Nitrogen and Phosphorus Co-Doped Carbon Dots for the Growth Promotion of Water Spinach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N, P−CDs
2.2. Characterization of N, P−CDs
2.3. Selection and Cultivation of Water Spinach
2.4. Morphology Determination of Water Spinach
2.5. Determination of Nutritional Quality and Photosynthetic Pigments
2.6. Statistical Analysis
3. Results
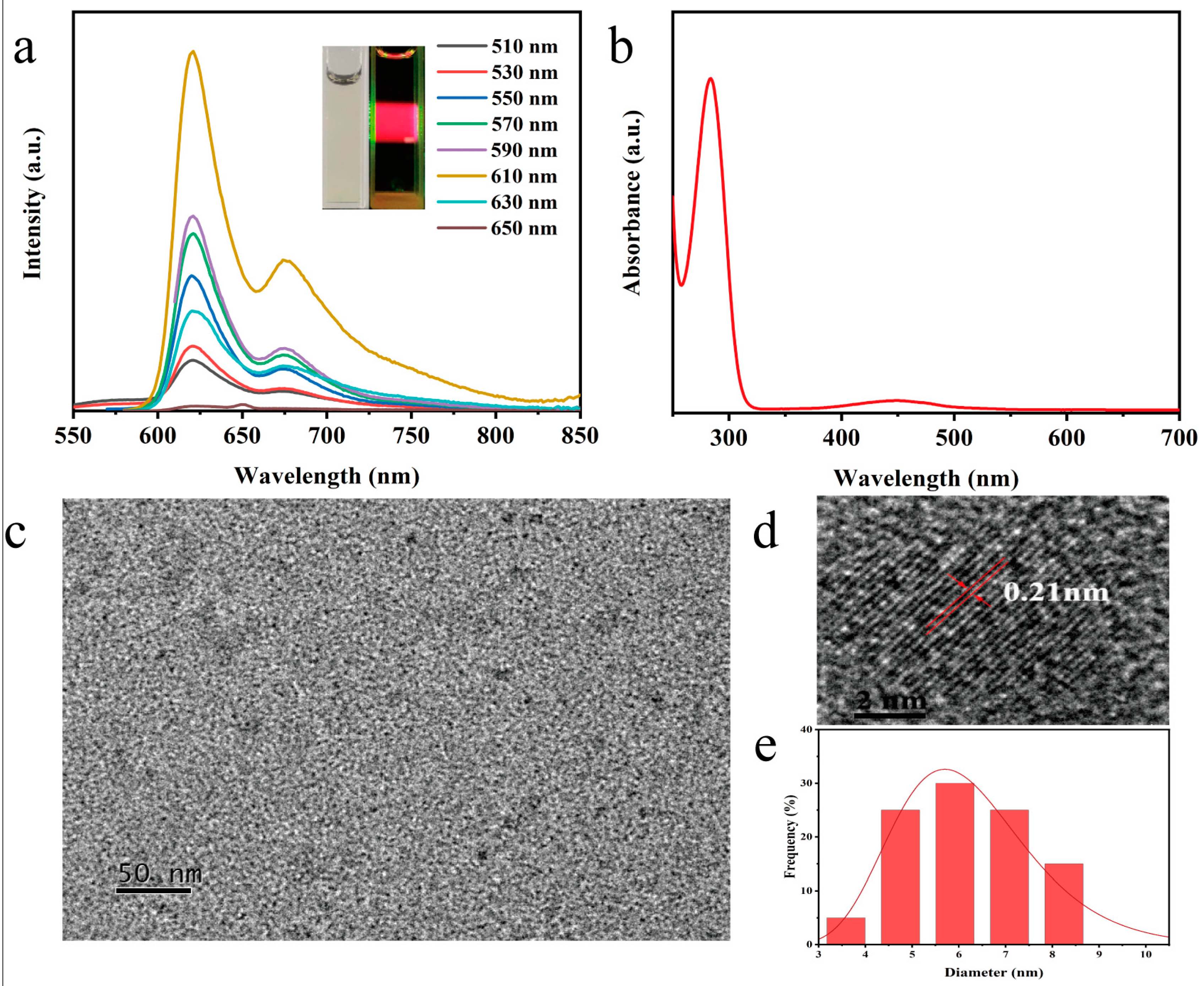
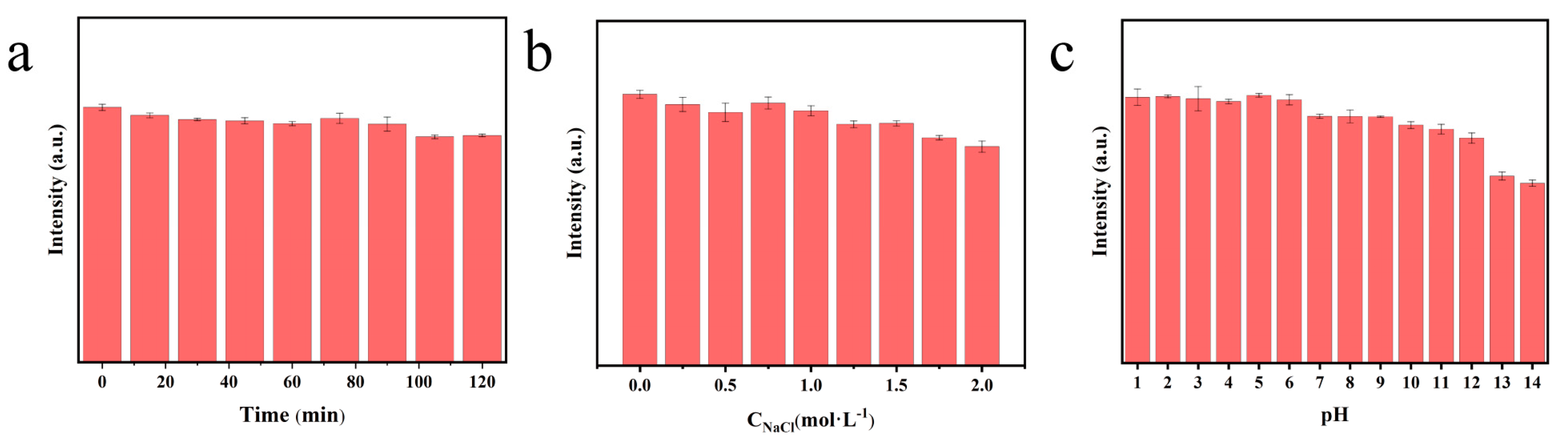
3.1. Spectroscopic Characterization of N, P−CDs
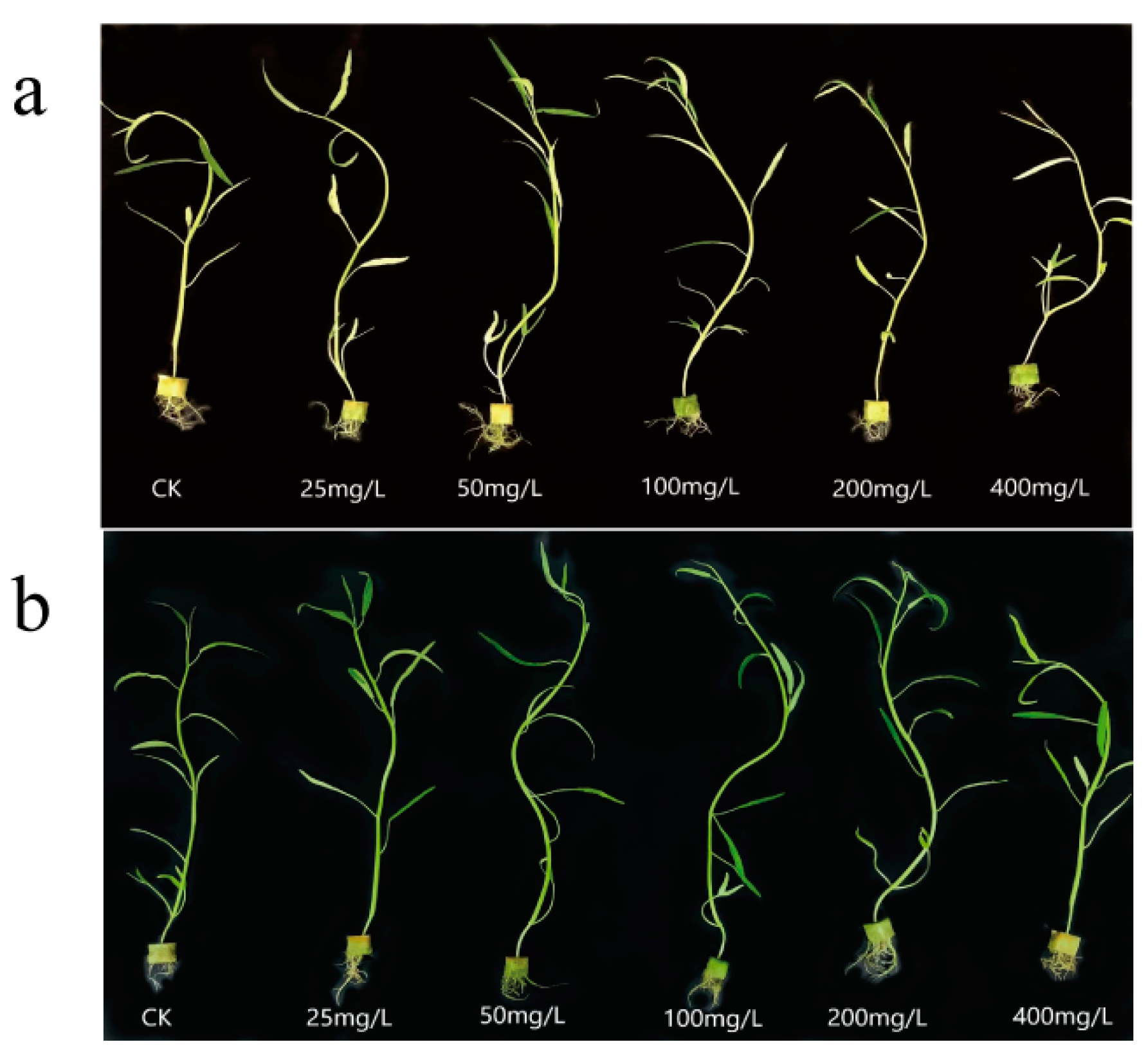
3.2. Effect of N, P−CDs on the Growth of Water Spinach
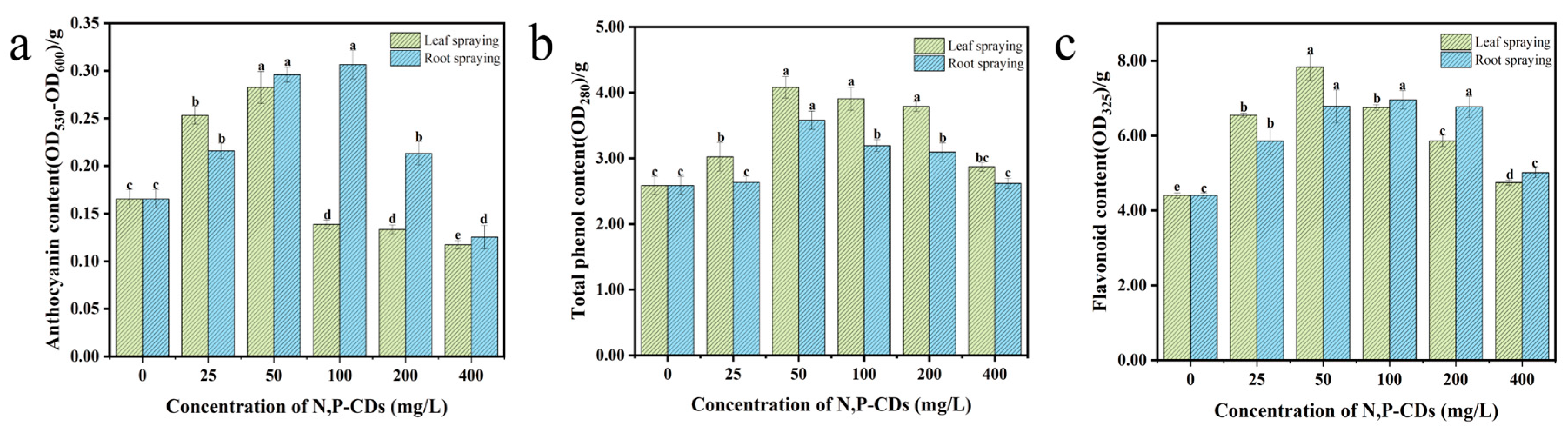
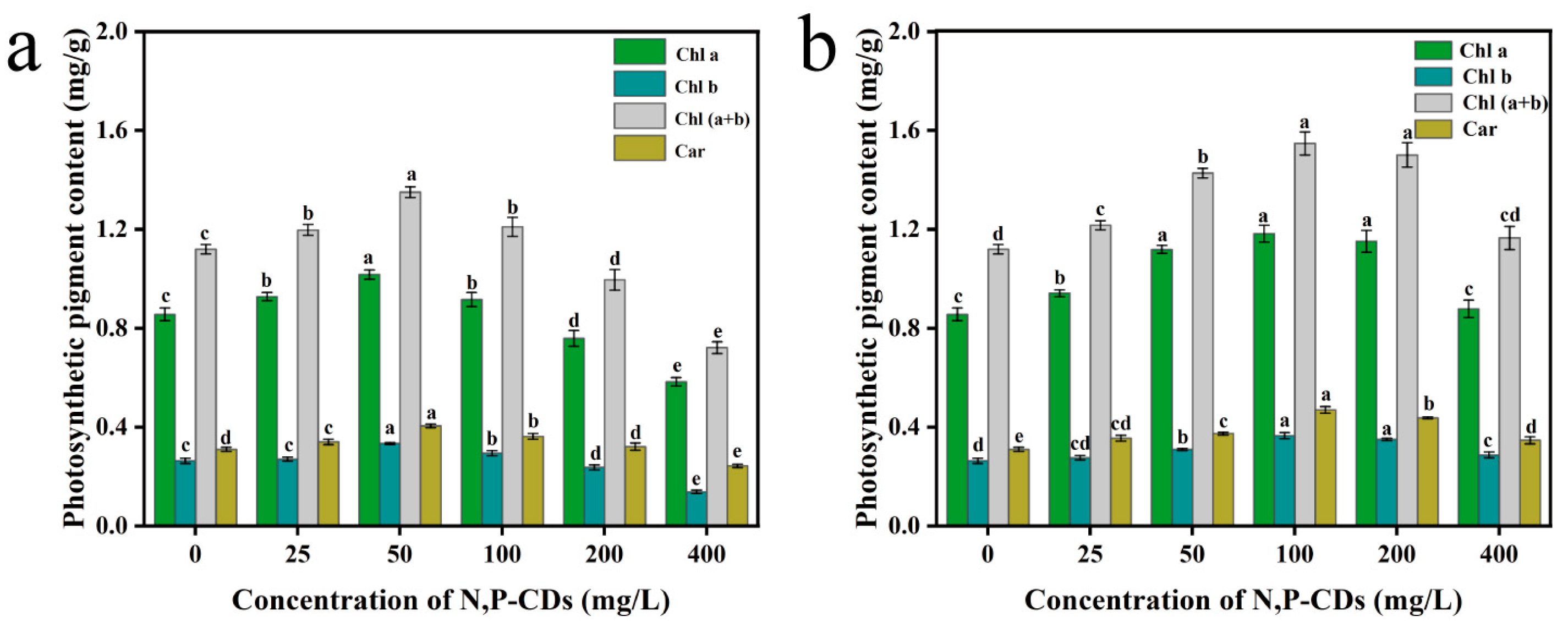
3.3. Effect of N, P−CDs on the Photosynthetic Properties of Water Spinach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalala, G.; Kambashi, B.; Everaert, N.; Beckers, Y.; Richel, A.; Pachikian, B.; Neyrinck, A.M.; Delzenne, N.M.; Bindelle, J. Characterization of fructans and dietary fibre profiles in raw and steamed vegetables. Int. J. Food Sci. Nutr. 2017, 69, 682–689. [Google Scholar]
- Calatayud, M.; Abbeele, P.V.D.; Ghyselinck, J.; Marzorati, M.; Rohs, E.; Birkett, A. Comparative Effect of 22 Dietary Sources of Fiber on Gut Microbiota of Healthy Humans In Vitro. Front. Nutr. 2021, 8, 700571. [Google Scholar]
- Barbosa, C.L.; Brettschneider, A.-K.; Haftenberger, M.; Lehmann, F.; Richter, A.; Mensink, G.B. Food and nutrient intake of children and adolescents living in Germany: Results from EsKiMo II. Proc. Nutr. Soc. 2020, 79, OCE2. [Google Scholar]
- Wang, A.; Luo, J.; Zhang, T.; Zhang, D. Dietary Vitamin C and Vitamin C Derived from Vegetables Are Inversely Associated with the Risk of Depressive Symptoms among the General Population. Antioxidants 2021, 10, 1984. [Google Scholar] [CrossRef]
- Sivadas, S.; Mohanty, A.K.; Rajesh, S.; Muthuvel, S.K.; Vasanthi, H.R. Molecular modelling and biological evaluation of phyto-molecules as potential activators of gluconolactone oxidase (GULO). J. Biomol. Struct. Dyn. 2023, 3, 1–13. [Google Scholar]
- Kathi, S.; Laza, H.; Singh, S.; Thompson, L.; Li, W.; Simpson, C. Increasing vitamin C through agronomic biofortification of arugula microgreens. Sci. Rep. 2022, 12, 13093. [Google Scholar]
- Bidar, G.; Pelfrêne, A.; Schwartz, C.; Waterlot, C.; Sahmer, K.; Marot, F.; Douay, F. Urban kitchen gardens: Effect of the soil contamination and parameters on the trace element accumulation in vegetables–A review. Sci. Total Environ. 2020, 738, 139569. [Google Scholar]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar]
- Scharff, L.B.; Saltenis, V.L.R.; Jensen, P.E.; Baekelandt, A.; Burgess, A.J.; Burow, M.; Ceriotti, A.; Cohan, J.; Geu-Flores, F.; Halkier, B.A.; et al. Prospects to improve the nutritional quality of crops. Food Energy Secur. 2021, 11, e327. [Google Scholar]
- Jusuf, B.N.; Sambudi, N.S.; Isnaeni; Samsuri, S. Microwave-assisted synthesis of carbon dots from eggshell membrane ashes by using sodium hydroxide and their usage for degradation of methylene blue. J. Environ. Chem. Eng. 2018, 6, 7426–7433. [Google Scholar]
- Cao, Y.; Fan, D.; Lin, S.; Mu, L.; Ng, F.T.; Pan, Q. Phase change materials based on comb-like polynorbornenes and octadecylamine-functionalized graphene oxide nanosheets for thermal energy storage. Chem. Eng. J. 2020, 389, 124318. [Google Scholar]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar]
- Sawalha, S.; Silvestri, A.; Criado, A.; Bettini, S.; Prato, M.; Valli, L. Tailoring the sensing abilities of carbon nanodots obtained from olive solid wastes. Carbon 2020, 167, 696–708. [Google Scholar]
- Hallaji, Z.; Bagheri, Z.; Tavassoli, Z.; Ranjbar, B. Fluorescent carbon dot as an optical amplifier in modern agriculture. Sustain. Mater. Technol. 2022, 34, e00493. [Google Scholar]
- Zhang, M.; Hu, L.; Wang, H.; Song, Y.; Liu, Y.; Li, H.; Shao, M.; Huang, H.; Kang, Z. One-step hydrothermal synthesis of chiral carbon dots and their effects on mung bean plant growth. Nanoscale 2018, 10, 12734–12742. [Google Scholar]
- Su, L.-X.; Ma, X.-L.; Zhao, K.-K.; Shen, C.-L.; Lou, Q.; Yin, D.-M.; Shan, C.-X. Carbon Nanodots for Enhancing the Stress Resistance of Peanut Plants. ACS Omega 2018, 3, 17770–17777. [Google Scholar] [CrossRef]
- Amarasinghe, T.; Madhusha, C.; Munaweera, I.; Kottegoda, N. Review on Mechanisms of Phosphate Solubilization in Rock Phosphate Fertilizer. Commun. Soil Sci. Plant Anal. 2022, 53, 944–960. [Google Scholar] [CrossRef]
- Pan, X.; Fu, F.; Xie, Z.; Li, W.; Yang, X.; Kang, Y.; Qu, S.; Zheng, Y.; Li, Q.; Zhang, H.; et al. Light-nutrition coupling effect of degradable fluorescent carbon dots on lettuce. Environ. Sci. Nano 2023, 10, 539–551. [Google Scholar]
- Krein, D.D.C.; Rosseto, M.; Cemin, F.; Massuda, L.A.; Dettmer, A. Recent trends and technologies for reduced environmental impacts of fertilizers: A review. Int. J. Environ. Sci. Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, W.; Zhao, Y. Postharvest Physiological and Biochemical Experimental Guidance for Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007; pp. 38–49. [Google Scholar]
- Yu, S.-J.; Lu, S.-Y.; Tan, D.-F.; Zhu, Y.-F. Nitrogen and phosphorus co-doped carbon dots for developing highly flame retardant poly (vinyl alcohol) composite films. Eur. Polym. J. 2021, 164, 110970. [Google Scholar]
- Perikala, M.; Bhardwaj, A. Engineering Photo-Luminescent Centers of Carbon Dots to Achieve Higher Quantum Yields. ACS Appl. Electron. Mater. 2020, 2, 2470–2478. [Google Scholar] [CrossRef]
- Kou, E.; Yao, Y.; Yang, X.; Song, S.; Li, W.; Kang, Y.; Qu, S.; Dong, R.; Pan, X.; Li, D.; et al. Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato. ACS Sustain. Chem. Eng. 2021, 9, 944–953. [Google Scholar] [CrossRef]
- Ju, Y.J.; Li, N.; Liu, S.G.; Liang, J.Y.; Gao, X.; Fan, Y.Z.; Li, N.B. Proton-controlled synthesis of red-emitting carbon dots and application for hematin detection in human erythrocytes. Anal. Bioanal. Chem. 2019, 411, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, Z.; Liu, X.; Zhang, R.; Xiao, X. Rapid and low-temperature synthesis of N, P co-doped yellow emitting carbon dots and their applications as antibacterial agent and detection probe to Sudan Red I. Mater. Sci. Eng. C 2020, 119, 111468. [Google Scholar]
- Li, Y.; Pan, X.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect. J. Hazard. Mater. 2020, 410, 124534. [Google Scholar]
- Shao, D.; Zhu, W.; Xin, G.; Lian, J.; Sawyer, S. Inorganic vacancy-ordered perovskite Cs2SnCl6:Bi/GaN heterojunction photodiode for narrowband, visible-blind UV detection. Appl. Phys. Lett. 2019, 115, 121106. [Google Scholar]
- Fan, H.; Lv, J.; Chen, W.; Dong, X.; Bian, W. Preparation of nitrogen and phosphorus co-doped carbon dots and its application for morin detection. Chem. Res. Appl. 2018, 30, 577–582. [Google Scholar]
- Chen, J.; Wei, J.-S.; Zhang, P.; Niu, X.-Q.; Zhao, W.; Zhu, Z.-Y.; Ding, H.; Xiong, H.-M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar]
- Dong, G.; Yu-Liang, Z.; Jing, S.; Hong-Jun, F. One-Step Synthesis of Specific pH-responsive Carbon Quantum Dots and Their Luminescence Mechanism. J. Inorg. Mater. 2019, 34, 1309–1315. [Google Scholar]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Lu, H.-L.; Nkoh, J.N.; Baquy, M.A.-A.; Dong, G.; Li, J.-Y.; Xu, R.-K. Plants alter surface charge and functional groups of their roots to adapt to acidic soil conditions. Environ. Pollut. 2020, 267, 115590. [Google Scholar] [PubMed]
- Li, W.; Zheng, Y.; Zhang, H.; Liu, Z.; Su, W.; Chen, S.; Liu, Y.; Zhuang, J.; Lei, B. Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants. ACS Appl. Mater. Interfaces 2016, 8, 19939–19945. [Google Scholar]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar]
- Khatri, K.; Rathore, M.S. Salt and osmotic stress-induced changes in physio-chemical responses, PSII photochemistry and chlorophyll a fluorescence in peanut. Plant Stress 2022, 3, 100063. [Google Scholar]
- Liao, C.; Nolte, K.; Brown, D.G.; Lay, J.; Agrawal, A. The carbon cost of agricultural production in the global land rush. Glob. Environ. Chang. 2023, 80, 102679. [Google Scholar]
- Cánovas, C.R.; Macías, F.; Pérez-López, R.; Basallote, M.D.; Millán-Becerro, R. Valorization of wastes from the fertilizer industry: Current status and future trends. J. Clean. Prod. 2018, 174, 678–690. [Google Scholar]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.-J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 400–408. [Google Scholar]
- Mascoli, V.; Bersanini, L.; Croce, R. Far-red absorption and light-use efficiency trade-offs in chlorophyll f photosynthesis. Nat. Plants 2020, 6, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, S.; Zhang, H.; Zhang, X.; Zhuang, J.; Hu, C.; Liu, Y.; Lei, B.; Ma, L.; Wang, X. Enhanced Biological Photosynthetic Efficiency Using Light-Harvesting Engineering with Dual-Emissive Carbon Dots. Adv. Funct. Mater. 2018, 28, 4004. [Google Scholar]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [PubMed]
- Bellasio, C. A generalised dynamic model of leaf-level C3 photosynthesis combining light and dark reactions with stomatal behaviour. Photosynth. Res. 2018, 141, 99–118. [Google Scholar] [PubMed]
- Hitchcock, A.; Hunter, C.N.; Sobotka, R.; Komenda, J.; Dann, M.; Leister, D. Redesigning the photosynthetic light reactions to enhance photosynthesis–The PhotoRedesign consortium. Plant J. 2021, 109, 23–34. [Google Scholar] [CrossRef]
- Guirguis, A.; Yang, W.; Conlan, X.A.; Kong, L.; Cahill, D.M.; Wang, Y. Boosting Plant Photosynthesis with Carbon Dots: A Critical Review of Performance and Prospects. Small 2023, 6, e2300671. [Google Scholar]
- Zhao, L.-S.; Huokko, T.; Wilson, S.; Simpson, D.M.; Wang, Q.; Ruban, A.V.; Mullineaux, C.W.; Zhang, Y.-Z.; Liu, L.-N. Structural variability, coordination and adaptation of a native photosynthetic machinery. Nat. Plants 2020, 6, 869–882. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the Photosynthetic Apparatus Functions by Chlorophyll Fluorescence and P700 Absorbance in C3 and C4 Plants under Physiological Conditions and under Salt Stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar]
- Borisova-Mubarakshina, M.M.; Vetoshkina, D.V.; Ivanov, B.N. Antioxidant and signaling functions of the plastoquinone pool in higher plants. Physiol. Plant. 2019, 166, 181–198. [Google Scholar]
- Lee, K.Y.; Park, S.J.; Lee, K.A.; Kim, S.H.; Kim, H.; Meroz, Y.; Shin, K. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat. Biotechnol. 2018, 36, 530–535. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; She, M.; Cai, X.; Li, X.; Huang, Y.; Lei, H.; Tan, Z. Nitrogen and Phosphorus Co-Doped Carbon Dots for the Growth Promotion of Water Spinach. Symmetry 2023, 15, 1532. https://doi.org/10.3390/sym15081532
Yu F, She M, Cai X, Li X, Huang Y, Lei H, Tan Z. Nitrogen and Phosphorus Co-Doped Carbon Dots for the Growth Promotion of Water Spinach. Symmetry. 2023; 15(8):1532. https://doi.org/10.3390/sym15081532
Chicago/Turabian StyleYu, Fan, Mengqi She, Xia Cai, Xiaoyan Li, Yuan Huang, Hongwei Lei, and Zuojun Tan. 2023. "Nitrogen and Phosphorus Co-Doped Carbon Dots for the Growth Promotion of Water Spinach" Symmetry 15, no. 8: 1532. https://doi.org/10.3390/sym15081532




