict Plant Functional Diversity in a Naturally Fragmented Forest. J. Plant Ecol. 2016, 11, rtw119. [Google Scholar] [CrossRef]
- Hein, S.; Pfenning, B.; Hovestadt, T.; Poethke, H.-J. Patch Density, Movement Pattern, and Realised Dispersal Distances in a Patch-Matrix Landscape—A Simulation Study. Ecol. Model. 2004, 174, 411–420. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Shi, H.; Han, L. Effect of Forest Connectivity on the Dispersal of Species: A Case Study in the Bogda World Natural Heritage Site, Xinjiang, China. Ecol. Indic. 2021, 125, 107576. [Google Scholar] [CrossRef]
- Kindlmann, P.; Burel, F. Connectivity Measures: A Review. Landsc. Ecol. 2008, 23, 879–890. [Google Scholar] [CrossRef]
- Keeley, A.T.H.; Beier, P.; Jenness, J.S. Connectivity Metrics for Conservation Planning and Monitoring. Biol. Conserv. 2021, 255, 109008. [Google Scholar] [CrossRef]
- Rivas, C.A.; Guerrero-Casado, J.; Navarro-Cerrillo, R.M. A New Combined Index to Assess the Fragmentation Status of a Forest Patch Based on Its Size, Shape Complexity, and Isolation. Diversity 2022, 14, 896. [Google Scholar] [CrossRef]
- Peptenatu, D.; Andronache, I.; Ahammer, H.; Radulovic, M.; Costanza, J.K.; Jelinek, H.F.; Di Ieva, A.; Koyama, K.; Grecu, A.; Gruia, A.K.; et al. A New Fractal Index to Classify Forest Fragmentation and Disorder. Landsc. Ecol. 2023, 38, 1373–1393. [Google Scholar] [CrossRef]
- Ma, D.; Stoica, A.D.; Wang, X.-L. Power-Law Scaling and Fractal Nature of Medium-Range Order in Metallic Glasses. Nat. Mater. 2009, 8, 30–34. [Google Scholar] [CrossRef]
- Noss, R.F. Assessing and Monitoring Forest Biodiversity: A Suggested Framework and Indicators. For. Ecol. Manag. 1999, 115, 135–146. [Google Scholar] [CrossRef]
- Sarkar, N.; Chaudhuri, B.B. An Efficient Approach to Estimate Fractal Dimension of Textural Images. Pattern Recognit. 1992, 25, 1035–1041. [Google Scholar] [CrossRef]
- Sarkar, N.; Chaudhuri, B.B. An Efficient Differential Box-Counting Approach to Compute Fractal Dimension of Image. IEEE Trans. Syst. Man, Cybern. 1994, 24, 115–120. [Google Scholar] [CrossRef]
- Jin, X.C.; Ong, S.H.; Jayasooriah. A Practical Method for Estimating Fractal Dimension. Pattern Recognit. Lett. 1995, 16, 457–464. [Google Scholar] [CrossRef]
- Mayrhofer-Reinhartshuber, M.; Kainz, P.; Ahammer, H. Image Pyramids as a New Approach for the Determination of Fractal Dimensions. In Proceedings of the 2nd International Conference on Pattern Recognition Applications and Methods, Barcelona, Spain, 15–18 February 2013; pp. 239–243. [Google Scholar]
- Mayrhofer-Reinhartshuber, M.; Ahammer, H. Pyramidal Fractal Dimension for High Resolution Images. Chaos 2016, 26, 073109. [Google Scholar] [CrossRef]
- Allain, C.; Cloitre, M. Characterizing the Lacunarity of Random and Deterministic Fractal Sets. Phys. Rev. A 1991, 44, 3552–3558. [Google Scholar] [CrossRef]
- Sengupta, K.; Vinoy, K.J. A New Measure of Lacunarity for Generalized Fractals and Its Impact in the Electromagnetic Behavior of Koch Dipole Antennas. Fractals 2006, 14, 271–282. [Google Scholar] [CrossRef]
- Roy, A.; Perfect, E. Lacunarity Analyses of Multifractal and Natural Grayscale Patterns. Fractals 2014, 22, 1440003. [Google Scholar] [CrossRef]
- Peleg, S.; Naor, J.; Hartley, R.; Avnir, D. Multiple Resolution Texture Analysis and Classification. IEEE Trans. Pattern Anal. Mach. Intell. 1984, PAMI-6, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ahammer, H. Higuchi Dimension of Digital Images. PLoS ONE 2011, 6, e24796. [Google Scholar] [CrossRef]
- Ahammer, H.; Sabathiel, N.; Reiss, M.A. Is a Two-Dimensional Generalization of the Higuchi Algorithm Really Necessary? Chaos 2015, 25, 073104. [Google Scholar] [CrossRef]
- Voss, R.F.; Wyatt, J.C.Y. Multifractals and the Local Connected Fractal Dimension. In Applications of Fractals and Chaos; Crilly, A.J., Earnshaw, R.A., Jones, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 171–192. ISBN 978-3-642-78099-8. [Google Scholar]
- Andronache, I.C.; Ahammer, H.; Jelinek, H.F.; Peptenatu, D.; Ciobotaru, A.-M.; Draghici, C.C.; Pintilii, R.D.; Simion, A.G.; Teodorescu, C. Fractal Analysis for Studying the Evolution of Forests. Chaos Solitons Fractals 2016, 91, 310–318. [Google Scholar] [CrossRef]
- Ahammer, H.; DeVaney, T.T.J.; Tritthart, H.A. How Much Resolution Is Enough?: Influence of Downscaling the Pixel Resolution of Digital Images on the Generalised Dimensions. Phys. D Nonlinear Phenom. 2003, 181, 147–156. [Google Scholar] [CrossRef]
- Amigó, J.; Balogh, S.; Hernández, S. A Brief Review of Generalized Entropies. Entropy 2018, 20, 813. [Google Scholar] [CrossRef]
- Andronache, I.; Marin, M.; Fischer, R.; Ahammer, H.; Radulovic, M.; Ciobotaru, A.-M.; Jelinek, H.F.; Di Ieva, A.; Pintilii, R.-D.; Drăghici, C.-C.; et al. Dynamics of Forest Fragmentation and Connectivity Using Particle and Fractal Analysis. Sci. Rep. 2019, 9, 12228. [Google Scholar] [CrossRef] [PubMed]
- Ciobotaru, A.-M.; Andronache, I.; Ahammer, H.; Radulovic, M.; Peptenatu, D.; Pintilii, R.-D.; Drăghici, C.-C.; Marin, M.; Carboni, D.; Mariotti, G.; et al. Application of Fractal and Gray-Level Co-Occurrence Matrix Indices to Assess the Forest Dynamics in the Curvature Carpathians—Romania. Sustainability 2019, 11, 6927. [Google Scholar] [CrossRef]
- Ciobotaru, A.-M.; Andronache, I.; Ahammer, H.; Jelinek, H.F.; Radulovic, M.; Pintilii, R.-D.; Peptenatu, D.; Drăghici, C.-C.; Simion, A.-G.; Papuc, R.-M.; et al. Recent Deforestation Pattern Changes (2000–2017) in the Central Carpathians: A Gray-Level Co-Occurrence Matrix and Fractal Analysis Approach. Forests 2019, 10, 308. [Google Scholar] [CrossRef]
- Diaconu, D.; Andronache, I.; Pintilii; Bretcan, P.; Simion, A.G.; Draghici, C.-C.; Gruia, K.A.; Grecu, A.; Marin, M.; Peptenatu, D. Using Fractal Fragmentation and Compaction Index in Analysis of the Deforestation Process in Bucegi Mountains Group, Romania. Carpathian J. Earth Environ. Sci. 2019, 14, 431–438. [Google Scholar] [CrossRef]
- Drăghici, C.C.; Andronache, I.; Ahammer, H.; Peptenatu, D.; Pintilii, R.-D.; Ciobotaru, A.-M.; Simion, A.G.; Dobrea, R.C.; Diaconu, D.C.; Vișan, M.-C.; et al. Spatial Evolution of Forest Areas in the Northern Carpathian Mountains of Romania. Acta Montan. Slovaca 2017, 22, 95–106. [Google Scholar]
- Pintilii, R.-D.; Andronache, I.; Diaconu, D.C.; Dobrea, R.C.; Zeleňáková, M.; Fensholt, R.; Peptenatu, D.; Drăghici, C.-C.; Ciobotaru, A.-M. Using Fractal Analysis in Modeling the Dynamics of Forest Areas and Economic Impact Assessment: Maramures, County, Romania, as a Case Study. Forests 2017, 8, 25. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature, 3rd ed.; W. H. Freeman and Comp.: New York, NY, USA, 1983. [Google Scholar]
- de Melo, R.H.C.; Conci, A. Succolarity: Defining a Method to Calculate This Fractal Measure. In Proceedings of the 2008 15th International Conference on Systems, Signals and Image Processing, Bratislava, Slovakia, 25–28 June 2008; pp. 291–294. [Google Scholar]
- de Melo, R.H.C.; Conci, A. How Succolarity Could Be Used as Another Fractal Measure in Image Analysis. Telecommun. Syst. 2013, 52, 1643–1655. [Google Scholar] [CrossRef]
- Simion, A.G.; Andronache, I.; Ahammer, H.; Marin, M.; Loghin, V.; Nedelcu, I.D.; Popa, C.M.; Peptenatu, D.; Jelinek, H.F. Particularities of Forest Dynamics Using Higuchi Dimension. Parâng Mountains as a Case Study. Fractal Fract. 2021, 5, 96. [Google Scholar] [CrossRef]
- Volk, X.K.; Gattringer, J.P.; Otte, A.; Harvolk-Schöning, S. Connectivity Analysis as a Tool for Assessing Restoration Success. Landsc. Ecol. 2018, 33, 371–387. [Google Scholar] [CrossRef]
- Cotovelea, A.; Sofletea, N.; Ionescu, G.; Ionescu, O. Genetic Approaches for Romanian Brown Bear (Ursus Arctos) Conservation. Bull. Transilv. Univ. Bras. Ser. II For. Wood Ind. Agric. Food Eng. 2013, 6, 19–26. [Google Scholar]
- Gînscă, C.; Stoica, B.; Neruja, M.L. Managing Human-Bear Conflicts in Brașov and Harghita Counties, Romania. AES Bioflux 2021, 13, 21–25. [Google Scholar]
- Morales-González, A.; Ruiz-Villar, H.; Ordiz, A.; Penteriani, V. Large Carnivores Living alongside Humans: Brown Bears in Human-Modified Landscapes. Glob. Ecol. Conserv. 2020, 22, e00937. [Google Scholar] [CrossRef]
- Penteriani, V.; López-Bao, J.V.; Bettega, C.; Dalerum, F.; del Mar Delgado, M.; Jerina, K.; Kojola, I.; Krofel, M.; Ordiz, A. Consequences of Brown Bear Viewing Tourism: A Review. Biol. Conserv. 2017, 206, 169–180. [Google Scholar] [CrossRef]
- Stăncioiu, P.T.; Micu, I.; Stăncioiu, N.H.; Dănilă, G. Why Would Romanian Carnivores Be at a Crossroads? Bucov. For. 2019, 19, 159–167. [Google Scholar] [CrossRef][Green Version]
- Stăncioiu, P.T.; Dutcă, I.; Bălăcescu, M.C.; Ungurean, Ș.V. Coexistence with Bears in Romania: A Local Community Perspective. Sustainability 2019, 11, 7167. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- De Melo, R.H.C.; De A. Vieira, E.; Conci, A. Characterizing the Lacunarity of Objects and Image Sets and Its Use as a Technique for the Analysis of Textural Patterns. In Advanced Concepts for Intelligent Vision Systems; Blanc-Talon, J., Philips, W., Popescu, D., Scheunders, P., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4179, pp. 208–219. ISBN 978-3-540-44630-9. [Google Scholar]
- Ahammer, H.; Reiss, M.A.; Hackhofer, M.; Andronache, I.; Radulovic, M.; Labra-Spröhnle, F.; Jelinek, H.F. ComsystanJ: A Collection of Fiji/ImageJ2 Plugins for Nonlinear and Complexity Analysis in 1D, 2D and 3D. PLoS ONE 2023, 18, e0292217. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.L.; Gollub, J.P. Chaotic Dynamics: An Introduction, 2nd ed.; Cambridge University Press: Cambridge, UK, 1996; ISBN 978-0-521-47106-0. [Google Scholar]
- Landini, G.; Murray, P.I.; Misson, G.P. Local Connected Fractal Dimensions and Lacunarity Analyses of 60 Degrees Fluorescein Angiograms. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2749–2755. [Google Scholar]
- Karperien, A. FracLac for ImageJ 2013. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2094424 (accessed on 14 November 2023).
- Loke, L.H.L.; Chisholm, R.A. Measuring Habitat Complexity and Spatial Heterogeneity in Ecology. Ecol. Lett. 2022, 25, 2269–2288. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cai, J.; Perfect, E.; Wei, W.; Zhang, Q.; Meng, Q. Fractal Dimension, Lacunarity and Succolarity Analyses on CT Images of Reservoir Rocks for Permeability Prediction. J. Hydrol. 2019, 579, 124198. [Google Scholar] [CrossRef]
- Xia, Y.; Cai, J.; Wei, W. Fractal Structural Parameters from Images: Fractal Dimension, Lacunarity, and Succolarity. In Modelling of Flow and Transport in Fractal Porous Media; Elsevier: Amsterdam, The Netherlands, 2021; pp. 11–24. ISBN 978-0-12-817797-6. [Google Scholar]
- Liritzis, I.; Andronache, I.; Stevenson, C. A Novel Approach to Documenting Water Diffusion in Ancient Obsidian Artifacts via the Complexity Analysis of Microscope Images. J. Archaeol. Sci. 2024, 161, 105896. [Google Scholar] [CrossRef]
- Zou, S.; Xu, P.; Xie, C.; Deng, X.; Tang, H. Characterization of Two-Phase Flow from Pore-Scale Imaging Using Fractal Geometry under Water-Wet and Mixed-Wet Conditions. Energies 2022, 15, 2036. [Google Scholar] [CrossRef]
- Ayad, A.; Bakkali, S. Fractal Assessment of the Disturbances of Phosphate Series Using Lacunarity and Succolarity Analysis on Geoelectrical Images (Sidi Chennane, Morocco). Complexity 2019, 2019, 9404567. [Google Scholar] [CrossRef]
- Bian, H.; Xia, Y.; Lu, C.; Qin, X.; Meng, Q.; Lu, H. Pore Structure Fractal Characterization and Permeability Simulation of Natural Gas Hydrate Reservoir Based on CT Images. Geofluids 2020, 2020, 6934691. [Google Scholar] [CrossRef]
- N’Diaye, M.; Degeratu, C.; Bouler, J.-M.; Chappard, D. Biomaterial Porosity Determined by Fractal Dimensions, Succolarity and Lacunarity on Microcomputed Tomographic Images. Mater. Sci. Eng. C 2013, 33, 2025–2030. [Google Scholar] [CrossRef]
- Sangeetha, S.; Sujatha, C.M.; Manamalli, D. Characterization of Trabecular Architecture in Femur Bone Radiographs Using Succolarity. In Proceedings of the 2013 39th Annual Northeast Bioengineering Conference, Syracuse, NY, USA, 5–7 April 2013; pp. 225–226. [Google Scholar]
- Ichim, L.; Dobrescu, R. Characterization of Tumor Angiogenesis Using Fractal Measures. In Proceedings of the 2013 19th International Conference on Control Systems and Computer Science, Bucharest, Romania, 29–31 May 2013; pp. 345–349. [Google Scholar]
- Ţălu, Ş.; Abdolghaderi, S.; Pinto, E.P.; Matos, R.S.; Salerno, M. Advanced Fractal Analysis of Nanoscale Topography of Ag/DLC Composite Synthesized by RF-PECVD. Surf. Eng. 2020, 36, 713–719. [Google Scholar] [CrossRef]
- Mah, S.A.; Avci, R.; Du, P.; Vanderwinden, J.-M.; Cheng, L.K. Deciphering Stomach Myoelectrical Slow Wave Conduction Patterns via Confocal Imaging of Gastric Pacemaker Cells and Fractal Geometry. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 3514–3517. [Google Scholar]
- Landini, G.; Rippin, J.W. How important is tumour shape? Quantification of the epithelial-connective tissue interface in oral lesions using local connected fractal dimension analysis. J. Pathol. 1996, 179, 210–217. [Google Scholar] [CrossRef]
- Holmen, S.; Galappaththi-Arachchige, H.N.; Kleppa, E.; Pillay, P.; Naicker, T.; Taylor, M.; Onsrud, M.; Kjetland, E.F.; Albregtsen, F. Characteristics of Blood Vessels in Female Genital Schistosomiasis: Paving the Way for Objective Diagnostics at the Point of Care. PLoS Negl. Trop. Dis. 2016, 10, e0004628. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.C.; Laiwalla, A.N.; Liou, R.; Gonzalez, N.R. Angiographic Structural Differentiation between Native Arteriogenesis and Therapeutic Synangiosis in Intracranial Arterial Steno-Occlusive Disease. AJNR Am. J. Neuroradiol. 2016, 37, 1086–1091. [Google Scholar] [CrossRef]
- Sijilmassi, O.; López Alonso, J.-M.; Del Río Sevilla, A.; Barrio Asensio, M.D.C. Multifractal Analysis of Embryonic Eye Tissues from Female Mice with Folic Acid Deficiency. Part II: Local Connected Fractal Dimension Analysis. Chaos Solitons Fractals 2020, 138, 109887. [Google Scholar] [CrossRef]
- Swaid, B.; Bilotta, E.; Pantano, P.; Lucente, R. Thresholding Urban Connectivity by Local Connected Fractal Dimensions and Lacunarity Analyses. In Proceedings of the 13th European Conference on Artificial Life (ECAL 2015), York, UK, 20–24 July 2015. [Google Scholar]
- Urgilez-Clavijo, A.; Rivas-Tabares, D.A.; Martín-Sotoca, J.J.; Tarquis Alfonso, A.M. Local Fractal Connections to Characterize the Spatial Processes of Deforestation in the Ecuadorian Amazon. Entropy 2021, 23, 748. [Google Scholar] [CrossRef]
- Andronache, I.; Fensholt, R.; Ahammer, H.; Ciobotaru, A.-M.; Pintilii, R.-D.; Peptenatu, D.; Drăghici, C.-C.; Diaconu, D.C.; Radulović, M.; Pulighe, G.; et al. Assessment of Textural Differentiations in Forest Resources in Romania Using Fractal Analysis. Forests 2017, 8, 54. [Google Scholar] [CrossRef]
- Diaconu, D.C.; Papuc, R.M.; Peptenatu, D.; Andronache, I.; Marin, M.; Dobrea, R.C.; Drăghici, C.C.; Pintilii, R.-D.; Grecu, A. Use of Fractal Analysis in the Evaluation of Deforested Areas in Romania. In Advances in Forest Management under Global Change; Zhang, L., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Pintilii, R.-D.; Andronache, I.C.; Simion, A.-G.; Draghici, C.-C.; Peptenatu, D.; Ciobotaru, A.-M.; Dobrea, R.-C.; Papuc, R.-M. Determining Forest Fund Evolution by Fractal Analysis (Suceava-Romania). Urban. Archit. Constr. 2016, 7, 31–42. [Google Scholar]
- Fernández, N.; Selva, N.; Yuste, C.; Okarma, H.; Jakubiec, Z. Brown Bears at the Edge: Modeling Habitat Constrains at the Periphery of the Carpathian Population. Biol. Conserv. 2012, 153, 134–142. [Google Scholar] [CrossRef]
- Mikoláš, M.; Tejkal, M.; Kuemmerle, T.; Griffiths, P.; Svoboda, M.; Hlásny, T.; Leitão, P.J.; Morrissey, R.C. Forest Management Impacts on Capercaillie (Tetrao urogallus) Habitat Distribution and Connectivity in the Carpathians. Landsc. Ecol. 2017, 32, 163–179. [Google Scholar] [CrossRef]
- Iosif, R.; Pop, M.I.; Chiriac, S.; Sandu, R.M.; Berde, L.; Szabó, S.; Rozylowicz, L.; Popescu, V.D. Den Structure and Selection of Denning Habitat by Brown Bears in the Romanian Carpathians. Ursus 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Sin, T.; Gazzola, A.; Chiriac, S.; Rîșnoveanu, G. Wolf Diet and Prey Selection in the South-Eastern Carpathian Mountains, Romania. PLoS ONE 2019, 14, e0225424. [Google Scholar] [CrossRef] [PubMed]
- Stăncioiu, P.T.; Niță, M.D.; Fedorca, M. Capercaillie (Tetrao urogallus) Habitat in Romania—A Landscape Perspective Revealed by Cold War Spy Satellite Images. Sci. Total Environ. 2021, 781, 146763. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andronache, I. Analysis of Forest Fragmentation and Connectivity Using Fractal Dimension and Succolarity. Land 2024, 13, 138. https://doi.org/10.3390/land13020138
Andronache I. Analysis of Forest Fragmentation and Connectivity Using Fractal Dimension and Succolarity. Land. 2024; 13(2):138. https://doi.org/10.3390/land13020138
Chicago/Turabian StyleAndronache, Ion. 2024. "Analysis of Forest Fragmentation and Connectivity Using Fractal Dimension and Succolarity" Land 13, no. 2: 138. https://doi.org/10.3390/land13020138
APA StyleAndronache, I. (2024). Analysis of Forest Fragmentation and Connectivity Using Fractal Dimension and Succolarity. Land, 13(2), 138. https://doi.org/10.3390/land13020138






