Novel Application of Mineral By-Products Obtained from the Combustion of Bituminous Coal–Fly Ash in Chemical Engineering
Abstract
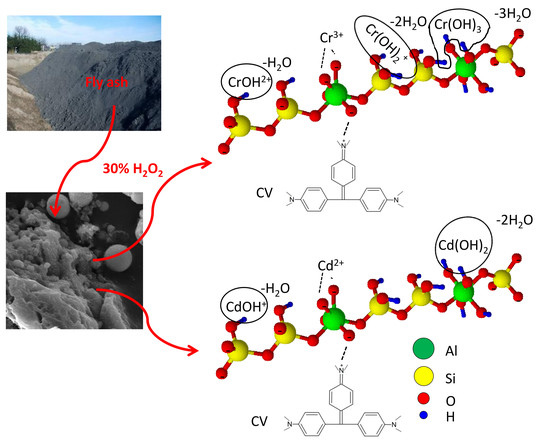
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fly Ash Sample and Its Modification
2.3. Sorption of Analytes
2.4. Determination of Analytes
3. Results and Discussion
3.1. Surface Morphology and Chemical Composition of FA and FA-H2O2
3.2. FT-IR Measurements of FA and FA-H2O2
3.3. Thermal Gravimetric Analysis of FA and FA-H2O2
3.4. Sorption Studies
3.4.1. Influence of pH
3.4.2. Effect of Initial Concentration
3.4.3. Adsorption Isotherms in a Bi-Component System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.B.; Wu, H.W. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard. Mater. 2006, 136, 482–501. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Ruhl, L.; Vengosh, A.; Dwyer, G.S.; Hsu-Kim, H.; Deonarine, A. Environmental Impacts of the Coal Ash Spill in Kingston. Tennessee: An 18-Month Survey. Environ. Sci. Technol. 2010, 44, 9272–9278. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Ma, Q.; Zhu, Z.H. Characteristics of coal fly ash and adsorption application. Fuel 2008, 87, 3469–3473. [Google Scholar] [CrossRef]
- Wu, P.; Li, J.; Zhuang, X.; Querol, X.; Moreno, N.; Li, B.; Ge, D.; Zhao, S.; Ma, X.; Cordoba, P.; et al. Mineralogical and environmental geochemistry of coal combustion products from Shenhuo and Yihua Power Plants in Xinjiang Autonomous Region, northwest China. Minerals 2019, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Seredin, V.V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Wijaya, N.; Zhang, L.; Ninomiya, Y.; Hocking, R. Synchrotron-Based XANES Speciation of Chromium in the Oxy-Fuel Fly Ash Collected from Lab-Scale Drop-Tube Furnace. Environ. Sci. Technol. 2011, 45, 6640–6646. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Guo, F.; Shao, Z.; Wu, J. Zeolite synthesized from coal fly ash produced by a gasification process for Ni2+ removal from water. Minerals 2018, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Maliyekkal, S.M.; Anshup; Antony, K.R.; Pradeep, T. High yield combustion synthesis of nanomagnesia and its application for fluoride removal. Sci. Total Environ. 2010, 408, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Reddya, D.H.K.; Seshaiaha, K.; Reddy, A.V.R.; Madhava Rao, M.; Wang, M.C. Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: Equilibrium and kinetic studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, X.; Wu, Y.; Liu, X. Adsorption of anionic dyes from aqueous solution on fly ash. J. Hazard. Mater. 2010, 181, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhenga, L.; Danga, Z.; Yi, X.; Zhanga, H. Equilibrium and kinetic studies of adsorption of Cd (II) from aqueous solution using modified corn stalk. J. Hazard. Mater. 2010, 176, 650–656. [Google Scholar] [CrossRef]
- Sočo, E.; Kalembkiewicz, J. Removal of copper (II) and zinc (II) ions from aqueous solution by chemical treatment of coal fly ash. Croat. Chem. Acta 2015, 88, 267–279. [Google Scholar] [CrossRef]
- Papciak, D.; Domoń, A.; Puszkarewicz, A.; Kaleta, J. The Use of Chalcedonite as a Biosorption Bed in the Treatment of Groundwater. Appl. Sci. 2019, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Sočo, E.; Kalembkiewicz, J. Kinetic and Thermodynamic Studies on the Adsorption of Cadmium (II) Ions from Aqueous Solution by Synthetic Zeolite from Coal Fly Ash. In Fly Ash: Properties, Analysis and Performance; Parker, J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 159–176. ISBN 978-1-53610-516-2. [Google Scholar]
- Puszkarewicz, A.; Kaleta, J.; Papciak, D. Adsorption of Phenol from Water on Natural Minerals. J. Ecol. Eng. 2018, 19, 132–138. [Google Scholar] [CrossRef]
- Moutsatsou, A.; Stamatakis, E.; Hatzizotzia, K.; Protonotarios, V. The utilization of Ca-rich and Ca-Si-rich fly ashes in zeolites production. Fuel 2006, 85, 657–663. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Waniak-Nowicka, H.; Czímerová, A. Textural properties v. CEC and EGME retention of Na-X zeolite prepared from fly ash at room temperature. Int. J. Miner. Process. 2007, 82, 57–68. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Yu, C.-C.; Yeh, C.-M. Adsorption of Cu2+ from water using raw and modified coal fly ashes. Fuel 2008, 87, 1355–1359. [Google Scholar] [CrossRef]
- An, C.; Huang, G. Stepwise adsorption of phenanthrene at the fly ash-water interface as affected by solution chemistry: Experimental and modeling studies. Environ. Sci. Technol. 2012, 46, 12742–12750. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Oh, D.; Kim, K. A study on removal characteristics of heavy metals from aqueous solution by fly ash. J. Hazard. Mater. 2005, 127, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, A.D.; Stournaras, C.J.; Panias, D.; Paspaliaris, I. Adsorption of Pb (II), Zn (II) and Cr (III) on coal fly ash porous pellets. Miner. Eng. 2011, 24, 1495–1501. [Google Scholar] [CrossRef]
- Visa, M.; Isac, L.; Duta, A. Fly ash adsorbents for multi-cation wastewater treatment. Appl. Surf. Sci. 2012, 258, 6345–6352. [Google Scholar] [CrossRef]
- Janoš, P.; Buchtova, H.; Rýznarová, M. Sorption of Dyes from Aqueous Solutions onto Fly Ash. Water Res. 2003, 37, 4938–4944. [Google Scholar] [CrossRef]
- Dizge, N.; Aydiner, C.; Demirbas, E.; Kobya, M.; Kara, S. Adsorption of reactive dyes from aqueous solutions by fly ash: Kinetic and equilibrium studies. J. Hazard. Mater. 2008, 150, 737–746. [Google Scholar] [CrossRef]
- Amodu, O.S.; Ojumu, T.V.; Ntwampe, S.K.; Ayanda, O.S. Rapid Adsorption of Crystal Violet onto Magnetic Zeolite Synthesized from Fly Ash and Magnetite Nanoparticles. J. Encapsul. Adsorpt. Sci. 2015, 5, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Sarma, G.K.; Gupta, S.S.; Bhattacharyya, K.G. Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag. 2016, 71, 1–10. [Google Scholar] [CrossRef]
- WHO/SDE/WSH/03.04/04 (2003) Chromium in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003.
- Kumar, R.; Chawla, J. Removal of cadmium ion from water/wastewater by nano-metal oxides: A review. Water Qual. Expo. Health 2014, 5, 215–226. [Google Scholar] [CrossRef]
- Yaacoubi, H.; Zidani, O.; Mouflih, M.; Gourai, M.; Sebti, S. Removal of cadmium from water using natural phosphate as adsorbent. Procedia Eng. 2014, 83, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Sahinkaya, E.; Kilic, A.; Altun, M.; Komnitsas, K.; Lens, P.N.L. Hexavalent chromium reduction in a sulfur reducing packed-bed bioreactor. J. Hazard. Mater. 2012, 219, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Bazdanis, G.; Bartzas, G. Efficiency of composite permeable reactive barriers for the removal of Cr (VI) from leachates. Desalin. Water Treat. 2016, 57, 8990–9000. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bartzas, G.; Paspaliaris, I. Modeling of reaction front progress in fly ash permeable reactive barriers. Environ. Forensics 2006, 7, 219–231. [Google Scholar] [CrossRef]
- Sočo, E.; Kalembkiewicz, J. Comparison of adsorption of Cd (II) and Pb (II) ions on pure and chemically modified fly ashes. Chem. Proc. Eng. 2016, 37, 215–234. [Google Scholar] [CrossRef]
- Styszko-Grochowiak, K.; Gołaś, J.; Jankowski, H.; Koziński, S. Characterization of the coal fly ash for the purpose of improvement of industrial on-line measurement of unburned carbon content. Fuel 2004, 83, 1847–1853. [Google Scholar] [CrossRef]
- Aslam, Z.; Shawabkeh, R.A.; Hussein, I.A.; Al-Baghli, N.; Eic, M. Synthesis of activated carbon from oil fly ash for removal of H2S from gas stream. Appl. Surf. Sci. 2015, 327, 107–115. [Google Scholar] [CrossRef]
- Szala, B.; Bajda, T.; Matusik, J.; Zięba, K.; Kijak, B. BTX sorption on Na-P1 organo-zeolite as a process controlled by the amount of adsorbed HDTMA. Microporous Mesoporous Mater. 2015, 202, 115–123. [Google Scholar] [CrossRef]
- Deng, H.; Yu, X. Adsorption of fluoride, arsenate and phosphate in aqueous solution by cerium impregnated fibrous protein. Chem. Eng. J. 2012, 184, 205–212. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, F.; Sun, B.; Jia, P.; Graham, I.T. Structural Characterizations of Aluminosilicates in Two Types of Fly Ash Samples from Shanxi Province, North China. Minerals 2019, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Mozgawa, W.; Jastrzębski, W.; Handke, M. Cation-terminated structural clusters as a model for the interpretation of zeolite vibrational spectra. J. Mol. Struct. 2006, 792, 163–169. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Alevizos, G. Adsorption of scandium and neodymium on biochar derived after low-temperature pyrolysis of sawdust. Minerals 2017, 7, 200. [Google Scholar] [CrossRef] [Green Version]
- Al-Asheh, S.; Banat, F.; Al-Omari, R.; Duvnjak, Z. Predictions of binary sorption isotherms for the sorption of heavy metals by pine bark using single isotherm data. Chemosphere 2000, 41, 659–665. [Google Scholar] [CrossRef]
- Hamdaouia, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon Part, I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Noroozi, B.; Sorial, G.A. Applicable models for multi-component adsorption of dyes: A review. J. Environ. Sci. 2013, 25, 419–429. [Google Scholar] [CrossRef]
- Wang, L. Application of activated carbon derived from ‘waste’ bamboo culms for the adsorption of azo disperse dye: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2012, 102, 79–87. [Google Scholar] [CrossRef]
- Vieira, R.S.; Guibal, E.; Silva, E.A.; Beppu, M.M. Adsorption and desorption of binary mixtures of copper and mercury ions on natural and crosslinked chitosan membranes. Adsorption 2007, 13, 603–611. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Kanellopoulos, N.K. Prediction of binary adsorption isotherms of Cu2+, Cd2+ and Pb2+ on calcium alginate beads from single adsorption data. J. Hazard. Mater. 2009, 162, 1347–1354. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Padilla-Ortega, E.; Regules-Martínez, M.C.; Leyva-Ramos, R.; Ocampo-Perez, R.; Carranza-Alvarez, C. Single and competitive adsorption of Cd(II) and Pb(II) ions from aqueous solutions onto industrial chili seeds (Capsicum annuum) waste. Sustain. Environ. Res. 2017, 27, 61–69. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Flores-Cano, J.V. Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem. Eng. J. 2013, 225, 535–546. [Google Scholar] [CrossRef]












| Adsorption Bands | Assignment | Interpretation |
|---|---|---|
| 3500–3000 cm−1 | Stretching (–OH) and bending (H–O–H) vibrations | Water molecules adsorbed on the fly ash surface |
| 2920 and 2850 cm−1 | Stretching vibrations of –OH | Presence of hydrated aluminosilicates |
| 1630 cm−1 | Bending vibrations of –OH and bending vibrations of H–O–H | |
| 1450 cm−1 | Asymmetric stretching vibrations of bridge bounds C–O | Carboxyl–carbonate structures |
| 1050 cm−1 | Asymmetric stretching vibrations of bridge bounds Si–O–Si and Si–O–Al | Tetrahedral or aluminum and silicon–oxygen bridges, typical for aluminosilicate framework structures |
| 800 cm−1 | Symmetric stretching vibration Al, Si–O | Quartz |
| Process | Type of Reaction | Range of Reaction Temperature (°C) |
|---|---|---|
| Evaporation of water (moisture, hydration water) | Endothermic | 50–150 |
| Oxidation of residue of organic matter | Exothermic | 250–400 |
| Dehydration of calcium hydroxide | Endothermic | 400–600 |
| Decomposition of calcium carbonate | Exothermic | 400–600 |
| Dehydration of coordinated and structural water/dehydroxylation of FA and FA–H2O2 | Endothermic | 520 |
| Recrystallization | Exothermic | 600 |
| Decomposition of mineral structure | Endothermic | 650–800 |
| Isotherm Model | Equation 1 | Parameter | Cd(II)–CV | Cr(III)–CV | ||
|---|---|---|---|---|---|---|
| Cd(II) | CV | Cr(III) | CV | |||
| Extended Langmuir | χ2/DoF | 0.9 | 0.8 | 27.1 | 31.5 | |
| R2 | 0.984 | 0.992 | 0.773 | 0.698 | ||
| Extended Langmuir–Freundlich | χ2/DoF | 33.3 | 45.8 | 47.9 | 0.6 | |
| R2 | 0.399 | 0.559 | 0.352 | 0.994 | ||
| Jain–Snoeyink | χ2/DoF | 36.7 | 0.04 | 0.2 | 34.6 | |
| R2 | 0.297 | 0.996 | 0.997 | 0.402 | ||
| when | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sočo, E.; Papciak, D.; Michel, M.M. Novel Application of Mineral By-Products Obtained from the Combustion of Bituminous Coal–Fly Ash in Chemical Engineering. Minerals 2020, 10, 66. https://doi.org/10.3390/min10010066
Sočo E, Papciak D, Michel MM. Novel Application of Mineral By-Products Obtained from the Combustion of Bituminous Coal–Fly Ash in Chemical Engineering. Minerals. 2020; 10(1):66. https://doi.org/10.3390/min10010066
Chicago/Turabian StyleSočo, Eleonora, Dorota Papciak, and Magdalena M. Michel. 2020. "Novel Application of Mineral By-Products Obtained from the Combustion of Bituminous Coal–Fly Ash in Chemical Engineering" Minerals 10, no. 1: 66. https://doi.org/10.3390/min10010066