Early- and Late-Light Embryonic Stimulation Modulates Similarly Chicks’ Ability to Filter out Distractors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
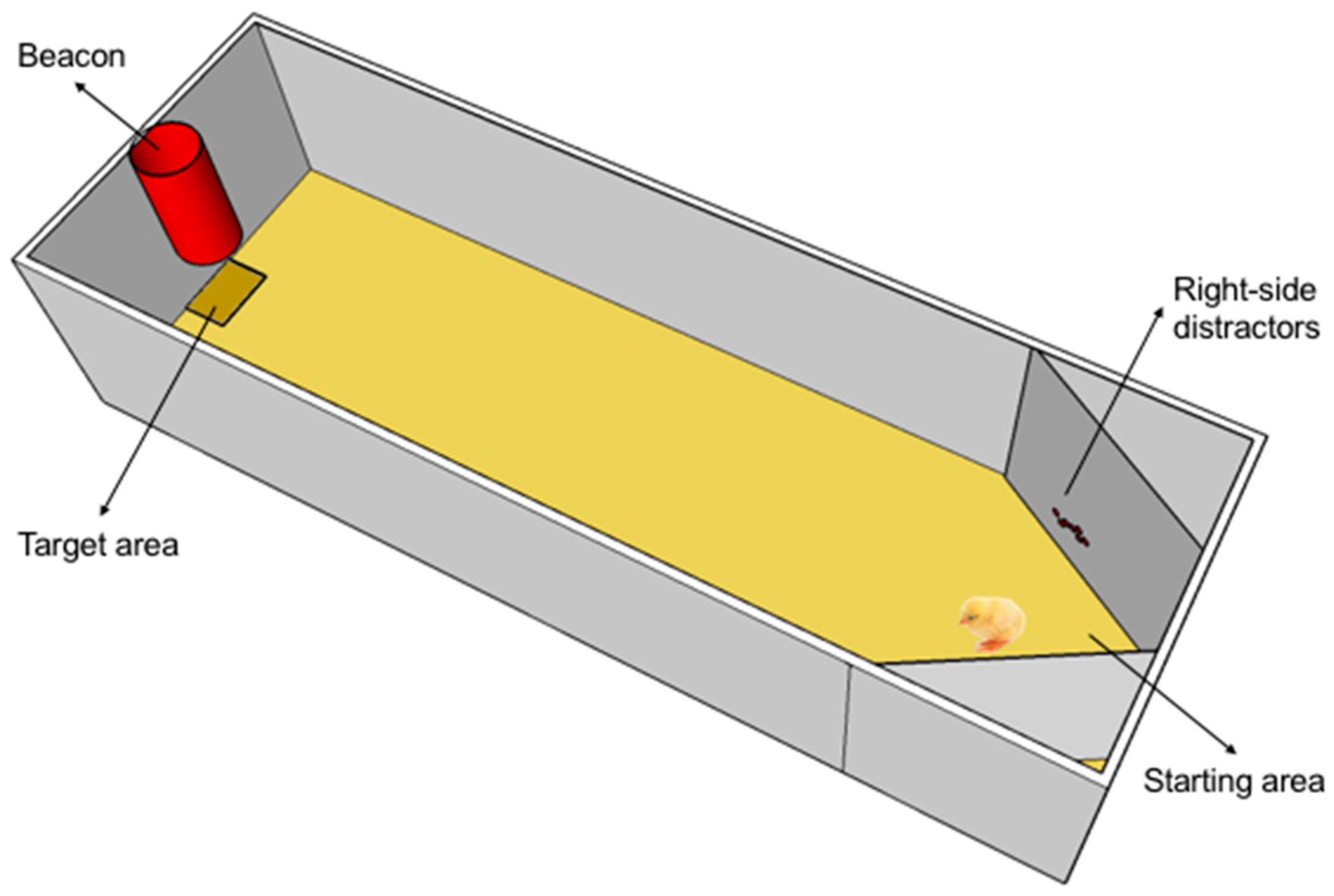
2.2. Apparatus
2.3. Procedure
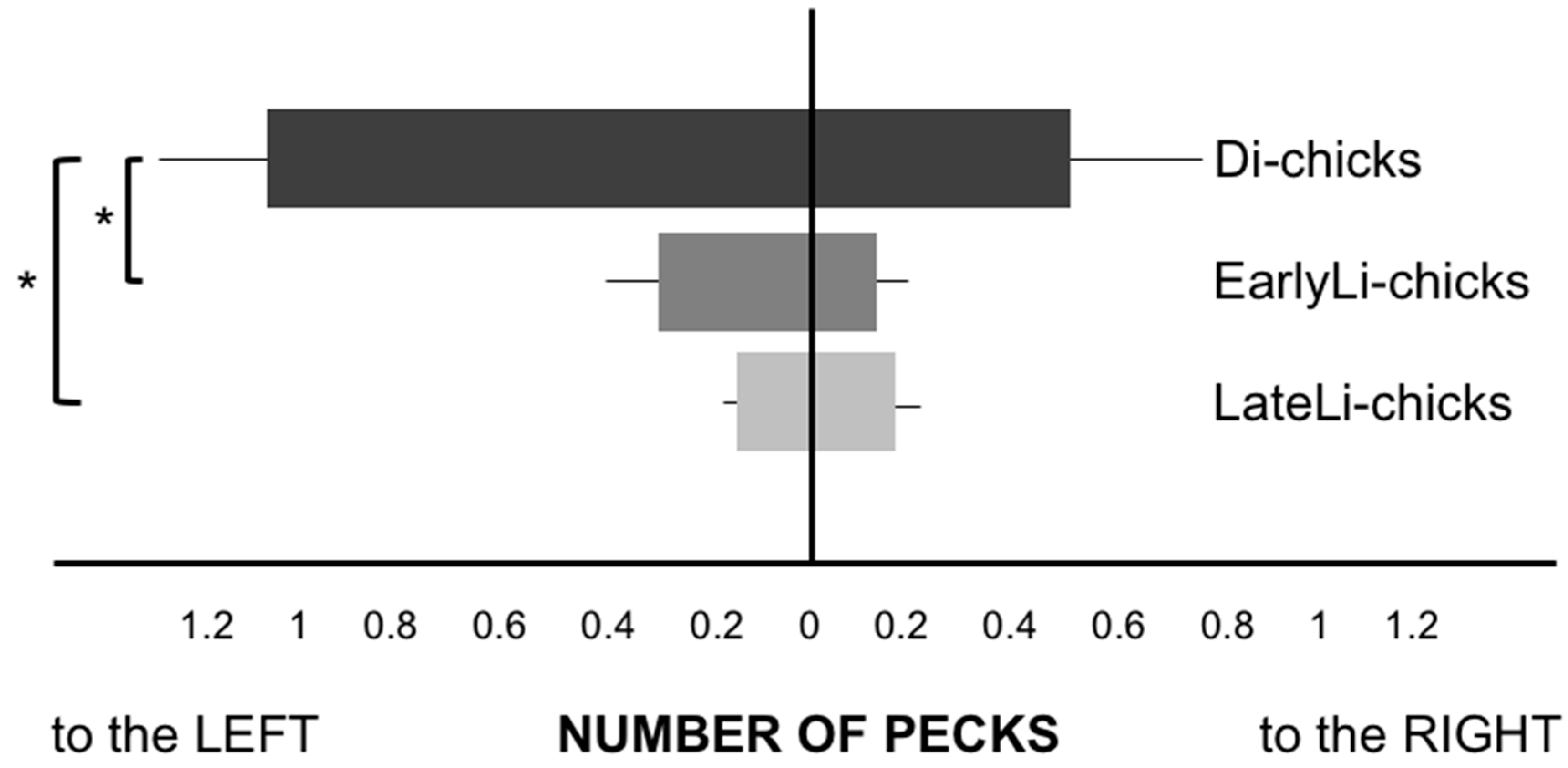
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Chiandetti, C. Manipulation of strength of cerebral lateralization via embryonic light stimulation in birds. In Lateralized Brain Functions. Methods in Human and Non-Human Species; Rogers, L.J., Vallortigara, G., Eds.; Humana Press: New York, NY, USA, 2017; pp. 611–631. [Google Scholar]
- Vallortigara, G.; Versace, E. Laterality at the Neural, Cognitive, and Behavioral Levels. In APA Handbook of Comparative Psychology: Vol. 1. Basic Concepts, Methods, Neural Substrate, and Behavior; Call, J., Ed.; American Psychological Association: Washington DC, USA, 2017; pp. 557–577. [Google Scholar]
- Kuan, Y.-S.; Gamse, J.T.; Schreiber, A.M.; Halpern, M.E. Selective asymmetry in a conserved forebrain to midbrain projection. J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Roussigné, M.; Blader, P.; Wilson, S.W. Breaking symmetry: The zebrafish as a model for understanding left-right asymmetry in the developing brain. Dev. Neurobiol. 2012, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Gamse, J.T.; Thisse, C.; Thisse, B.; Halpern, M.E. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development 2003, 130, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Concha, M.L.; Bianco, I.H.; Wilson, S.W. Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 2012, 13, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.J.; Osorio, D.; Budaev, S. Light during embryonic development modulates patterns of lateralization strongly and similarly in both zebrafish and chick. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Budaev, S.; Andrew, R.J. Patterns of early embryonic light exposure determine behavioural asymmetries in zebrafish: A habenular hypothesis. Behav. Brain Res. 2009, 200, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Budaev, S.; Andrew, R. Shyness and behavioural asymmetries in larval zebrafish (Brachydanio rerio) developed in light and dark. Behaviour 2009, 146, 1037–1052. [Google Scholar] [CrossRef]
- De Borsetti, N.H.; Dean, B.J.; Bain, E.J.; Clanton, J.A.; Taylor, R.W.; Gamse, J.T. Light and melatonin schedule neuronal differentiation in the habenular nuclei. Dev. Biol. 2011, 358, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.A.; Miklósi, A.; Watkins, J.; Bianco, I.H.; Wilson, S.W.; Andrew, R.J. Fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 2005, 15, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Johnson, R.L.; Stern, C.D.; Kuehn, M.R.; Tabin, C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 1995, 82, 803–814. [Google Scholar] [CrossRef]
- Rogers, L.J. Light experience and asymmetry of brain function in chickens. Nature 1982, 297, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Light input and the reversal of functional lateralization in the chicken brain. Behav. Brain Res. 1990, 38, 211–221. [Google Scholar] [CrossRef]
- Rogers, L.J.; Deng, C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 1999, 98, 277–287. [Google Scholar] [CrossRef]
- Ströckens, F.; Güntürkün, O. Cryptochrome 1b: A possible inducer of visual lateralization in pigeons? Eur. J. Neurosci. 2016, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Anson, J.M. Lateralisation of function in the chicken fore-brain. Pharmacol. Biochem. Behav. 1979, 10, 679–686. [Google Scholar] [CrossRef]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. R. Soc. B Biol. Sci. 2004, 271, S420–S422. [Google Scholar] [CrossRef] [PubMed]
- Chiandetti, C.; Vallortigara, G. Effects of embryonic light stimulation on the ability to discriminate left from right in the domestic chick. Behav. Brain Res. 2009, 198, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Chiandetti, C. Pseudoneglect and embryonic light stimulation in the avian brain. Behav. Neurosci. 2011, 125, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.; Güntürkün, O. Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon’s (Columba livia) visual system. Behav. Neurosci. 1999, 113, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Mascetti, G.G.; Vallortigara, G. Why do birds sleep with one eye open? Light exposure of the chick embryo as a determinant of monocular sleep. Curr. Biol. 2001, 11, 971–974. [Google Scholar] [CrossRef]
- Vallortigara, G.; Andrew, R.J. Lateralization of response by chicks to change in a model partner. Anim. Behav. 1991, 41, 187–194. [Google Scholar] [CrossRef]
- Vallortigara, G.; Andrew, R.J. Olfactory lateralization in the chick. Neuropsychologia 1994, 32, 417–423. [Google Scholar] [CrossRef]
- Vallortigara, G. Right hemisphere advantage for social recognition in the chick. Neuropsychologia 1992, 30, 761–768. [Google Scholar] [CrossRef]
- Andrew, R.J.; Johnston, A.N.B.; Robins, A.; Rogers, L.J. Light experience and the development of behavioural lateralisation in chicks. II. Choice of familiar versus unfamiliar model social partner. Behav. Brain Res. 2004, 155, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.N.B.; Rogers, L.J. Light exposure of chick embryo influences lateralized recall of imprinting memory. Behav. Neurosci. 1999, 113, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.J. Origins of asymmetry in the CNS. Semin. Cell Dev. Biol. 2009, 20, 485–490. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left & right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [PubMed]
- Rogers, L.J. Asymmetry of Brain and Behavior in Animals: Its Development, Function, and Human Relevance. Genesis 2014, 52, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.; Ströckens, F. Functional and structural comparison of visual lateralization in birds—Similar but still different. Front. Psychol. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiandetti, C.; Galliussi, J.; Andrew, R.J.; Vallortigara, G. Early-light embryonic stimulation suggests a second route, via gene activation, to cerebral lateralization in vertebrates. Sci. Rep. 2013, 3, 2701. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Tomonari, S.; Takagi, A.; Akamatsu, S.; Noji, S.; Ohuchi, H. A non-canonical photopigment, melanopsin, is expressed in the differentiating ganglion, horizontal, and bipolar cells of the chicken retina. Dev. Dyn. 2005, 234, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.; Römling, J. The impact of asymmetrical light input on cerebral hemispheric specialization and interhemispheric cooperation. Nat. Commun. 2012, 3, 696. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. The two hemispheres of the avian brain: Their differing roles in perceptual processing and the expression of behavior. J. Ornithol. 2012, 153, 61–74. [Google Scholar] [CrossRef]
- Agetsuma, M.; Aizawa, H.; Aoki, T.; Nakayama, R.; Takahoko, M.; Goto, M.; Sassa, T.; Amo, R.; Shiraki, T.; Kawakami, K.; et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010, 13, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, A.; Andrew, R.J. Right eye use associated with decision to bite in zebrafish. Behav. Brain Res. 1999, 105, 199–205. [Google Scholar] [CrossRef]
- Miklósi, A.; Andrew, R.J.; Gasparini, S. Role of right hemifield in visual control of approach to target in zebrafish. Behav. Brain Res. 2001, 122, 57–65. [Google Scholar] [CrossRef]
- Omura, Y.; Oguri, M. Early development of the pineal photoreceptors prior to the retinal differentiation in the embryonic rainbow trout, Oncorhynchus mykiss (Teleostei). Arch. Histol. Cytol. 1993, 56, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Östholm, T.; Brännäs, E.; van Veen, T. The pineal organ is the first differentiated light receptor in the embryonic salmon, Salmo salar L. Cell Tissue Res. 1987, 249, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Guglielmotti, V.; Bentivoglio, M. Diencephalic asymmetries. Neurosci. Biobehav. Rev. 1996, 20, 637–643. [Google Scholar] [CrossRef]
- Braitenberg, V.; Kemali, M. Exceptions to bilateral symmetry in the epithalamus of lower vertebrates. J. Comp. Neurol. 1970, 138, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bianco, I.H.; Wilson, S.W. The habenular nuclei: A conserved asymmetric relay station in the vertebrate brain. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Gurusinghe, C.J.; Ehrlich, D. Sex-dependent structural asymmetry of the medial habenular nucleus of the chicken brain. Cell. Tissue Res. 1985, 240, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Fejér, Z.; Röhlich, P.; Szél, Á.; Dávid, C.; Zádori, A.; Manzano, M.J.; Vígh, B. Comparative ultrastructure and cytochemistry of the avian pineal organ. Microsc. Res. Tech. 2001, 53, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Vígh, B.; Röhlich, P.; Görcs, T.; Maria, M.J.; Szél, Á.; Fejér, Z.; Vígh-Teichmann, I. The pineal organ as a folded retina: Immunocytochemical localization of opsins. Biol. Cell 1998, 90, 653–659. [Google Scholar] [PubMed]
- Nakane, Y. Intrinsic photosensitivity of a deep brain photoreceptor. Curr. Biol. 2014, 24, R596–R597. [Google Scholar] [CrossRef] [PubMed]
- Nakane, Y.; Ikegami, K.; Ono, H.; Yamamoto, N.; Yoshida, S.; Hirunagi, K.; Ebihara, S.; Kubo, Y.; Yoshimura, T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. USA 2010, 107, 15264–15268. [Google Scholar] [CrossRef] [PubMed]
- Letzner, S.; Patzke, N.; Verhaal, J.; Manns, M. Shaping a lateralized brain: Asymmetrical light experience modulates access to visual interhemispheric information in pigeons. Sci. Rep. 2014, 4, 4253. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M. Alone in the dark? Modeling the conditions for visual experience in human fetuses. Dev. Psychobiol. 2011, 53, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, M.; Nagata, N.; Ikeno, S.; Terakawa, N. The relationship between the response to external light stimulation and behavioral states in the human fetus: How it differs from vibroacoustic stimulation. Early Hum. Dev. 2000, 58, 153–165. [Google Scholar] [CrossRef]
- Geschwind, N.; Galaburda, A.M. Cerebral Lateralization: Biological Mechanisms, Associations and Pathology; MIT Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Tran, U.S.; Stieger, S.; Voracek, M. Latent variable analysis indicates that seasonal anisotropy accounts for the higher prevalence of left-handedness in men. Cortex 2014, 57, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.J. Neural and Behavioural Plasticity: The Use of the Domestic Chick as a Model; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Vallortigara, G.; Regolin, L.; Zucca, P. Secondary imprinting in the domestic chick: Binocular and lateralized monocular performance. Int. J. Comp. Psychol. 2000, 13, 119–136. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiandetti, C.; Lemaire, B.S.; Versace, E.; Vallortigara, G. Early- and Late-Light Embryonic Stimulation Modulates Similarly Chicks’ Ability to Filter out Distractors. Symmetry 2017, 9, 84. https://doi.org/10.3390/sym9060084
Chiandetti C, Lemaire BS, Versace E, Vallortigara G. Early- and Late-Light Embryonic Stimulation Modulates Similarly Chicks’ Ability to Filter out Distractors. Symmetry. 2017; 9(6):84. https://doi.org/10.3390/sym9060084
Chicago/Turabian StyleChiandetti, Cinzia, Bastien S. Lemaire, Elisabetta Versace, and Giorgio Vallortigara. 2017. "Early- and Late-Light Embryonic Stimulation Modulates Similarly Chicks’ Ability to Filter out Distractors" Symmetry 9, no. 6: 84. https://doi.org/10.3390/sym9060084





