Abstract
The application of graph theory in chemical and molecular structure research has far exceeded people’s expectations, and it has recently grown exponentially. In the molecular graph, atoms are represented by vertices and bonds by edges. Topological indices help us to predict many physico-chemical properties of the concerned molecular compound. In this article, we compute Generalized first and multiplicative Zagreb indices, the multiplicative version of the atomic bond connectivity index, and the Generalized multiplicative Geometric Arithmetic index for silicon-carbon and second.
1. Introduction
For quite a few years, Chemical Graph theory has been assuming an imperative part in mathematical chemistry, quantitative structure-activity relationships (QSAR) and structure-property relationships (QSPR), and closeness/assorted variety investigation of sub-atomic libraries [1]. Essentially, molecular descriptors utilized as a part of these research fields are obtained from the graph of molecule, which speak to use some method to calculate numbers associated with molecular graph then using these number to describe molecule [1,2].
In chemical graph theory, a graph of molecule is a simple connected graph, in which atoms and chemical bonds are represented by vertices and edges respectively. A graph is connected if there is a connection between any pair of vertices. A network is a connected graph that has no multiple edge and loop. The number of vertices that are connected to a fixed vertex v is called the degree of v. The distance between two vertices is the length of the shortest path between them. The concept of valence in chemistry and the concept of degree in a graph is somehow closely related. For details on bases of graph theory, we refer to the book [3]. Throughout this paper, G denotes connected graph, V and E denote the vertex set and the edge set, respectively, and denotes the degree of a vertex.
The topological index of the graph of a chemical compound is the number associated with it. In 1947 Weiner laid the foundation of the topological index when he was approximating the boiling point of alkanes and introduced the Weiner index [4]. Till now, more than 140 topological indices are defined, but none of them is enough to determine all physico-chemical properties of understudy molecule. However, these indices together can do this to some extent. Later, in 1975, Milan Randić introduced Randić index [5]. In 1998, Bollobas and Erdos [6] and Amic et al. [7] defined the generalized Randić index, which got attention from both chemists and mathematicians [8]. The Randić index is one of the most popular, studied, and applied topological indices. Many reviews, papers, and books [9,10,11,12,13] are written on this simple graph invariant.
In 1972, Gutman introduced the first and the second Zagreb indices in [14].
These indices have applied to study molecular chirality, complexity, hetero-systems, and ZE-isomerism [15].
Some indices are The first and second multiplicative Zagreb indices [16] are related to Wiener’s work and defined as:
and the Narumi-Katayama index [17]:
In computational chemistry, these types of indices are the focus of considerable research, like the Wiener index [18,19,20]. For example, in 2011. Gutman [18] studied and characterized the multiplicative first and second Zagreb indices for trees and determined the unique trees that give maximum and minimum values for M1(G) and M2(G), respectively. In [20], authors extended Gutman’s result and defined the following index for k-trees:
Notice that for s = 1, 2 the above defined index is the Narumi-Katayama and Zagreb index, respectively. Based on the successful consideration of multiplicative Zagreb indices, M. Eliasi et al. [21] defined a new version of the multiplicative first Zagreb index as:
Furthering the study of topological indices, the first and second hyper-Zagreb indices of a graph [22] are defined as:
In [23], Kulli et al. defined the first and second generalized multiplicative Zagreb indices:
Multiplicative sum connectivity and multiplicative product connectivity indices [24] are defined as:
Note that for , first and second generalized multiplicative Zagreb indices are first and second multiplicative Zagreb indices, respectively, and for , first and second generalized multiplicative Zagreb indices are first and second hyper multiplicative Zagreb indices, respectively. For first and second generalized multiplicative Zagreb indices are multiplicative sum connectivity and multiplicative product connectivity indices.
Multiplicative atomic bond connectivity index, multiplicative Geometric arithmetic index, and generalized multiplicative Geometric arithmetic index are defined as
In this paper, we compute Generalized first and second multiplicative Zagreb indices, multiplicative version of Atomic bond connectivity index and Generalized multiplicative Geometric Arithmetic index for silicon-carbon and . Now we discuss the graph of and
In Figure 1, one unit of is shown. Molecular graph of is shown in Figure 2, in which p denotes the number of cells attached in a single row and q denotes the number of total rows where each row contains p cells. In Figure 3 and Figure 4, we demonstrate how cells are connected in one row (chain) and how one row is connected to another row.
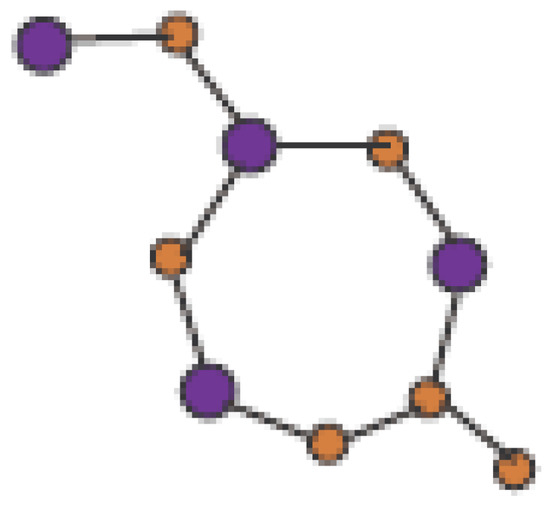
Figure 1.
Unit Cell of .
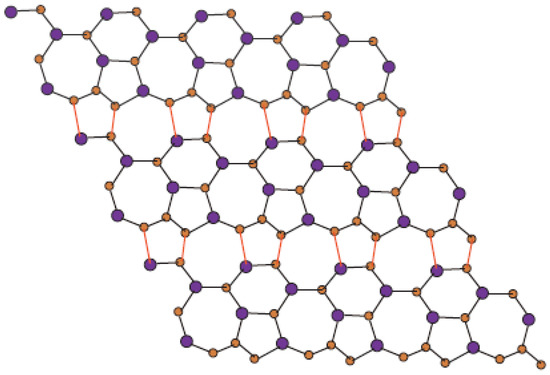
Figure 2.
Sheet of for p = 4 and q = 3.
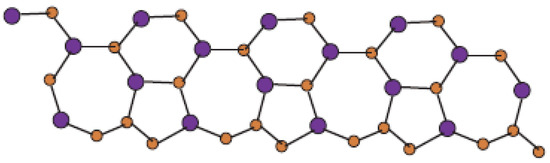
Figure 3.
Sheet of for p = 4 and q = 1.
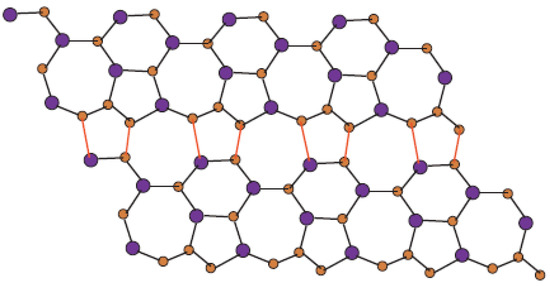
Figure 4.
Sheet of for p = 4 and q = 2.
In Figure 1, Figure 2, Figure 3 and Figure 4, carbon atoms are shown as brown, and silicon atoms Si are shown as blue.
In Figure 5, one unit of is given. By connecting p cells in a row and then connecting q rows where each row contains p cells, we get molecular graph of . The molecular graph of is shown in Figure 6 for p = 3 and q = 4. Figure 7 and Figure 8 demonstrate how cells are connected in a row (chain) and how a row is connected to another row. We will use to represent this molecular graph.
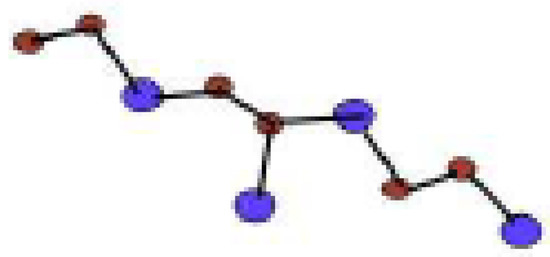
Figure 5.
One Unit of .
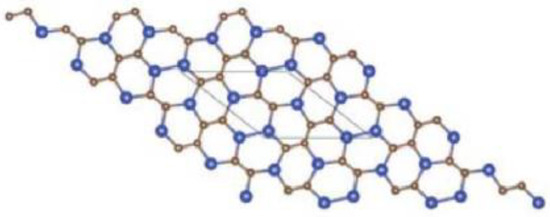
Figure 6.
Sheet of for p = 3 and q = 3.
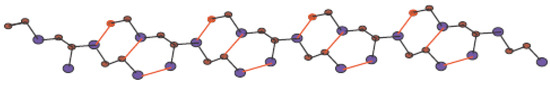
Figure 7.
Sheet of for p = 5 and q = 1.
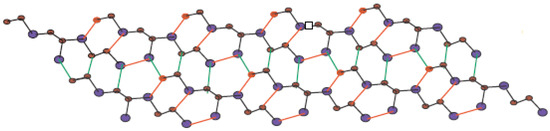
Figure 8.
Sheet of for p = 5 and q = 2.
2. Computational Results
In this section we give our main results.
Theorem 1.
Let be the Silicon Carbide. Then
Proof.
Let G be the graph of . From the graph of (Figure 1, Figure 2, Figure 3 and Figure 4), we can see that the total number of vertices are , and total number of edges are .
The edge set of with has following five partitions:
Additionally, it has
Now,
and
☐
Theorem 2.
Let be the Silicon Carbide. Then,
Proof.
Taking , in Theorem 1, we get our desired results. ☐
Theorem 3.
Let be the Silicon Carbide. Then,
Proof.
Taking , in Theorem 1, we get our desired results. ☐
Theorem 4.
Let be the Silicon Carbide. Then,
Proof.
Taking , in Theorem 1, we get our desired results. ☐
Theorem 5.
Let be the Silicon Carbide. Then,
Proof.
☐
Theorem 6.
Let be the Silicon Carbide. Then,
Proof.
Let G be the graph of . From the graph of (Figure 5, Figure 6, Figure 7 and Figure 8), we can see that the total number of vertices are and total number of edges are .
The edge set of with has following five partitions:
Additionally, it has
Now,
and
☐
Theorem 7.
Let be the Silicon Carbide. Then,
Proof.
Taking , in Theorem 6, we get our desired results. ☐
Theorem 8.
Let be the Silicon Carbide. Then,
Proof.
Taking , in Theorem 6, we get our desired results. ☐
Theorem 9.
Let be the Silicon Carbide. Then,
Proof.
Taking , in Theorem 6, we get our desired results. ☐
Theorem 10.
Let be the Silicon Carbide. Then,
Proof.
☐
3. Remarks
Multiplicative, degree-based topological indices for silicon-carbon have been investigated here. Our results can help us to understand the physical features, chemical reactivity, and biological activities of silicon-carbon. For example, the atom-bond connectivity (ABC) index provides a very good correlation for computing the strain energy of molecules [25]. ABC is used to describe the heats of formation of alkanes, resulting in a good quantitative structure-property relationship (QSPR) model (r = 0.9970) [26]. The GA index has as much predictive power as that of the Randic index, so the GA index is more useful than the Randic index [25]. The first and second Zagreb indices were found to occur for the computation of the total π-electron energy of molecules and have a strong relationship with Weiner index [27]. The computation of distance-based and counting-related topological indices for these symmetrical graphs is an open challenge and is yet to be investigated. Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8 are taken from [28].
Author Contributions
Y.C.K. did formal Analysis of paper. A.u.R.V. computed the results. W.N. proposed the methodology and wrote the paper. M.A.R. supervised and S.M.K. validates the results.
Funding
This research is supported by the funds of Dong A University.
Acknowledgments
We thank the reviewers for their suggestions, which have helped us to improve the quality of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Todeschini, R.; Consonni, V. New local vertex invariants and molecular descriptors based on functions of the vertex degrees. Match Commun. Math. Comput. Chem. 2010, 64, 359–372. [Google Scholar]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics: Volume I: Alphabetical Listing/Volume II: Appendices, References; John Wiley & Sons: New York, NY, USA, 2009; Volume 41. [Google Scholar]
- West, D.B. Introduction to Graph Theory; Prentice-Hall: Upper Saddle River, NJ, USA, 2001; Volume 2. [Google Scholar]
- Wiener, H. Structural determination of paraffin boiling points. J. Am. Chem. Soc. 1947, 69, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Randic, M. Characterization of molecular branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar] [CrossRef]
- Bollobás, B.; Erdös, P. Graphs of extremal weights. Ars Comb. 1998, 50, 225–233. [Google Scholar] [CrossRef]
- Amić, D.; Bešlo, D.; Lučić, B.; Nikolić, S.; Trinajstić, N. The vertex-connectivity index revisited. J. Chem. Inf. Comput. Sci. 1998, 38, 819–822. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Shi, Y.; Xu, T.; Gutman, I. On molecular graphs with smallest and greatest zeroth-order general Randic index. Match Commun. Math. Comput. Chem. 2005, 54, 425–434. [Google Scholar]
- Li, X.; Gutman, I.; Randić, M. Mathematical Aspects of Randić-Type Molecular Structure Descriptors; Nankai University, Faculty of Science: Tianjin, China, 2006. [Google Scholar]
- Randić, M. On history of the Randić index and emerging hostility toward chemical graph theory. Match Commun. Math. Comput. Chem. 2008, 59, 5–124. [Google Scholar]
- Randić, M. The connectivity index 25 years after. J. Mol. Gr. Model. 2001, 20, 19–35. [Google Scholar] [CrossRef]
- Gutman, I.; Furtula, B. Recent Results in the Theory of Randić Index; University of Kragujevac and Faculty of Science Kragujevac: Kragujevac, Serbia, 2008. [Google Scholar]
- Li, X.; Shi, Y. A survey on the Randic index. Match Commun. Math. Comput. Chem. 2008, 59, 127–156. [Google Scholar]
- Gutman, I.; Trinajstić, N. Graph theory and molecular orbitals. Total π-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 1972, 17, 535–538. [Google Scholar] [CrossRef]
- Eliasi, M.; Vukicevic, D. Comparing the multiplicative Zagreb indices. Match Commun. Math. Comput. Chem. 2013, 69, 765–773. [Google Scholar]
- Gutman, I.; Ruscić, B.; Trinajstić, N.; Wilcox, C.F., Jr. Graph theory and molecular orbitals. XII. Acyclic polyenes. J. Chem. Phys. 1975, 62, 3399–3405. [Google Scholar] [CrossRef]
- Narumi, H.; Katayama, M. Simple Topological Index: A Newly Devised Index Characterizing the Topological Nature of Structural Isomers of Saturated Hydrocarbons; Hokkaido University, Memoirs of the Faculty of Engineering: Hokkaido, Japan, 1984; Volume 16, pp. 209–214. [Google Scholar]
- Gutman, I. Multiplicative Zagreb indices of trees. Bull. Soc. Math. Banja Luka 2011, 18, 17–23. [Google Scholar]
- Todeschini, R.; Ballabio, D.; Consonni, V. Novel molecular descriptors based on functions of new vertex degrees. In Novel Molecular Structure Descriptors—Theory and Applications I; Gutman, I., Furtula, B., Eds.; University of Kragujevac: Kragujevac, Serbia, 2010; pp. 73–100. [Google Scholar]
- Wang, S.; Wei, B. Multiplicative Zagreb indices of k-trees. Discret. Appl. Math. 2015, 180, 168–175. [Google Scholar] [CrossRef]
- Eliasi, M.; Iranmanesh, A.; Gutman, I. Multiplicative versions of first Zagreb index. Match Commun. Math. Comput. Chem. 2012, 68, 217. [Google Scholar]
- Kulli, V.R. Multiplicative Hyper-Zagreb Indices and Coindices of Graphs: Computing These Indices of Some Nanostructures. Int. Res. J. Pure Algebra 2016, 6, 2248–9037. [Google Scholar]
- Kulli, V.R.; Stone, B.; Wang, S.; Wei, B. Generalised multiplicative indices of polycyclic aromatic hydrocarbons and benzenoid systems. Zeitschrift für Naturforschung A 2017, 72, 573–576. [Google Scholar] [CrossRef]
- Kulli, V.R. Multiplicative connectivity indices of TUC4C8 [m, n] and TUC4 [m, n] nanotubes. J. Comput. Math. Sci. 2016, 7, 599–605. [Google Scholar]
- Imran, M.; Ali, M.A.; Ahmad, S.; Siddiqui, M.K.; Baig, A.Q. Topological Characterization of the Symmetrical Structure of Bismuth Tri-Iodide. Symmetry 2018, 10, 201. [Google Scholar] [CrossRef]
- Estrada, E.; Torres, L.; Rodriguez, L.; Gutman, I. An atom-bond connectivity index: Modelling the enthalpy of formation of alkanes. NISCAIR-CSIR 1998, 37A, 849–855. [Google Scholar]
- Zhou, B.; Gutman, I. Relations between Wiener, hyper-Wiener and Zagreb indices. Chem. Phys. Lett. 2004, 394, 93–95. [Google Scholar] [CrossRef]
- Imran, M.; Siddiqui, M.K.; Naeem, M.; Iqbal, M.A. On Topological Properties of Symmetric Chemical Structures. Symmetry 2018, 10, 173. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).