Preparation of Knockout Extract by Immunoaffinity Column and Its Application
Abstract
:1. Introduction
2. Preparation of the Immunoaffinity Column against Ginsenoside Rb1 (G-Rb1), and the G-Rb1-KO Extract
2.1. Preparation of Anti-G-Rb1mAb
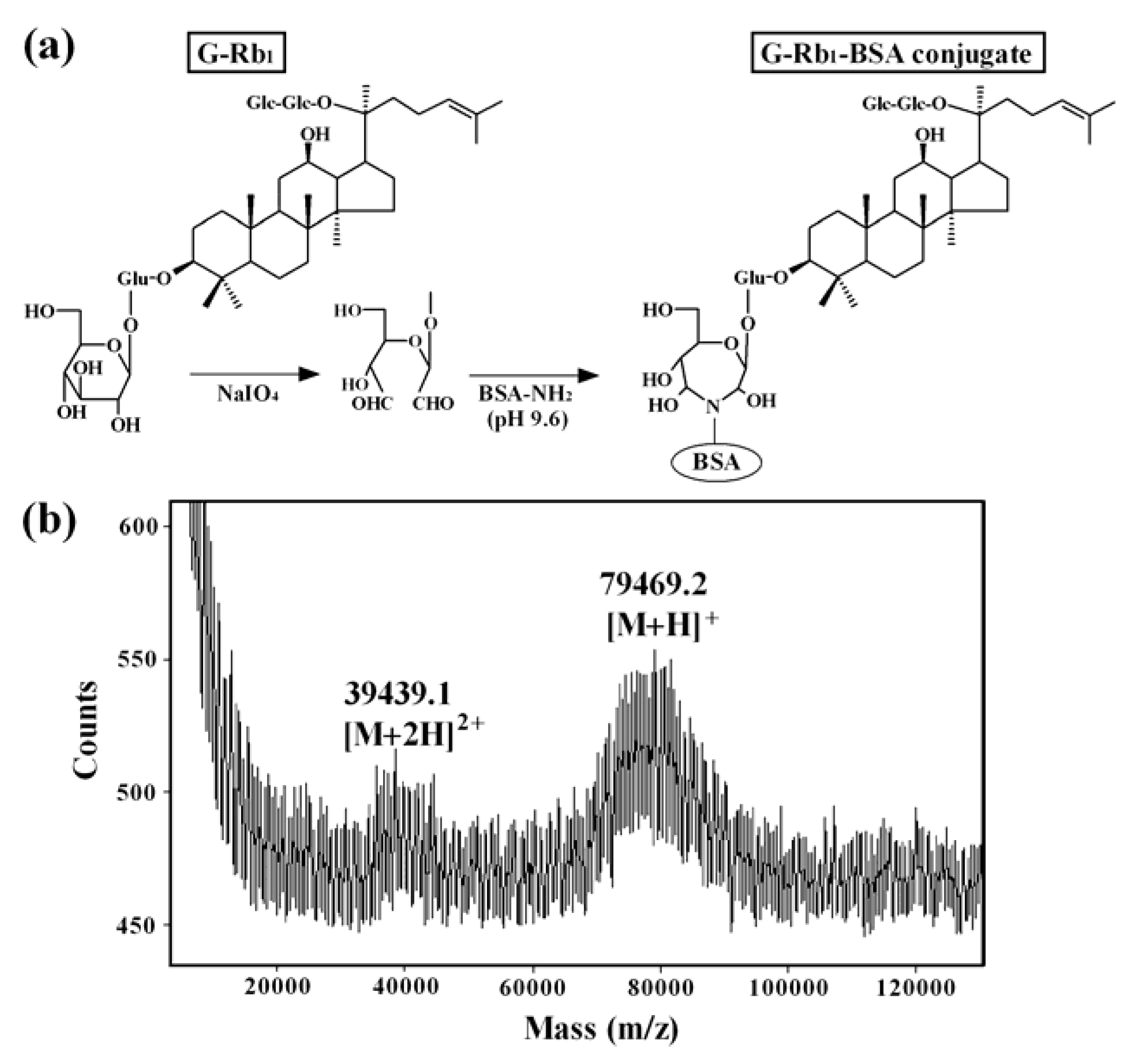
2.2. Preparation of the Anti-G-Rb1 mAb-Coupled Immunoaffinity Column

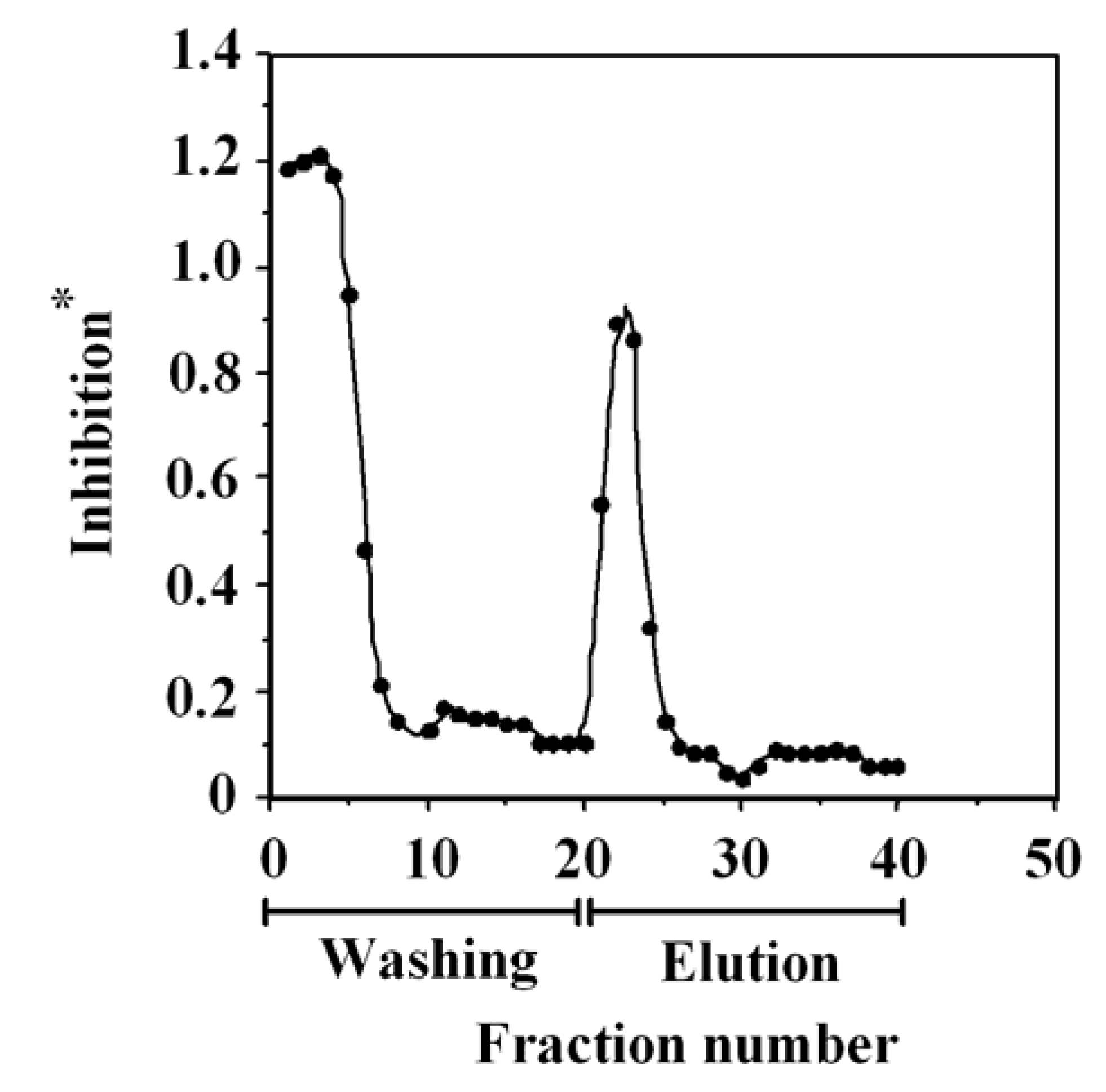
2.3. One-Step Purification of G-Rb1 from Ginseng Extract Using the Anti-G-Rb1 mAb-Coupled Immunoaffinity Column
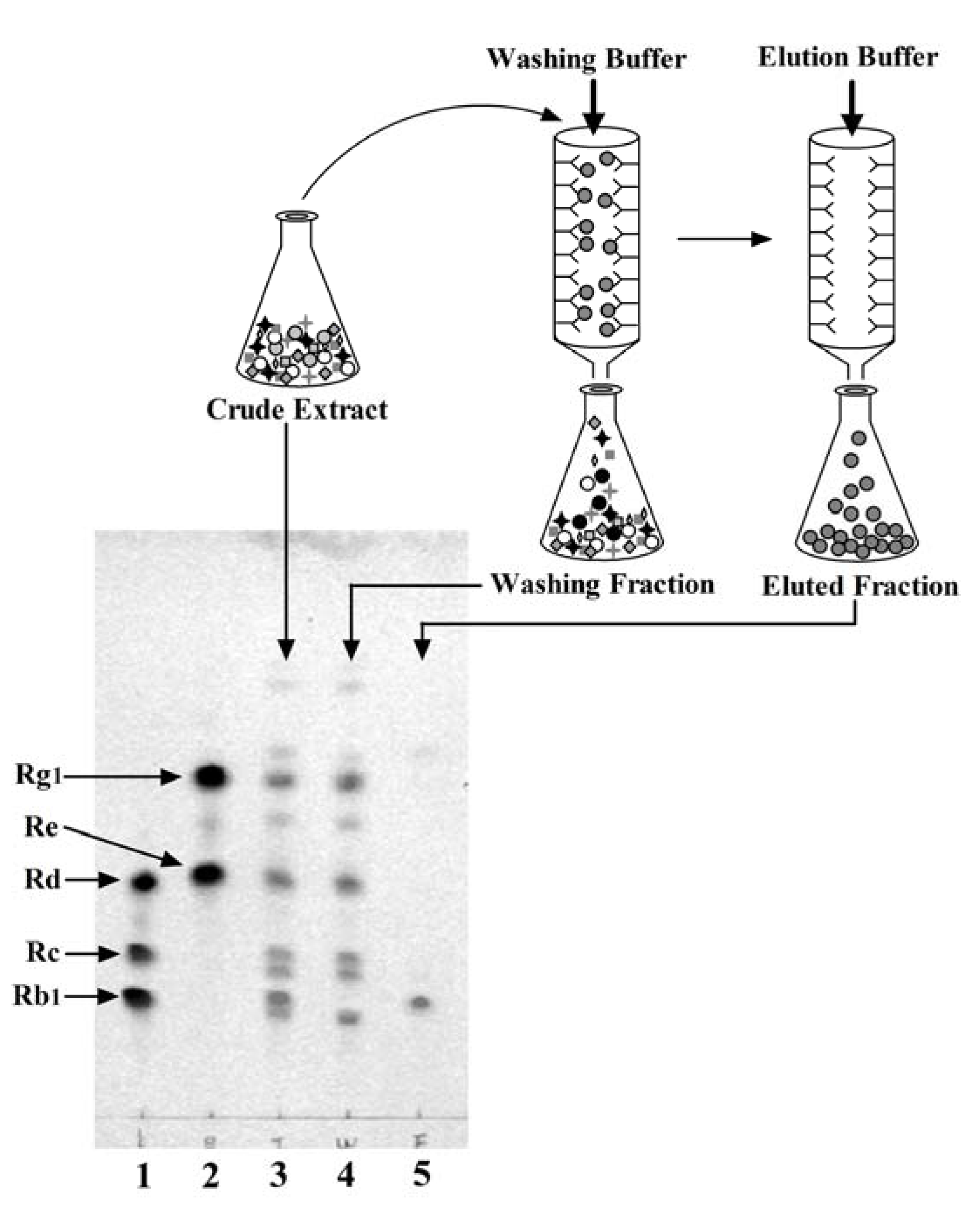
2.4. Preparation of the G-Rb1-KO Extract Using the Anti-G-Rb1 mAb-Coupled Immunoaffinity Column
3. Glycyrrhizin (GC)-KO Extract and its Application in Cell-Based Analysis
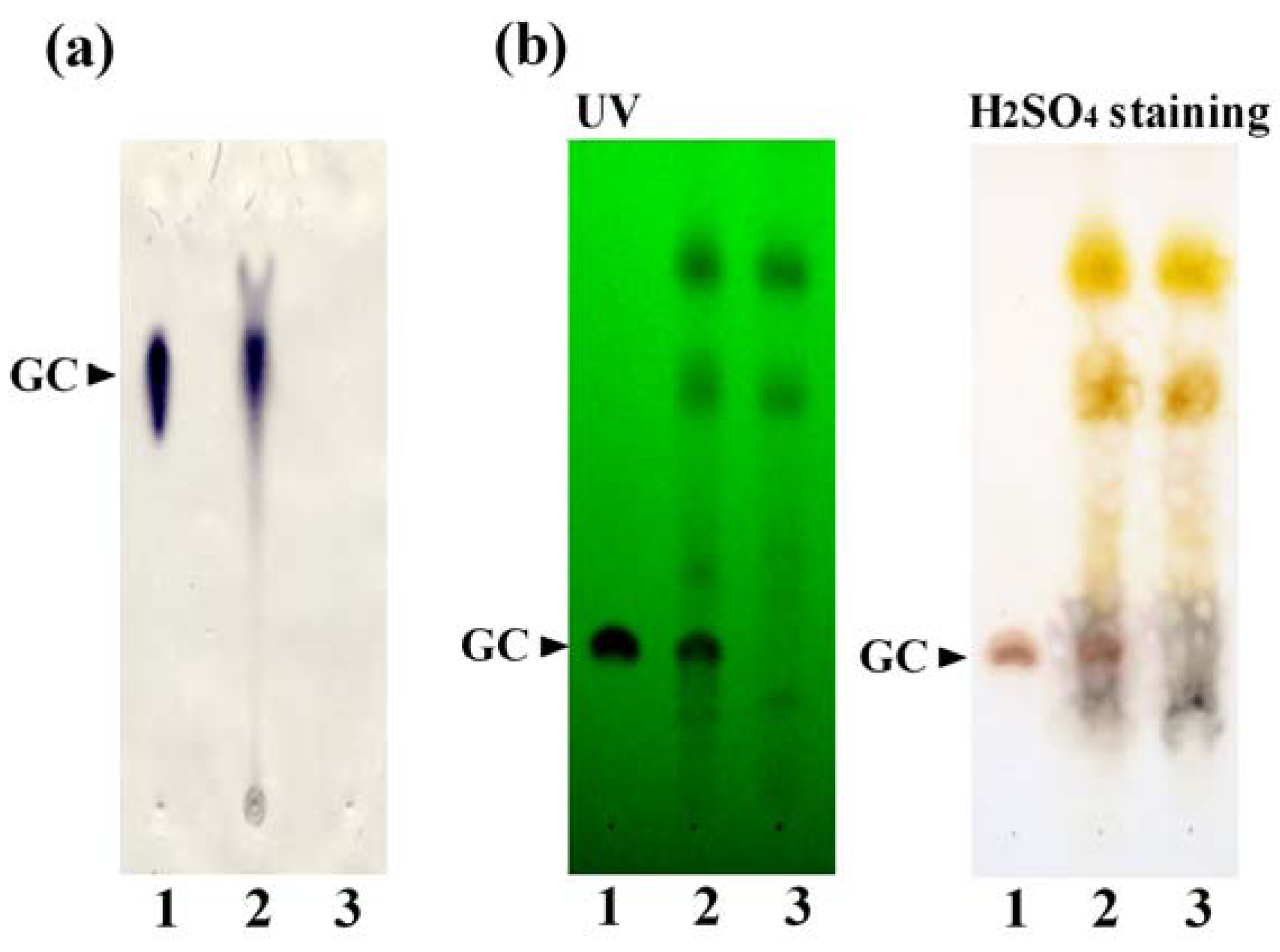
3.1. Preparation of the GC-KO Extract and its Characterization
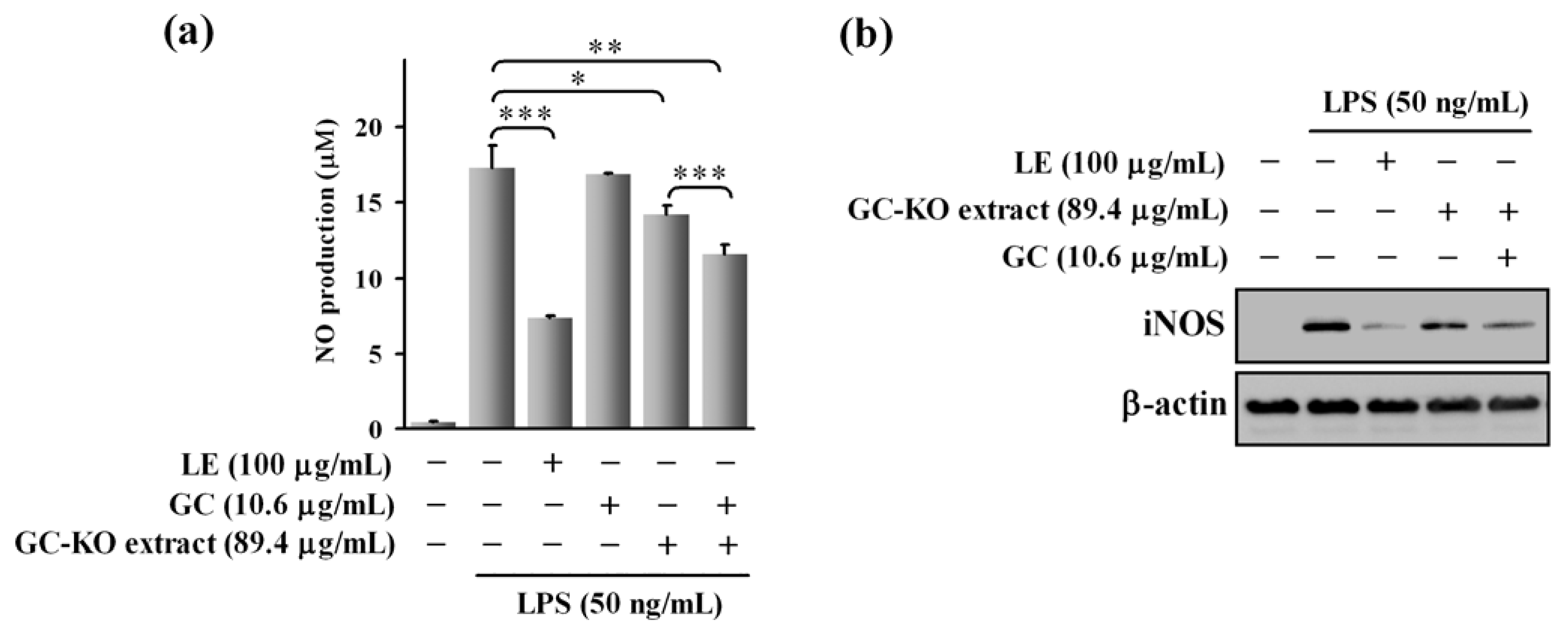
3.2. Cell-Based Assay Using the GC-KO Extract
4. Conclusions
Acknowledgments
References
- Sakata, R.; Shoyama, Y.; Murakami, H. Production of monoclonal antibodies and enzyme immunoassay for typical adenylate cyclase activator, Forskolin. Cytotechnology 1994, 16, 101–108. [Google Scholar]
- Xuan, L.; Tanaka, H.; Xu, Y.; Shoyama, Y. Preparation of monoclonal antibody against crocin and its characterization. Cytotechnology 1999, 29, 65–70. [Google Scholar]
- Lu, Z.; Morinaga, O.; Tanaka, H.; Shoyama, Y. A quantitative ELISA using monoclonal antibody to survey paeoniflorin and albiflorin in crude drugs and traditional Chinese herbal medicines. Biol. Pharm. Bull. 2003, 26, 862–866. [Google Scholar]
- Tanaka, H.; Fukuda, N.; Shoyama, Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rb1 and its characterization. Cytotechnology 1999, 29, 115–120. [Google Scholar]
- Fukuda, N.; Tanaka, H.; Shoyama, Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rg1 and its characterization. Monoclonal antibody for a ginseng saponin. Cytotechnology 2000, 34, 197–204. [Google Scholar]
- Zhu, S.; Shimokawa, S.; Tanaka, H.; Shoyama, Y. Development of an assay system for saikosaponin a using anti-saikosaponin a monoclonal antibodies. Biol. Pharm. Bull. 2004, 27, 66–71. [Google Scholar]
- Shan, S.J.; Tanaka, H.; Shoyama, Y. Enzyme-linked immunosorbent assay for glycyrrhizin using anti-glycyrrhizin monoclonal antibody and an eastern blotting technique for glucuronides of glycyrrhetic acid. Anal. Chem. 2001, 73, 5784–5790. [Google Scholar]
- Ishiyama, M.; Shoyama, Y.; Murakami, H.; Shinohara, H. Production of monoclonal antibodies and development of an ELISA for solamargine. Cytotechnology 1996, 18, 153–158. [Google Scholar]
- Shoyama, Y.; Fukada, T.; Murakami, H. Production of monoclonal antibodies and ELISA for thebaine and codeine. Cytotechnology 1996, 19, 55–61. [Google Scholar]
- Kim, J.S.; Tanaka, H.; Shoyama, Y. Immunoquantitative analysis for berberine and its related compounds using monoclonal antibodies in herbal medicines. Analyst 2004, 129, 87–91. [Google Scholar] [CrossRef]
- Morinaga, O.; Tanaka, H.; Shoyama, Y. Production of monoclonal antibody against a major purgative component, sennoside A, its characterization and ELISA. Analyst 2000, 125, 1109–1113. [Google Scholar]
- Morinaga, O.; Nakajima, S.; Tanaka, H.; Shoyama, Y. Production of monoclonal antibodies against a major purgative component, sennoside B, their characterization and use in ELISA. Analyst 2001, 126, 1372–1376. [Google Scholar]
- Tanaka, H.; Goto, Y.; Shoyama, Y. Monoclonal antibody based enzyme immunoassay for marihuana (cannabinoid) compounds. Immunoassay 1996, 17, 321–342. [Google Scholar]
- Loungratana, P.; Tanaka, H.; Shoyama, Y. Production of monoclonal antibody against ginkgolic acids in Ginkgo biloba Linn. Am. J. Chin. Med. 2004, 32, 33–48. [Google Scholar]
- Yanagihara, H.; Sakata, R.; Minami, H.; Shoyama, Y.; Murakami, H. Immunoaffinity column chromatography against forskolin using an anti-forskolin monoclonal antibody and its application. Anal. Chim. Acta 1996, 335, 63–70. [Google Scholar]
- Putalun, W.; Tanaka, H.; Yukihira, S. Rapid separation of solasodine glycosides by an immunoaffinity column using anti-solamargine monoclonal antibody. Cytotechnology 1999, 31, 151–156. [Google Scholar]
- Fukuda, N.; Tanaka, H.; Shoyama, H. Isolation of the pharmacologically active saponin ginsenoside Rb1 from ginseng by immunoaffinity column chromatography. J. Nat. Prod. 2000, 63, 283–285. [Google Scholar]
- Xu, J.; Tanaka, H.; Shoyama, Y. One-step immunochromatographic separation and ELISA quantification of glycyrrhizin from traditional Chinese medicines. J. Chromatogr. B 2007, 850, 53–58. [Google Scholar] [CrossRef]
- Yun, T.K. Brief introduction of Panax ginseng C.A. Meyer. J. Korean Med. Sci. 2001, 16, S3–S5. [Google Scholar]
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar]
- Choi, K.T. Botanical characteristics, pharmacological and medicinal components of Korean Panax ginseng C.A. Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar]
- Washida, D.; Kitanaka, S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem. Pharm. Bull. 2003, 51, 1314–1317. [Google Scholar] [CrossRef]
- Mook-Jung, I.; Hong, H.S.; Boo, J.H.; Lee, K.H.; Yun, S.H.; Cheong., M.Y.; Joo, I.; Huh, K.; Jung, M.W. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J. Neurosci. Res. 2001, 63, 509–515. [Google Scholar]
- Lim, J.H.; Wen, T.C.; Matsuda, S.; Tanaka, J.; Maeda, N.; Peng, H.; Aburaya, J.; Ishihara, K.; Sakanaka, M. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci. Res. 1997, 28, 191–200. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J. Effects of ginsenoside Rb1 and Rg1 on synaptosomal free calcium level, ATPase and calmodulin in rat hippocampus. Chin. Med. J. (Engl.) 1995, 108, 544–547. [Google Scholar]
- Jiang, K.Y.; Qian, Z.N. Effects of Panax notoginseng saponins on posthypoxic cell damage of neurons in vitro. Zhongguo Yao Li Xue Bao 1995, 16, 399–402. [Google Scholar]
- Fukuda, N.; Tanaka, H.; Shoyama, Y. Applications of ELISA, western blotting and immunoaffinity concentration for survey of ginsenosides in crude drugs of Panax species and traditional Chinese herbal medicines. Analyst 2000, 125, 1425–1429. [Google Scholar] [CrossRef]
- Wengatz, I.; Schmid, R.D.; Kreißig, S.; Wittmann, C.; Hock, B.; Ingendoh, A.; Hillenkamp, F. Determination of the hapten density of immuno-conjugates by matrix-assisted UV laser desorption/ionization mass spectrometry. Anal. Lett. 1992, 25, 1983–1997. [Google Scholar] [CrossRef]
- Shoyama, Y.; Sakata, R.; Isobe, R.; Murakami, H. Direct determination of forskolin-bovine serum albumin conjugate by matrix-assisted laser desorption ionization mass spectrometry. Org. Mass Spect. 1993, 28, 987–988. [Google Scholar] [CrossRef]
- Shoyama, Y.; Fukada, T.; Tanaka, T.; Kusai, A.; Nojima, K. Direct determination of opium alkaloid-bovine serum albumin conjugate by matrix-assisted laser desorption ionization mass spectrometry. Biol. Pharm. Bull. 1993, 16, 1051–1053. [Google Scholar] [CrossRef]
- Goto, Y.; Shima, Y.; Morimoto, S.; Shoyama, Y.; Murakami, H.; Kusai, A.; Nojima, K. Determination of tetrahydrocannabinolic acid-carrier protein conjugate by matrix-assisted laser desorption ionization mass spectrometry and antibody formation. Org. Mass Spectr. 1994, 29, 668–671. [Google Scholar] [CrossRef]
- Galfrè, G.; Milstein, C. Preparation of monoclonal antibodies: Strategies and procedures. Methods Enzymol. 1981, 73, 3–46. [Google Scholar]
- Goding, J.W. Antibody production by hybridomas. J. Immunol. Methods 1980, 39, 285–308. [Google Scholar] [CrossRef]
- Morinaga, O.; Tanaka, H.; Shoyama, Y. Detection and quantification of ginsenoside Re in ginseng samples by a chromatographic immunostaining method using monoclonal antibody against ginsenoside Re. J. Chromatogr. B 2006, 830, 100–104. [Google Scholar] [CrossRef]
- Tanaka, H.; Fukuda, N.; Yahara, S.; Isoda, S.; Yuan, C.S.; Shoyama, Y. Isolation of ginsenoside Rb1 from Kalopanax pictus by eastern blotting using anti-ginsenoside Rb1 monoclonal antibody. Phytother. Res. 2005, 19, 255–258. [Google Scholar] [CrossRef]
- Tanaka, H.; Fukuda, N.; Shoyama, Y. Eastern blotting and immunoaffinity concentration using monoclonal antibody for ginseng saponins in the field of traditional Chinese medicines. J. Agric. Food Chem. 2007, 55, 3783–3787. [Google Scholar] [CrossRef]
- Wang, C.A.; Shoyama, Y. Herbal medicine: Identification, analysis, and evaluationstrategies. In Textbook of Complementary and Alternative Medicine, 2nd ed.; Yuan, C.S., Bieber, E.J., Bauer, B.A., Eds.; Informa Healthcare: London, UK, 2006; pp. 51–70. [Google Scholar]
- Kim, S.C.; Byun, S.H.; Yang, C.H.; Kim, C.Y.; Kim, J.W.; Kim, S.G. Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage). Toxicology 2004, 197, 239–251. [Google Scholar] [CrossRef]
- Fuchikami, J.; Isohama, Y.; Sakaguchi, M.; Matsuda, M.; Kucota, T.; Akie, Y.; Fujino, A.; Miyata, T. Effect of glycyrrhizin on late asthmatic responses induced by antigen inhalation in guinea pigs. J. Pharmacol. Sci. 2004, 94, 251. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nixon, D.W. Licorice and cancer. Nutr. Cancer 2001, 39, 1–11. [Google Scholar]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Morinaga, O.; Fujino, A.; Tanaka, H.; Shoyama, Y. An on-membrane quantitative analysis system for glycyrrhizin in licorice roots and traditional Chinese medicines. Anal. Bioanal. Chem. 2005, 383, 668–672. [Google Scholar] [CrossRef]
- Uto, T.; Morinaga, O.; Tanaka, H.; Shoyama, Y. Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract onlipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem. Biophys. Res. Commun. 2012, 417, 473–478. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001, 11, 66–75. [Google Scholar] [CrossRef]
- Vincent, S.R. Nitric oxide neurons and neurotransmission. Prog. Neurobiol. 2010, 90, 246–255. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef]
- Gassull, M.A. The role of nutrition in the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2004, 20, 79–83. [Google Scholar] [CrossRef]
- Blantz, R.C.; Munger, K. Role of nitric oxide in inflammatory conditions. Nephron 2002, 90, 373–378. [Google Scholar] [CrossRef]
- Kröncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Fukuda, N.; Tanaka, H.; Shoyama, Y. Western blotting for ginseng saponins, ginsenosides using anti-ginsenoside Rb1 monoclonal antibody. Biol. Pharm. Bull. 1999, 22, 219–220. [Google Scholar] [CrossRef]
- Sritularak, B.; Morinaga, O.; Yuan, C.S.; Shoyama, Y.; Tanaka, H. Quantitative analysis of ginsenosides Rb1, Rg1, and Re in American ginseng berry and flower samples by ELISA using monoclonal antibodies. J. Nat. Med. 2009, 63, 360–363. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Uto, T.; Tung, N.H.; Morinaga, O.; Shoyama, Y. Preparation of Knockout Extract by Immunoaffinity Column and Its Application. Antibodies 2012, 1, 294-307. https://doi.org/10.3390/antib1030294
Uto T, Tung NH, Morinaga O, Shoyama Y. Preparation of Knockout Extract by Immunoaffinity Column and Its Application. Antibodies. 2012; 1(3):294-307. https://doi.org/10.3390/antib1030294
Chicago/Turabian StyleUto, Takuhiro, Nguyen Huu Tung, Osamu Morinaga, and Yukihiro Shoyama. 2012. "Preparation of Knockout Extract by Immunoaffinity Column and Its Application" Antibodies 1, no. 3: 294-307. https://doi.org/10.3390/antib1030294



