Two-Stage Origin of K-Enrichment in Ultrapotassic Magmatism Simulated by Melting of Experimentally Metasomatized Mantle
Abstract
:1. Introduction
2. Experimental Strategy
3. Materials and Methods
3.1. Starting Materials
3.2. Experimental and Analytical Techniques
4. Results
4.1. First-Stage Experiments
4.2. Second-Stage Experiments
5. Discussion
5.1. Comparison with Previous Experimental Studies
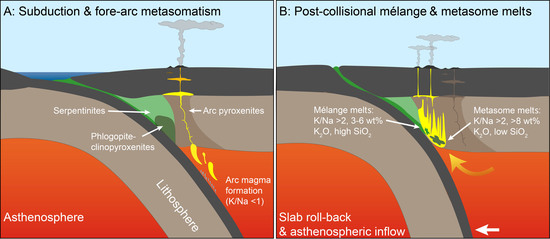
- (A)
- UP lavas form by melting of phlogopite pyroxenites and phlogopite peridotites that are abundant within the continental lithospheric mantle [58,59]; this has been successfully simulated by experiments with UP melts (Figure 2), K2O 6 wt%–12 wt%, MgO >3 wt%, and low SiO2 [15,16,60]. Infiltrating melts derived from sediments form phlogopite-bearing pyroxenites at the expense of peridotites following the reaction melt + olivine → phlogopite + pyroxene [23,35,61]. This process is concentrated in the fore-arc, where hydrous sediments melt and infiltrate the mantle wedge because of their low solidus temperatures and high volatile contents [34].
- (B)
- UP lavas form by direct melting and hybridization of crustal rocks with mantle peridotites in a single stage process at mantle depths [7,62]. Melt inclusions in garnets in ultrahigh-pressure paragneiss (5 GPa) are strongly enriched in potassium and resemble some types of shoshonitic magmas [63]. In this scenario, either the subducted crustal rocks mix with peridotites to form mélanges, or melts of subducted crustal rocks percolate and react with peridotites to form UP melts. This has previously been tested in experiments where a rhyolitic melt with as much as 6.4 wt% K2O was mixed with harzburgite [64], while others hybridized a phyllite (1.9 wt% K2O) with dunite [30,31]. In both cases, experimental melts contain <6 wt% K2O, and K/Na ratios of 4–5, much lower than the 12 wt% K2O and K/Na up to 10 of UP lavas [2]. It is noteworthy that crustal lithologies have a solidus up to 500 °C lower than that of peridotite [21]. Therefore, at mantle temperatures of >1200 °C, crustal lithologies generate Si-rich high degree melts in which K2O is diluted. This is evident from experiments hybridizing rhyolite and peridotite at 1200–1350 °C [19,64], where the maximum K2O mass fraction of the melt (~7.4 wt%) is close to that of the rhyolite component in the starting material (6.4 wt%). Even though these studies did not aim to reveal the origin of UP lavas, their work demonstrates that even high mass fractions of K2O in the starting material do not produce UP melts. In addition, K/Na ratios are lower than in the bulk starting material at >1100 °C, as melting of crustal rocks at high mantle temperatures does not form any Na-bearing phases that can increase K/Na ratios of the melt. All experimental melt compositions of sediment/peridotite hybridization so far fit this pattern [19,30,31,64], with K/Na values of 1–2 at ≥1100 °C (Figure 4A). In contrast, at <1100 °C, where temperatures are below that of the ambient mantle, experimental melts of either sedimentary rocks alone, or melts resulting from sediment/peridotite reaction, reach K/Na values that exceed those of the starting material. This difference in behavior is induced by the interaction between phengite stability and Na2O content of residual clinopyroxene in the sediment layer: as in the case of melting of phlogopite, the highest K/Na of experimental melts are reached after phengite breakdown (at 900 °C; Figure 4A) where all potassium is accommodated within the melt. However, at <1100 °C, the melts are generally Si-rich (SiO2 > 70 wt%), thus high K/Na values are bound to high Si- and low-Mg contents, unlike UP lavas. These results are in accordance with previous experiments by the authors of [30,31], showing that melts produced by sediment + peridotite hybridization are mildly potassic and Si-rich. These may explain the origin of high-K calc-alkaline to shoshonitic lavas [7]. However, hybridization and melting of sediment and peridotite fail to explain the origin of the most K-enriched lavas—lamproites—which form in the same areas as mildly potassic lavas [9].
5.2. First-Stage Experiments: Formation of Si-Rich Potassic Melts from a Phlogopite-Free Source
5.3. Second-Stage Experiments: Formation of High K/Na Melts from a Metasomatized, Phlogopite-Bearing Mantle
5.4. Implications for the Formation of Post-Collisional Lavas
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P. Igneous Rocks: A Classification and Glossary of Terms: Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks; Cambridge University Press: Cambridge, UK, 2005; ISBN 1139439391. [Google Scholar]
- Mitchell, R.H.; Bergman, S.C. Petrology of Lamproites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1991; ISBN 030643556X. [Google Scholar]
- Foley, S.; Venturelli, G.; Green, D.H.; Toscani, L. The ultrapotassic rocks: Characteristics, classification, and constraints for petrogenetic models. Earth-Sci. Rev. 1987, 24, 81–134. [Google Scholar] [CrossRef]
- Hofmann, A.W. Chemical differentiation of the Earth: The relationship between mantle, continental crust, and oceanic crust. Earth Planet. Sci. Lett. 1988, 90, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Novella, D.; Frost, D.J.; Hauri, E.H.; Bureau, H.; Raepsaet, C.; Roberge, M. The distribution of H2O between silicate melt and nominally anhydrous peridotite and the onset of hydrous melting in the deep upper mantle. Earth Planet. Sci. Lett. 2014, 400, 1–13. [Google Scholar] [CrossRef]
- Foley, S. Vein-plus-wall-rock melting mechanisms in the lithosphere and the origin of potassic alkaline magmas. Lithos 1992, 28, 435–453. [Google Scholar] [CrossRef]
- Campbell, I.H.; Stepanov, A.S.; Liang, H.-Y.; Allen, C.M.; Norman, M.D.; Zhang, Y.-Q.; Xie, Y.-W. The origin of shoshonites: New insights from the Tertiary high-potassium intrusions of eastern Tibet. Contrib. Mineral. Petrol. 2014, 167, 983. [Google Scholar] [CrossRef]
- Conticelli, S.; Avanzinelli, R.; Ammannati, E.; Casalini, M. The role of carbon from recycled sediments in the origin of ultrapotassic igneous rocks in the Central Mediterranean. Lithos 2015, 232, 174–196. [Google Scholar] [CrossRef]
- Prelević, D.; Foley, S.F.; Romer, R.; Conticelli, S. Mediterranean Tertiary lamproites derived from multiple source components in postcollisional geodynamics. Geochim. Cosmochim. Acta 2008, 72, 2125–2156. [Google Scholar] [CrossRef]
- Xu, B.; Griffin, W.L.; Xiong, Q.; Hou, Z.-Q.; O’Reilly, S.Y.; Guo, Z.; Pearson, N.J.; Gréau, Y.; Yang, Z.-M.; Zheng, Y.-C. Ultrapotassic rocks and xenoliths from South Tibet: Contrasting styles of interaction between lithospheric mantle and asthenosphere during continental collision. Geology 2017, 45, 51–54. [Google Scholar] [CrossRef]
- Lustrino, M. What ‘anorogenic’igneous rocks can tell us about the chemical composition of the upper mantle: Case studies from the circum-Mediterranean area. Geol. Mag. 2011, 148, 304–316. [Google Scholar] [CrossRef]
- Foley, S.F.; Prelevic, D.; Rehfeldt, T.; Jacob, D.E. Minor and trace elements in olivines as probes into early igneous and mantle melting processes. Earth Planet. Sci. Lett. 2013, 363, 181–191. [Google Scholar] [CrossRef]
- Foley, S. Petrological characterization of the source components of potassic magmas: Geochemical and experimental constraints. Lithos 1992, 28, 187–204. [Google Scholar] [CrossRef]
- Safonov, O.; Butvina, V.; Limanov, E. Phlogopite-Forming Reactions as Indicators of Metasomatism in the Lithospheric Mantle. Minerals 2019, 9, 685. [Google Scholar] [CrossRef] [Green Version]
- Condamine, P.; Médard, E.; Devidal, J.-L. Experimental melting of phlogopite-peridotite in the garnet stability field. Contrib. Mineral. Petrol. 2016, 171, 95. [Google Scholar] [CrossRef]
- Förster, M.W.; Prelević, D.; Schmück, H.R.; Buhre, S.; Veter, M.; Mertz-Kraus, R.; Foley, S.F.; Jacob, D.E. Melting and dynamic metasomatism of mixed harzburgite+ glimmerite mantle source: Implications for the genesis of orogenic potassic magmas. Chem. Geol. 2017, 455, 182–191. [Google Scholar] [CrossRef]
- Condamine, P.; Médard, E. Experimental melting of phlogopite-bearing mantle at 1 GPa: Implications for potassic magmatism. Earth Planet. Sci. Lett. 2014, 397, 80–92. [Google Scholar] [CrossRef]
- Foley, S.F.; Musselwhite, D.S.; van der Laan, S.R. Melt Compositions from Ultramafic Vein Assemblages in the Lithospheric Mantle: A Comparison of Cratonic and Non-Cratonic Settings. In Proceedings of the 7th International Kimberlite Conference Foley, Cape Town, South Africa, 11–17 April 1998; Red Roof Design Cape Town: Cape Town, South Africa, 1999. [Google Scholar]
- Mallik, A.; Dasgupta, R.; Tsuno, K.; Nelson, J. Effects of water, depth and temperature on partial melting of mantle-wedge fluxed by hydrous sediment-melt in subduction zones. Geochim. Cosmochim. Acta 2016, 195, 226–243. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Prelević, D.; Buhre, S.; Foley, S.F. Constraints on the sources of post-collisional K-rich magmatism: The roles of continental clastic sediments and terrigenous blueschists. Chem. Geol. 2017, 455, 192–207. [Google Scholar] [CrossRef]
- Hermann, J.; Spandler, C.; Hack, A.; Korsakov, A.V. Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos 2006, 92, 399–417. [Google Scholar] [CrossRef]
- Hermann, J.; Spandler, C.J. Sediment melts at sub-arc depths: An experimental study. J. Petrol. 2007, 49, 717–740. [Google Scholar] [CrossRef] [Green Version]
- Sekine, T.; Wyllie, P.J. Experimental simulation of mantle hybridization in subduction zones. J. Geol. 1983, 91, 511–528. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Sekine, T. The formation of mantle phlogopite in subduction zone hybridization. Contrib. Mineral. Petrol. 1982, 79, 375–380. [Google Scholar] [CrossRef]
- Plank, T.; Langmuir, C.H. The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem. Geol. 1998, 145, 325–394. [Google Scholar] [CrossRef]
- Tommasini, S.; Avanzinelli, R.; Conticelli, S. The Th/La and Sm/La conundrum of the Tethyan realm lamproites. Earth Planet. Sci. Lett. 2011, 301, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Avanzinelli, R.; Elliott, T.; Tommasini, S.; Conticelli, S. Constraints on the genesis of potassium-rich Italian volcanic rocks from U/Th disequilibrium. J. Petrol. 2007, 49, 195–223. [Google Scholar] [CrossRef]
- Prelević, D.; Jacob, D.E.; Foley, S.F. Recycling plus: A new recipe for the formation of Alpine–Himalayan orogenic mantle lithosphere. Earth Planet. Sci. Lett. 2013, 362, 187–197. [Google Scholar] [CrossRef]
- Prelević, D.; Akal, C.; Romer, R.L.; Foley, S.F. Lamproites as indicators of accretion and/or shallow subduction in the assembly of south-western Anatolia, Turkey. Terra Nova 2010, 22, 443–452. [Google Scholar] [CrossRef]
- Wang, Y.; Foley, S.F. Hybridization Melting between Continent-derived Sediment and Depleted Peridotite in Subduction Zones. J. Geophys. Res. Solid Earth 2018, 123, 3414–3429. [Google Scholar] [CrossRef]
- Wang, Y.; Foley, S.F.; Prelević, D. Potassium-rich magmatism from a phlogopite-free source. Geology 2017, 45, 467–470. [Google Scholar] [CrossRef]
- Lundstrom, C.C. An experimental investigation of the diffusive infiltration of alkalis into partially molten peridotite: Implications for mantle melting processes. Geochem. Geophys. Geosyst. 2003, 4. [Google Scholar] [CrossRef]
- Vigouroux, N.; Wallace, P.J.; Kent, A., Jr. Volatiles in high-K magmas from the western Trans-Mexican Volcanic Belt: Evidence for fluid fluxing and extreme enrichment of the mantle wedge by subduction processes. J. Petrol. 2008, 49, 1589–1618. [Google Scholar] [CrossRef]
- Gülmez, F.; Genç, Ş.C.; Prelević, D.; Tüysüz, O.; Karacik, Z.; Roden, M.F.; Billor, Z. Ultrapotassic volcanism from the waning stage of the Neotethyan subduction: A key study from the Izmir–Ankara–Erzincan Suture Belt, Central Northern Turkey. J. Petrol. 2016, 57, 561–593. [Google Scholar] [CrossRef]
- Sekine, T.; Wyllie, P.J. Phase relationships in the system KAlSiO4-Mg2SiO4-SiO2-H2O as a model for hybridization between hydrous siliceous melts and peridotite. Contrib. Mineral. Petrol. 1982, 79, 368–374. [Google Scholar] [CrossRef]
- Massonne, H.-J. Evidence for low-temperature ultrapotassic siliceous fluids in subduction zone environments from experiments in the system K2O MgO Al2O3 SiO2 H2O (KMASH). Lithos 1992, 28, 421–434. [Google Scholar] [CrossRef]
- Funk, S.P.; Luth, R.W. Melting phase relations of a mica–clinopyroxenite from the Milk River area, southern Alberta, Canada. Contrib. Mineral. Petrol. 2013, 166, 393–409. [Google Scholar] [CrossRef]
- Lloyd, F.E.; Arima, M.; Edgar, A.D. Partial melting of a phlogopite-clinopyroxenite nodule from south-west Uganda: An experimental study bearing on the origin of highly potassic continental rift volcanics. Contrib. Mineral. Petrol. 1985, 91, 321–329. [Google Scholar] [CrossRef]
- Bryant, J.A.; Yogodzinski, G.M.; Churikova, T.G. Melt-mantle interactions beneath the Kamchatka arc: Evidence from ultramafic xenoliths from Shiveluch volcano. Geochem. Geophys. Geosyst. 2007, 8. [Google Scholar] [CrossRef] [Green Version]
- Kargin, A.V.; Sazonova, L.V.; Nosova, A.A.; Lebedeva, N.M.; Tretyachenko, V.V.; Abersteiner, A. Cr-rich clinopyroxene megacrysts from the Grib kimberlite, Arkhangelsk province, Russia: Relation to clinopyroxene–phlogopite xenoliths and evidence for mantle metasomatism by kimberlite melts. Lithos 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Aulbach, S.; Sun, J.; Tappe, S.; Höfer, H.E.; Gerdes, A. Volatile-rich metasomatism in the Cratonic Mantle beneath SW Greenland: Link to Kimberlites and Mid-lithospheric discontinuities. J. Petrol. 2017, 58, 2311–2338. [Google Scholar] [CrossRef]
- Dawson, J.B.; Smith, J.V. The MARID (mica-amphibole-rutile-ilmenite-diopside) suite of xenoliths in kimberlite. Geochim. Cosmochim. Acta 1977, 41, 309–323. [Google Scholar] [CrossRef]
- Conticelli, S.; Guarnieri, L.; Farinelli, A.; Mattei, M.; Avanzinelli, R.; Bianchini, G.; Boari, E.; Tommasini, S.; Tiepolo, M.; Prelević, D. Trace elements and Sr–Nd–Pb isotopes of K-rich, shoshonitic, and calc-alkaline magmatism of the Western Mediterranean Region: Genesis of ultrapotassic to calc-alkaline magmatic associations in a post-collisional geodynamic setting. Lithos 2009, 107, 68–92. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Wilson, M.; Liu, J.; Mao, Q. Post-collisional, potassic and ultrapotassic magmatism of the northern Tibetan Plateau: Constraints on characteristics of the mantle source, geodynamic setting and uplift mechanisms. J. Petrol. 2006, 47, 1177–1220. [Google Scholar] [CrossRef]
- Sokol, K.; Prelevic, D.; Romer, R.; Bozovic, M.; van den Bogaard, P.; Stefanova, E.; Kostic, B.; Cokulov, N. Cretaceous ultrapotassic magmatism from the Sava-Vardar zone of the Balkans. Lithos 2019, 105268. [Google Scholar] [CrossRef]
- Huong, L.; Otter, L.; Förster, M.; Hauzenberger, C.; Krenn, K.; Alard, O.; Jochum, K. Femtosecond laser ablation-ICP-mass spectrometry and CHNS elemental analyzer reveal trace element characteristics of danburite from Mexico, Tanzania, and Vietnam. Minerals 2018, 8, 234. [Google Scholar] [CrossRef] [Green Version]
- Ziaja, K.; Foley, S.F.; White, R.W.; Buhre, S. Metamorphism and melting of picritic crust in the early Earth. Lithos 2014, 189, 173–184. [Google Scholar] [CrossRef]
- Gonzalez, C.M.; Gorczyk, W.; Gerya, T.V. Decarbonation of subducting slabs: Insight from petrological–thermomechanical modeling. Gondwana Res. 2016, 36, 314–332. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostandards Geoanalytical Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A new geochemical database for reference materials and isotopic standards. Geostandards Geoanalytical Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Griffin, W.L. GLITTER: Data reduction software for laser ablation ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Mineralogical Association of Canada: Quebec City, QC, Canada, 2008; pp. 308–311. [Google Scholar]
- Chiodini, G.; Marini, L. Hydrothermal gas equilibria: The H2O-H2-CO2-CO-CH4 system. Geochim. Cosmochim. Acta 1998, 62, 2673–2687. [Google Scholar] [CrossRef]
- Förster, M.W.; Prelević, D.; Buhre, S.; Mertz-Kraus, R.; Foley, S.F. An experimental study of the role of partial melts of sediments versus mantle melts in the sources of potassic magmatism. J. Asian Earth Sci. 2019, 177, 76–88. [Google Scholar] [CrossRef]
- Falloon, T.J.; Green, D.H.; O’Neill, H.S.C.; Hibberson, W.O. Experimental tests of low degree peridotite partial melt compositions: Implications for the nature of anhydrous near-solidus peridotite melts at 1 GPa. Earth Planet. Sci. Lett. 1997, 152, 149–162. [Google Scholar] [CrossRef]
- Pirard, C.; Hermann, J. Experimentally determined stability of alkali amphibole in metasomatised dunite at sub-arc pressures. Contrib. Mineral. Petrol. 2005, 169, 1. [Google Scholar] [CrossRef]
- Prelević, D.; Akal, C.; Foley, S.F.; Romer, R.L.; Stracke, A.; van den Bogaard, P. Ultrapotassic mafic rocks as geochemical proxies for post-collisional dynamics of orogenic lithospheric mantle: The case of southwestern Anatolia, Turkey. J. Petrol. 2012, 53, 1019–1055. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.; Zhu, D.-C.; Niu, Y.; DePaolo, D.J.; Harrison, T.M.; Mo, X.; Dong, G.; Zhou, S.; Sun, C. Postcollisional potassic and ultrapotassic rocks in southern Tibet: Mantle and crustal origins in response to India–Asia collision and convergence. Geochim. Cosmochim. Acta 2014, 143, 207–231. [Google Scholar] [CrossRef]
- Martin, A.M.; Médard, E.; Righter, K.; Lanzirotti, A. Intraplate mantle oxidation by volatile-rich silicic magmas. Lithos 2017, 292, 320–333. [Google Scholar] [CrossRef]
- Mattsson, H.B.; Nandedkar, R.H.; Ulmer, P. Petrogenesis of the melilititic and nephelinitic rock suites in the Lake Natron–Engaruka monogenetic volcanic field, northern Tanzania. Lithos 2013, 179, 175–192. [Google Scholar] [CrossRef]
- Förster, M.W.; Prelević, D.; Schmück, H.R.; Buhre, S.; Marschall, H.R.; Mertz-Kraus, R.; Jacob, D.E. Melting phlogopite-rich MARID: Lamproites and the role of alkalis in olivine-liquid Ni-partitioning. Chem. Geol. 2018, 476, 429–440. [Google Scholar] [CrossRef]
- Sekine, T.; Wyllie, P.J. The system granite-peridotite-H2O at 30 kbar, with applications to hybridization in subduction zone magmatism. Contrib. Mineral. Petrol. 1982, 81, 190–202. [Google Scholar] [CrossRef]
- Ersoy, E.Y.; Helvacı, C.; Palmer, M.R. Mantle source characteristics and melting models for the early-middle Miocene mafic volcanism in Western Anatolia: Implications for enrichment processes of mantle lithosphere and origin of K-rich volcanism in post-collisional settings. J. Volcanol. Geotherm. Res. 2010, 198, 112–128. [Google Scholar] [CrossRef]
- Stepanov, A.S.; Hermann, J.; Rubatto, D.; Korsakov, A.V.; Danyushevsky, L.V. Melting history of an ultrahigh-pressure paragneiss revealed by multiphase solid inclusions in garnet, Kokchetav massif, Kazakhstan. J. Petrol. 2016, 57, 1531–1554. [Google Scholar] [CrossRef] [Green Version]
- Mallik, A.; Nelson, J.; Dasgupta, R. Partial melting of fertile peridotite fluxed by hydrous rhyolitic melt at 2–3 GPa: Implications for mantle wedge hybridization by sediment melt and generation of ultrapotassic magmas in convergent margins. Contrib. Mineral. Petrol. 2015, 169, 48. [Google Scholar] [CrossRef]
- Foley, S.F. Experimental constraints on phlogopite chemistry in lamproites: 1. The effect of water activity and oxygen fugacity. Eur. J. Mineral. 1989, 1, 411–426. [Google Scholar] [CrossRef]
- Shaw, D.M. Trace element fractionation during anatexis. Geochim. Cosmochim. Acta 1970, 34, 237–243. [Google Scholar] [CrossRef]
- Sun, S.-S.; McDonough, W.-S. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Borghini, A.; Ferrero, S.; Wunder, B.; Laurent, O.; O’Brien, P.J.; Ziemann, M.A. Granitoid melt inclusions in orogenic peridotite and the origin of garnet clinopyroxenite. Geology 2018, 46, 1007–1010. [Google Scholar] [CrossRef]
- Peccerillo, A. The Roman Province. Cenozoic Volcanism in the Tyrrhenian Sea Region; Springer: Berlin/Heidelberg, Germany, 2017; pp. 81–124. [Google Scholar]
- Murphy, D.T.; Collerson, K.D.; Kamber, B.S. Lamproites from Gaussberg, Antarctica: Possible transition zone melts of Archaean subducted sediments. J. Petrol. 2002, 43, 981–1001. [Google Scholar] [CrossRef]
- Foley, S.F.; Jenner, G.A. Trace element partitioning in lamproitic magmas—The Gaussberg olivine leucitite. Lithos 2004, 75, 19–38. [Google Scholar] [CrossRef]
- Nelson, D.R. Isotopic characteristics of potassic rocks: Evidence for the involvement of subducted sediments in magma genesis. Lithos 1992, 28, 403–420. [Google Scholar] [CrossRef]
- Jaques, A.L.; Foley, S.F. Insights into the petrogenesis of the West Kimberley lamproites from trace elements in olivine. Mineral. Petrol. 2018. [Google Scholar] [CrossRef]






| # | Composition | T (°C) | P (GPa) | Melt (%) | Phases in Sediment Layer | Reaction Zone Phases | Phases in Dunite | Duration |
|---|---|---|---|---|---|---|---|---|
| 1 | Sediment/Dunite reaction | 800 | 3 | ~10 | Gt + Cpx + Phe + Ap + Cc + Coe + Si-rich melt | Cpx + Phl + Opx | Ol + Opx + Mgs | 7 d |
| 2 | Sediment/Dunite reaction | 850 | 3 | ~20 | Gt + Cpx + Phe + Ap + Si-rich melt | Phl + CPx + Opx + Ap + Fe–Ni Sulphides | Ol + Opx + Mgs | 13 d |
| 3 | Sediment/Dunite reaction | 900 | 3 | ~20 | Gt + Cpx + Si-rich melt | Phl + Cpx + OPx+ Ap + Fe–Ni Sulphides | Ol + Opx + Mgs | 14 d |
| 4 | Sediment/Dunite reaction | 1000 | 3 | ~30 | Gt + Si-rich melt | Phl + CPx + Opx | Ol + Opx | 4 d |
| 5 | Metasome melting (Reaction zone of experiment 3) | 1200 | 2 | ~20 | - | Ol + Opx + Phl + melt | - | 6 h |
| Sample | K/Na | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sediment bulk | 1.57 | 1.78(6) | 2.46(9) | 2.17(9) | 54.9(4) | 3.93(6) | 3.6(2) | 17.8(1) | 13.5(2) | 0.82(3) | 100 |
| Dunite bulk | - | 0.03 | 0.00 | 0.13 | 40.44 | 49.08 | 10.06 | 0.08 | 13.50 | 0.01 | 100 |
| 3 GPa/800 °C Sediment/Dunite reaction melt | 2.9(6) | 1.6(4) | 3.9(4) | 0.13(4) | 75(1) | 0.4(4) | 0.4(1) | 15.3(4) | 2.5(6) | 0.25(2) | 89(1) * |
| 3 GPa/850 °C Sediment/Dunite reaction melt | 6(2) | 1.0(3) | 5(1) | 0.03(4) | 76(1) | 0.12(7) | 0.15(5) | 14.6(8) | 1.6(5) | 0.25(5) | 88(2) * |
| 3 GPa/900 °C Sediment/Dunite reaction melt | 6(2) | 1.1(2) | 6.2(8) | 0.13(3) | 74.6(4) | 0.50(8) | 0.88(4) | 12.9(5) | 3.3(5) | 0.33(2) | 82(1) * |
| 3 GPa/1000 °C Sediment/Dunite reaction melt | 4.0(5) | 1.3(2) | 4.5(1) | 0.9(1) | 56(1) | 5.5(3) | 3.2(2) | 16.1(2) | 11.3(7) | 1.03(7) | 81(1) * |
| 2 GPa/1200 °C second-stage melting | 7.1(6) | 1.3(1) | 8.4(2) | 0.37(8) | 58(1) | 3(1) | 1.0(4) | 17.8(9) | 9(1) | 0.63(7) | 88(2) * |
| Olivine | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
| 3 GPa/900 °C Sediment/Dunite reaction | 0.04(5) | 0.01(2) | 0.13(2) | 40.7(4) | 52.3(3) | 8.5(2) | 0.01(1) | 0.06(3) | 0.01(1) | 101(1) |
| 3 GPa/1000 °C Sediment/Dunite reaction | 0.02(1) | 0.01(1) | 0.20(6) | 39.8(2) | 50.2(1) | 8.3(2) | 0.02(2) | 0.04(1) | 0.02(1) | 98.6(2) |
| 2 GPa/1200 °C second-stage melting | 0.002(3) | 0.01(1) | 0.06(1) | 41.6(2) | 54.6(4) | 1.97(6) | 0.01(1) | 0.33(3) | 0.01(1) | 98.7(4) |
| Phlogopite | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
| 3 GPa/800 °C Sediment/Dunite reaction | 0.30(7) | 8.40(9) | 0.10(3) | 43.6(7) | 26.0(5) | 1.13(9) | 12.7(4) | 0.6(5) | 0.59(3) | 95.0(9) |
| 3 GPa/850 °C Sediment/Dunite reaction | 0.15(4) | 9(1) | 0.04(3) | 43(1) | 24(2) | 3.7(5) | 13(1) | 0.2(3) | 0.7(2) | 94(1) |
| 3 GPa/900 °C Sediment/Dunite reaction | 0.5(1) | 9.5(2) | 0.10(3) | 41.5(6) | 24.1(5) | 3.7(1) | 14.1(4) | 0.2(4) | 1.4(1) | 95(1) |
| 2 GPa/1200 °C second-stage melting | 0.2(1) | 10.4(7) | 0.09(5) | 42(2) | 24(4) | 1.0(2) | 14.7(5) | 1(1) | 1.4(2) | 96(2) |
| Phengite | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
| 3 GPa/800 °C Sediment/Dunite reaction | 0.45(8) | 10.4(2) | 0.06(4) | 49.2(8) | 3.6(1) | 1.0(1) | 28.2(5) | 0.15(8) | 0.9(1) | 94.6(7) |
| 3 GPa/850 °C Sediment/Dunite reaction | 0.18(2) | 9.8(6) | 0.04(3) | 49.7(7) | 5.6(3) | 0.6(2) | 27.0(3) | 0.09(2) | 1.0(1) | 94.9(8) |
| Clinopyroxene | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
| 3 GPa/800 °C Sediment/Dunite reaction | 3.0(6) | 0.2(3) | 0.31(6) | 53(3) | 13(3) | 1.9(6) | 8(2) | 16(1) | 0.23(7) | 96(4) |
| 3 GPa/900 °C Sediment/Dunite reaction | 1.4(4) | 0.1(1) | 0.4(2) | 53.8(8) | 16(1) | 2.3(4) | 5(2) | 21(1) | 0.21(9) | 100.3(5) |
| 3 GPa/1000 °C Sediment/Dunite reaction | 0.84(8) | 0.03(4) | 0.71(6) | 51.9(6) | 14.9(7) | 3.1(3) | 5(1) | 21.9(2) | 0.22(6) | 98.6(4) |
| Orthopyroxene | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
| 3 GPa/900 °C Sediment/Dunite reaction | 0.03(4) | 0.4(9) | 0.30(7) | 57(1) | 36.7(6) | 5.1(4) | 1(1) | 0.15(2) | 0.08(4) | 100.9(4) |
| 3 GPa/1000 °C Sediment/Dunite reaction | 0.02(2) | 0.03(4) | 0.43(4) | 54(1) | 34.1(9) | 4.9(2) | 2.5(6) | 0.7(3) | 0.08(3) | 98(2) |
| 2 GPa/1200 °C second-stage melting | 0.16(4) | 0.01(1) | 0.12(4) | 54.5(6) | 35.1(7) | 1.41(8) | 5.8(7) | 1.3(4) | 0.21(6) | 99.8(4) |
| Garnet | Na2O | K2O | MnO | SiO2 | MgO | FeO | Al2O3 | CaO | TiO2 | Total |
| 3 GPa/800 °C Sediment/Dunite reaction | 0.2(1) | 0.2(3) | 7(2) | 39(4) | 5.9(5) | 12(1) | 21.3(6) | 11.7(8) | 0.9(2) | 99.4(1) |
| 3 GPa/900 °C Sediment/Dunite reaction | 0.07(4) | 0.02(2) | 5(2) | 40.7(8) | 13(3) | 11(4) | 22.8(6) | 9(2) | 0.5(3) | 101.3(7) |
| 3 GPa/1000 °C Sediment/Dunite reaction | 0.02(4) | 0.03(2) | 4.2(4) | 40.4(6) | 12(1) | 8.7(6) | 21.9(5) | 11.1(5) | 0.34(5) | 99.1(2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Förster, M.W.; Buhre, S.; Xu, B.; Prelević, D.; Mertz-Kraus, R.; Foley, S.F. Two-Stage Origin of K-Enrichment in Ultrapotassic Magmatism Simulated by Melting of Experimentally Metasomatized Mantle. Minerals 2020, 10, 41. https://doi.org/10.3390/min10010041
Förster MW, Buhre S, Xu B, Prelević D, Mertz-Kraus R, Foley SF. Two-Stage Origin of K-Enrichment in Ultrapotassic Magmatism Simulated by Melting of Experimentally Metasomatized Mantle. Minerals. 2020; 10(1):41. https://doi.org/10.3390/min10010041
Chicago/Turabian StyleFörster, Michael W., Stephan Buhre, Bo Xu, Dejan Prelević, Regina Mertz-Kraus, and Stephen F. Foley. 2020. "Two-Stage Origin of K-Enrichment in Ultrapotassic Magmatism Simulated by Melting of Experimentally Metasomatized Mantle" Minerals 10, no. 1: 41. https://doi.org/10.3390/min10010041