Prolinethioamides versus Prolinamides in Organocatalyzed Aldol Reactions—A Comparative Study
Abstract
:1. Introduction
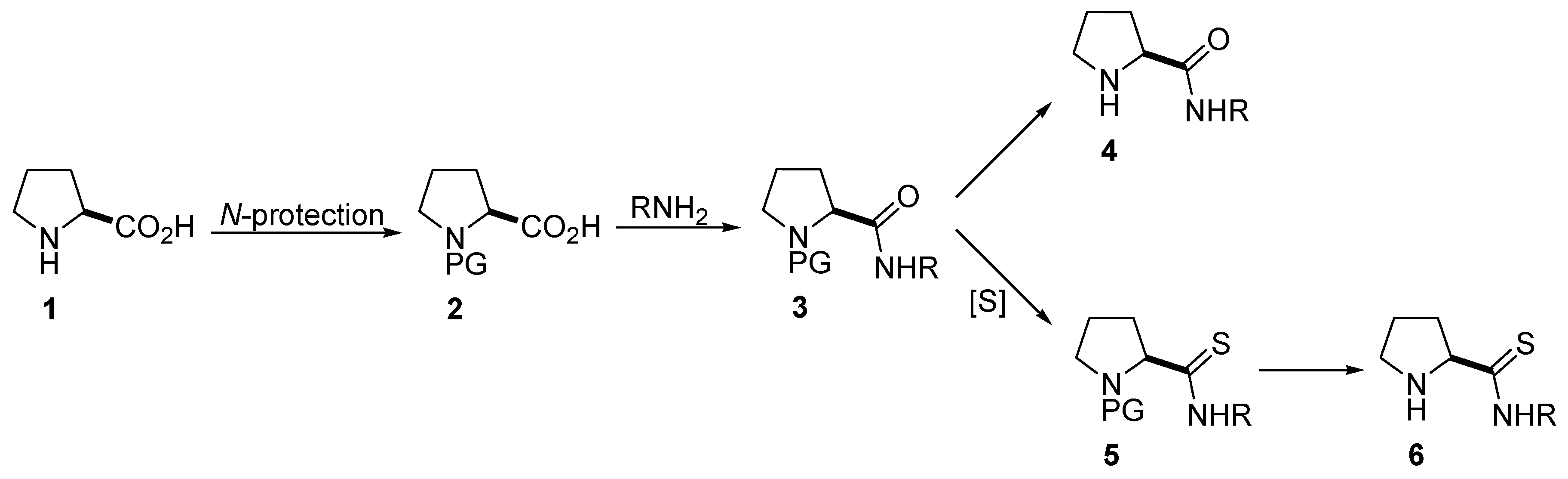
2. Synthesis of Catalysts
3. Acidity
4. L-Proline Amides and Thioamides as Organocatalysts
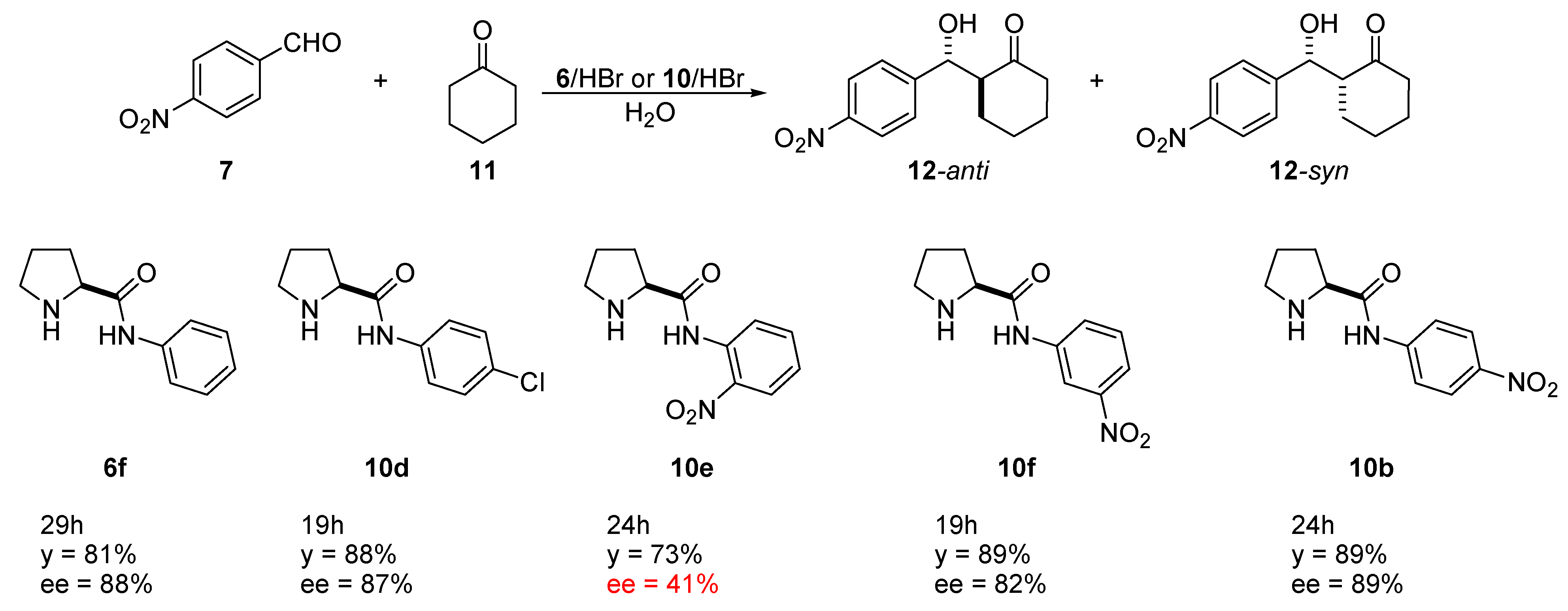
4.1. Aldol Reaction Catalyzed by Simple Aryl and Alkyl-Prolinamide Derivatives
4.2. Prolinamide Derivatives with an Additional Stereocenter
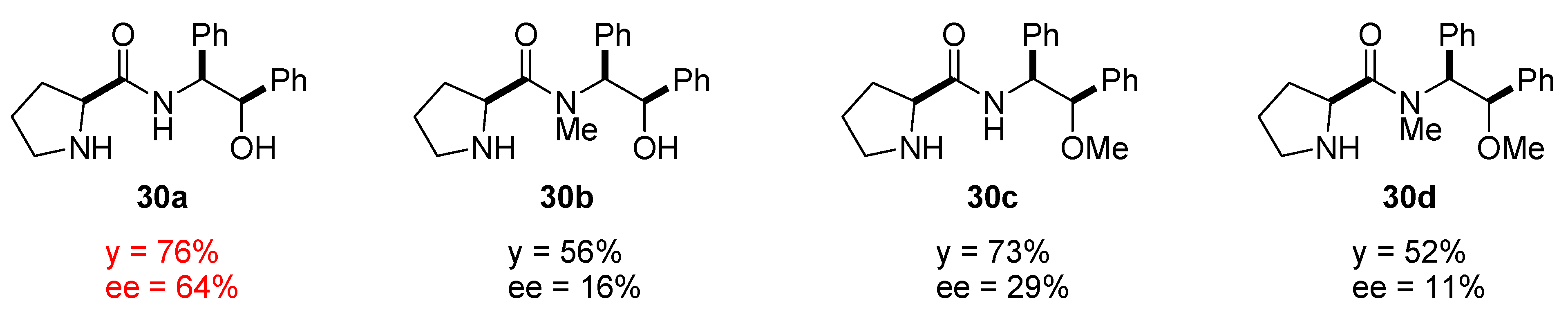
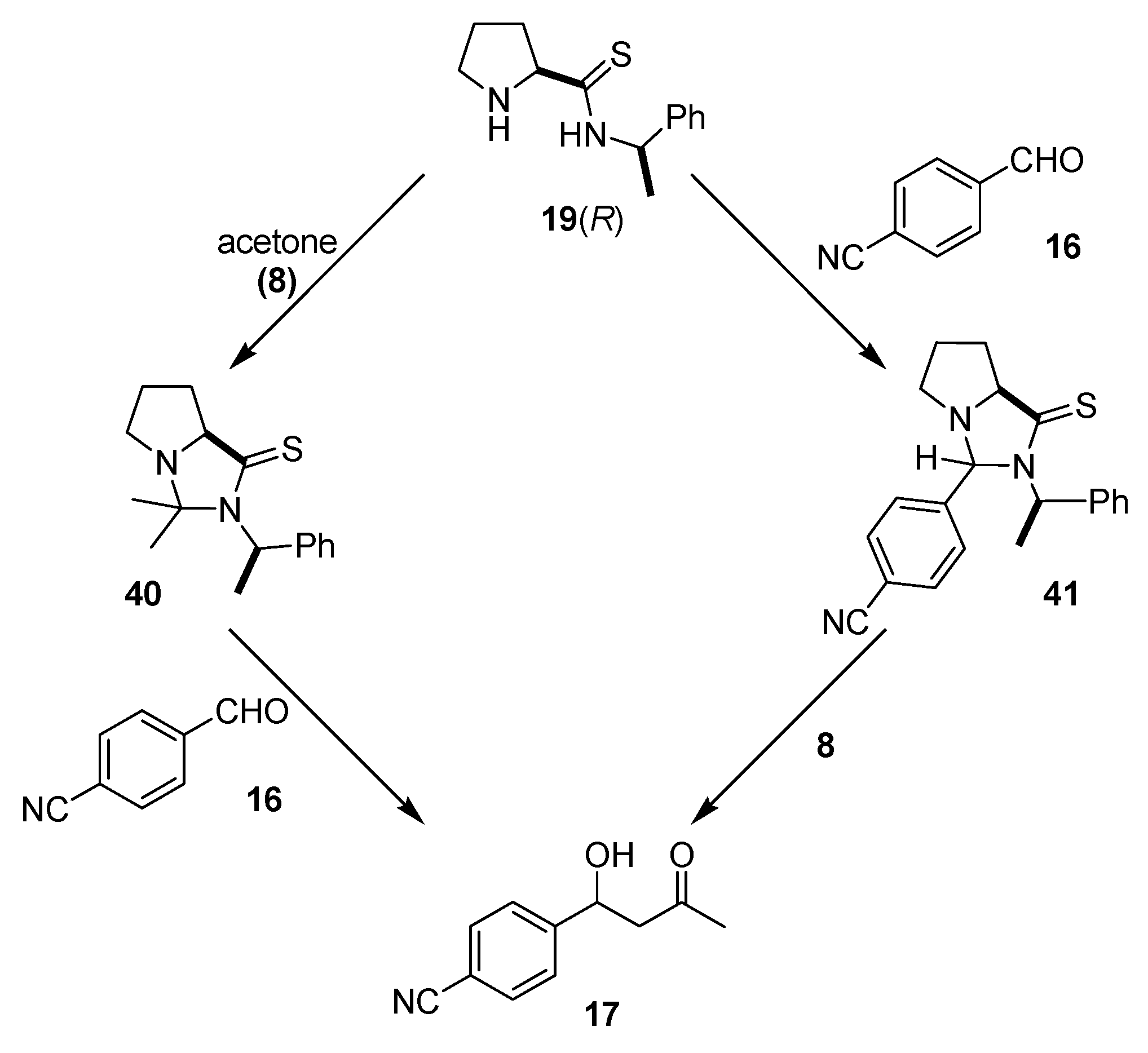
4.2.1. 1-Phenylethylamine Derived Prolinamides and Thioamides
4.2.2. L-Prolinamides and Thioamides with a Terminal Hydroxy Group. Effects of the Hydroxy Group on Catalyst Activity
5. Mechanism of the Aldol Reaction Catalyzed by Prolinamide Derivatives
6. Conclusions
Acknowledgements
References
- Erkkilä, A.; Majander, I.; Pihko, P.M. Iminium catalysis. Chem. Rev. 2007, 107, 5416–5470. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Yang, J.W.; Hoffmann, S.; List, B. Asymmetric enamine catalysis. Chem. Rev. 2007, 107, 5471–5569. [Google Scholar] [CrossRef]
- Berkessel, A.; Gröger, H. Asymmetric Organocatalysis; Wiley-VCH: Berlin, Germany, 2005; pp. 140–179. [Google Scholar]
- Tanaka, F.; Barbas, C.F., III. Aldol and Mannich-type reactions. In Enantioselective Organocatalysis; Dalko, P.I., Ed.; Wiley-VCH: Berlin, Germany, 2007; pp. 19–55. [Google Scholar]
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis—after the gold rush. Chem. Soc. Rev. 2009, 38, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Pihko, P.M.; Majander, I.; Erkkilä, A. Enamine catalysis. In Asymmetric Organocatalysis; List, B., Ed.; Springer-Verlag: Berlin, Germany, 2010; pp. 29–75. [Google Scholar]
- Geary, L.M.; Hultin, P.G. The state of the art in asymmetric induction: the aldol reaction as a case study. Tetrahedron: Asymmetry 2009, 20, 131–173. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Kucherenko, A.S.; Beletskaya, I.P. Organocatalysis of asymmetric aldol reaction. Catalysts and reagents. Russ. Chem. Rev. 2009, 78, 737–784. [Google Scholar] [CrossRef]
- Hajos, Z.G.; Parrish, D.R. Asymmetric synthesis of bicyclic intermediates of natural product chemistry. J. Org. Chem. 1974, 39, 1615–1621. [Google Scholar] [CrossRef]
- Eder, U.; Sauer, G.; Wiechert, R. New type of asymmetric cyclization to optically active steroid CD partial structures. Angew. Chem. Int. Ed. 1971, 10, 496–497. [Google Scholar] [CrossRef]
- List, B.; Lerner, R.; Barbas, C.F., III. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Bellis, E.; Kokotos, G. 4-Substituted prolines as oragnocatalysts for aldol reactions. Tetrahedron 2005, 61, 8669–8676. [Google Scholar] [CrossRef]
- Aratake, S.; Itoh, T.; Okano, T.; Nagae, N.; Sumiya, T.; Shoji, M.; Hayashi, Y. Highly diastereo- and enantioselective direct aldol reactions of aldehydes and ketones catalyzed by siloxyproline in the presence of water. Chem. Eur. J. 2007, 13, 10246–10256. [Google Scholar] [CrossRef]
- Hayashi, Y.; Aratake, S.; Okano, T.; Takahashi, J.; Sumiya, T.; Shoji, M. Combined proline-surfactant organocatalyst for the highly diastereo- and enantioselective aqueous direct cross-aldol reaction of aldehydes. Angew. Chem. Int. Ed. 2006, 45, 5527–5529. [Google Scholar] [CrossRef] [PubMed]
- Hartikka, A.; Arvidsson, P.I. Rational design of asymmetric organocatalysts—increased reactivity and solvent scope with a tetrazolic acid. Tetrahedron: Asymmetry 2004, 15, 1831–1834. [Google Scholar] [CrossRef]
- Torii, H.; Nakadai, M.; Ishihara, K.; Saito, S.; Yamamoto, H. Asymmetric direct aldol reaction assisted by water and a proline-derived tetrazole catalyst. Angew. Chem. Int. Ed. 2004, 43, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.E.T.; Brenner, S.E.; García-Fortanet, J.; Ley, S.V. An efficient, asymmetric organocatalyst-mediated conjugate addition of nitroalkanes to unsaturated cyclic and acyclic ketones. Org. Biomol. Chem. 2006, 4, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, F.; Cui, X.; Gong, L.-Z.; Mi, A.-Q.; Jiang, Y.-Z.; Wu, Y.-D. Enantioselective direct aldol reactions catalyzed by L-prolineamide derivatives. Proc. Natl. Acad. Sci. USA 2004, 101, 5755–5760. [Google Scholar] [CrossRef]
- Gryko, D.; Lipiński, R. L-Prolinethioamides–eficient organocatalysts for the direct asymmetric aldol reaction. Adv. Synth. Catal. 2005, 347, 1948–1952. [Google Scholar] [CrossRef]
- Gryko, D.; Lipiński, R. Direct asymmetric aldol reaction catalysed by L-proinethioamides. Eur. J. Org. Chem. 2006, 3864–3876. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Nájera, C. Prolinamides versus prolinethioamides as recyclabe catalysts in the enantioselective solvent-free inter- and intramolecular aldol reactions. Adv. Synth. Catal. 2008, 350, 2467–2472. [Google Scholar] [CrossRef]
- Bordwell, F.G.; Ji, G.Z. Effects of structural changes on acidities and hemolytic bond dissociation energies of the hydrogen-nitrogen bonds in amidines, carboxamides, and thiocarboxamides. J. Am. Chem. Soc. 1991, 113, 8398–8401. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Wang, H.-J.; Shi, J. Theoretical study on acidities of (S)-proline amide derivatives in DMSO and its implications for organocatalysis. J. Phys. Chem. A 2010, 114, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Aratake, S.; Itoh, T.; Okano, T.; Usui, T.; Shoji, M.; Hayashi, Y. Small organic molecule in enantioselective, direct aldol reaction “in water”. Chem. Commun. 2007, 2524–2526. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Tang, Z.; Cun, L.-F.; Mi, A.-Q.; Jiang, Y.-Z.; Gong, L.-Z. L-Proline amide-catalyzed direct asymmetric aldol reaction o aldehydes with chloroacetone. Tetrahedron 2006, 62, 346–351. [Google Scholar] [CrossRef]
- Chen, X.-H.; Luo, S.-W.; Tang, Z.; Cun, L.-F.; Mi, A.-Q.; Jiang, Y.-Z.; Gong, L.-Z. Organocatalyzed highly enantioselective direct aldol reactios of aldehydes with hydroxyacetone and fluoroacetone in aqueous media: the use of water to control regioselectivity. Chem. Eur. J. 2007, 13, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, J.N.; Saha, S. Highly diastereo- and enantioselective aldol reactions in common organic solvents using N-arylprolinamides as organocatalysts with enhanced acidity. Eur. J. Org. Chem. 2009, 739–748. [Google Scholar] [CrossRef]
- Chimni, S.S.; Singh, S.; Mahajan, D. Protonated (S)-prolinamide derivatives—water compatible organocatalysts for direct asymmetric aldol reaction. Tetrahedron: Asymmetry 2008, 19, 2276–2284. [Google Scholar] [CrossRef]
- Ji, C.; Peng, Y.; Huang, C.; Wang, N.; Jiang, Y. The influence of acidity on direct aldol reactions catalyzed by pyrrolidine/acid bifunctional organocatalyst. Synlett 2005, 986–990. [Google Scholar]
- Gryko, D.; Zimnicka, M.; Lipiński, R. Brønsted acids as additives for the direct asymmetric aldol reaction catalyzed by L-proinethioamides. Direct evidence for enamine-iminium catalysis. J. Org. Chem. 2007, 72, 964–970. [Google Scholar] [CrossRef]
- Gryko, D.; Saletra, W. Organocatalytic asymmetric aldol reaction in the presence of water. Org. Biomol. Chem. 2007, 5, 2148–2153. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Balaguer, A.N.; Nájera, C. Water versus solvent-free conditions for the enatioselective inter- and intramolecular aldol reaction employing L-prolinamides and L-prolinethioamides as organocatalysts. Adv. Synth. Catal. 2009, 351, 1123–1131. [Google Scholar]
- Wennemers, H. Peptides as asymmetric catalysts for aldol reactions. CHIMIA Int. J. Chem. 2007, 61, 276–278. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.-H.; Cun, L.-F.; Gong, L.-Z.; Mi, A.-Q.; Jiang, Y.-Z. Small peptides catalyze highly enantioselective direct aldol reactions of aldehydes with hydroxyacetone: Unprecedented regiocontrol in aqueous media. Org. Lett. 2004, 6, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, G.; Liu, L.; Chang, W.; Li, J. A novel proline-valinol thioamide small organic molecule highly enantioselective direct aldol reaction. Adv. Synth. Catal. 2009, 351, 2441–2448. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, F.; Yu, L.-T.; Cui, X.; Gong, L.-Z.; Mi, A.-Q.; Jiang, Y.-Z.; Wu, Y.-D. Novel small organic molecules for a highly enantioselective direct aldol reaction. J. Am. Chem. Soc. 2003, 125, 5262–5263. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Z.-H.; Chen, X.-H.; Cun, L.-F.; Mi, A.-Q.; Jiang, Y.-Z.; Gong, L.-Z. A highly efficient organocatalyst for direct aldol reactions of ketones with aldehydes. J. Am. Chem. Soc. 2005, 127, 9285–9289. [Google Scholar] [CrossRef] [PubMed]
- Maya, V.; Raj, M.; Singh, V. Highly enantioselective organocatalytic direct aldol reaction in an aqueous medium. Org. Lett. 2007, 9, 2593–2595. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.; Maya, V.; Ginotra, S.K.; Singh, V.K. Highly enantioselective direct aldol reaction catalyzed by organic molecules. Org. Lett. 2006, 8, 4097–4099. [Google Scholar] [CrossRef] [PubMed]
- Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C.F., III. Organocatalytic direct asymmetric aldol reactions in water. J. Am. Chem. Soc. 2006, 128, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Mase, N.; Watanabe, K.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C.F., III. Organocatalytic direct Michael reaction of ketones and aldehydes with β-nitrostyrene in brine. J. Am. Chem. Soc. 2006, 128, 4966–4967. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y. In water or in presence of water? Angew. Chem. Int. Ed. 2006, 48, 8103–8104. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Liu, L.; Chang, W.; Li, J. Highly efficient direct asymmetric aldol reactions catalyzed by a prolinethioamide derivative in aqueous media. Eur. J. Org. Chem. 2010, 5951–5954. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Houk, K.N.; Martin, H.J.; List, B. Quantum mechanical predictions of the stereoselectivities of proline-catalyzed asymmetric intermolecular aldol reactions. J. Am. Chem. Soc. 2003, 125, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Bahmanyar, S.; Houk, K.N. Transition states of amine-catalyzed aldol reactions involving enamine intermediates: Theoretical studies of mechanism, reactivity and stereoselectivity. J. Am. Chem. Soc. 2001, 123, 11273–11283. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Hoang, L.; Martin, H.J. New mechanistic studies on the proline-catalyzed aldol reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 5839–5842. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.; Metzger, J.O. ESI-MS study on the aldol reaction catalyzed by L-proline. Chem. Commun. 2006, 1539–1541. [Google Scholar] [CrossRef] [PubMed]
- Fuentes de Arriba, A.L.; Simón, L.; Raposo, C.; Alcázar, V.; Sanz, F.; Muñiz, F.M.; Morán, J.R. Imidazolidinone intermediates in prolinamide-catalyzed aldol reactions. Org. Biomol. Chem. 2010, 8, 2979–2985. [Google Scholar] [CrossRef] [PubMed]







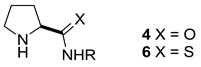
| R | pKa in DMSO | ||||||
|---|---|---|---|---|---|---|---|
| X | H | Me | Et | i-Pr | t-Bu | Ph | |
| O | (4a) 25.6 | (4b) 27.6 | (4c) 28.6 | (4d) 29.4 | (4e) 30.1 | (4f) 23.3 | |
| S | (6a) 19.0 | (6b) 20.6 | (6c) 20.4 | (6d) 21.3 | (6e) 22.5 | (6f) 16.7 | |

| Entry | R | Yield (%) | ee (%) | pKa | |
|---|---|---|---|---|---|
| 1 | OCH3 | (10a) | 78 | 31 | 24.0 |
| 2 | H | (6f) | 88 | 37 | 23.5 |
| 3 | NO2 | (10b) | 80 | 39 | - |
| 4 | CF3 | (10c) | 88 | 45 | 22.2 |

| Entry | R | c (M) a | Catalyst (mol%) | 23-meso:23-anti | Yield of 23 (ee) (%) | |
|---|---|---|---|---|---|---|
| 1 | F5C6 | (20) | 27 | 20 (19(R)) | n.d | 44 (99) |
| 2 | 2-ClC6H4 | (21) | 27 | 20 (19(R)) | 17:83 | 33 (94) |
| 3 | 4-NO2C6H4 | (7) | 0.01 (in H2O) | 10 (22) | 25:75 | 53 (n.d.) |
| Entry | Acid | pKa | Yield (%) | ee (%) |
|---|---|---|---|---|
| 1 | ─HCl | −8.00 | 0 | - |
| 2 | CF3CO2H | 0.26 | 81 | 94 |
| 3 | Cl3CCO2H | 0.65 | 10 | 93 |
| 4 | Cl2HCCO2H | 1.29 | 99 | 93 |
| 5 | ClH2CCO2H | 2.85 | 60 | 93 |
| 6 | 2-OHC6H4CO2H | 3.00 | 83 | 93 |
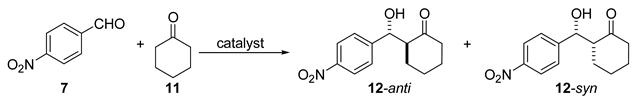
| Entry | Catalyst | Time (h) | Yield (%) | anti:syn | ee (%) | Reference |
|---|---|---|---|---|---|---|
| 1 | 19(R), (20 mol%) | traces | nd | nd | [30] | |
| 2 | 19(R)/TFA, (5 mol%) | 18 | 96 | 95:5 | 94 | [30] |
| 3 | 18(R)/HBr, (20 mol%) | 17 | 87 | 78:22 | 83 | [28] |
| 4 | 19(R)/HCl, (5 mol%) | 16 | 50 | 90:10 | 85 | [31] |
| 5 | 19(R)/4-CH3C6H4CO2H, (5 mol%) | 16 | 92 | 19:81 | 85 | [31] |
| Entry | Catalyst | Method | Time (h) | Conversion (%) | anti:syn Ratio | ee (%) anti | References |
|---|---|---|---|---|---|---|---|
| 1 | 26 | solvent-free | 3 | 99 | 70:30 | 58 | [32] |
| 2 | 27 | solvent-free | 1 | 99 | 89:11 | 88 | [32] |
| 3 | 27 | solvent-free | 8 | 98 | 94:6 | 93 | [32] |
| 4 | 26 | water | 3 | 97 | 77:23 | 71 | [21] |
| 5 | 27 | water | 3 | 99 | 91:9 | 89 | [21] |
| 6 | 27 | water | 8 a | 98 | 98:2 | 94 | [21] |
| Entry | Aldehyde | Time (h) | Yield (%) | anti:syn Ratio | ee (%) anti |
|---|---|---|---|---|---|
| 1 | 4-NO2C6H4 | 8 | 99 | 94:6 | 93 |
| 2 | 4-CNC6H4 | 24 | 93 | 95:5 | 92 |
| 3 | 4-ClC6H4 | 48 | 70 | 97:3 | 94 |
| 4 | C6H5 | 72 | 33 | 97:3 | 90 |
| 5 | 4-CH3C6H4 | 6 d | 30 | 93:7 | 84 |

| Entry | Catalyst | Time (h) | Yield (%) | ee (%) |
|---|---|---|---|---|
| 1 | 28 | 12 | 25 | 20 |
| 2 | 28/PhCO2H | 12 | 59 | 35 |
| 3 | 29 | 12 | 73 | 77 |
| 4 | 29/PhCO2H | 8 | 72 | 85 |
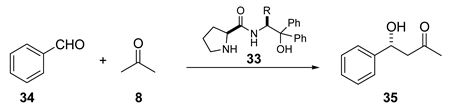
| Entry | Temperature | R | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H 33a | Me 33b | i-Pr 33c | i-Bu 33d | sec-Bu 33e | Bn 33f | Ph 33g | |||
| 1 | rt | ee (%) | - | 88 | 89 | 92 | 65 | 92 | 84 |
| yield (%) | - | 72 | 65 | 68 | 65 | 69 | 71 | ||
| 2 | ─ 40 | ee (%) | 89 | 99 | 92 | 99 | 79 | 95 | 99 |
| yield (%) | 64 | 62 (48) * | 62 | 52 (48) | 55 | 53 | 77 (22) | ||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gryko, D.; Chromiński, M.; Pielacińska, D.J. Prolinethioamides versus Prolinamides in Organocatalyzed Aldol Reactions—A Comparative Study. Symmetry 2011, 3, 265-282. https://doi.org/10.3390/sym3020265
Gryko D, Chromiński M, Pielacińska DJ. Prolinethioamides versus Prolinamides in Organocatalyzed Aldol Reactions—A Comparative Study. Symmetry. 2011; 3(2):265-282. https://doi.org/10.3390/sym3020265
Chicago/Turabian StyleGryko, Dorota, Mikołaj Chromiński, and Dominika J. Pielacińska. 2011. "Prolinethioamides versus Prolinamides in Organocatalyzed Aldol Reactions—A Comparative Study" Symmetry 3, no. 2: 265-282. https://doi.org/10.3390/sym3020265






