The Influence of Fly Ash on Mechanical Properties of Clay-Based Ceramics
Abstract
:1. Introduction
2. Materials and Methods
- fly ash from a fluidized bed combustion boiler operating at 850 °C (FFA),
- fly ash from a pulverized combustion boiler operating at 1400 °C (PFA).
3. Results and Discussion
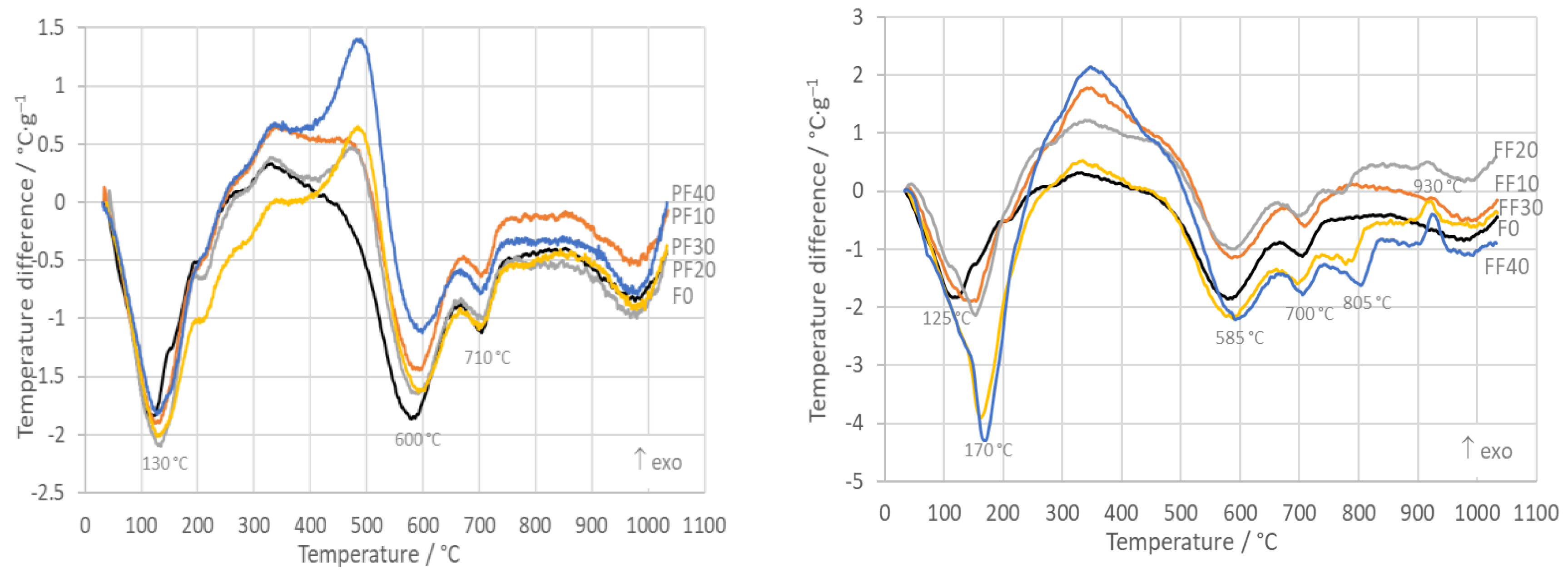
3.1. DTA and TG
3.2. Thermal Expansion
3.3. Bulk Density
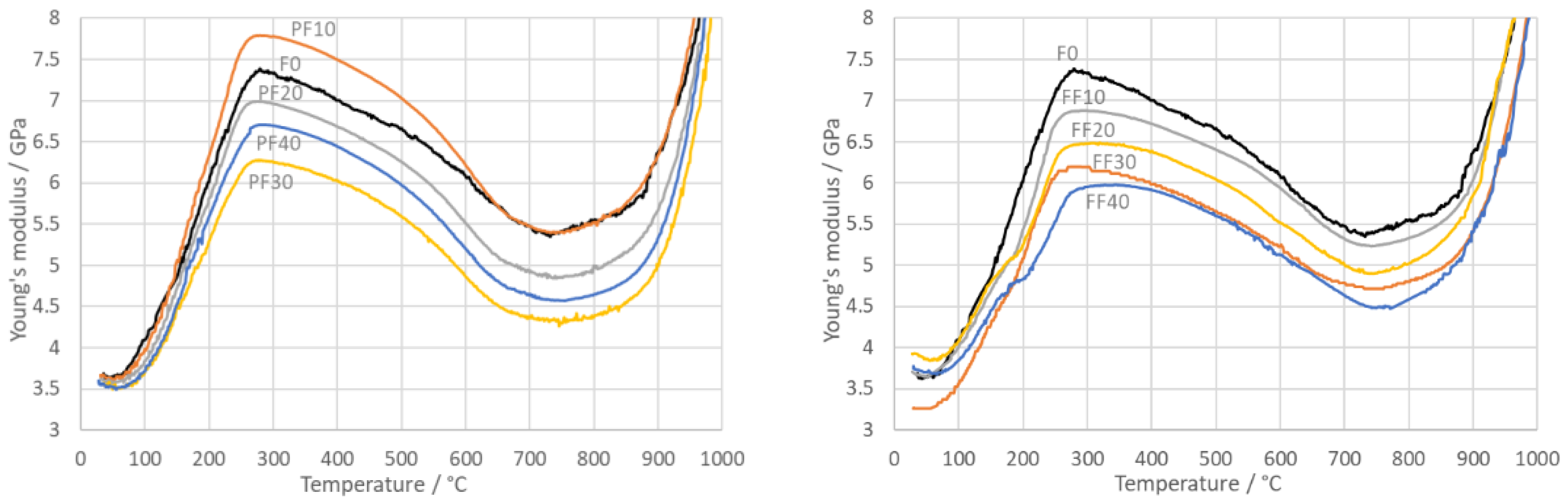
3.4. Young’s Modulus
3.5. Mechanical Strength
4. Conclusions
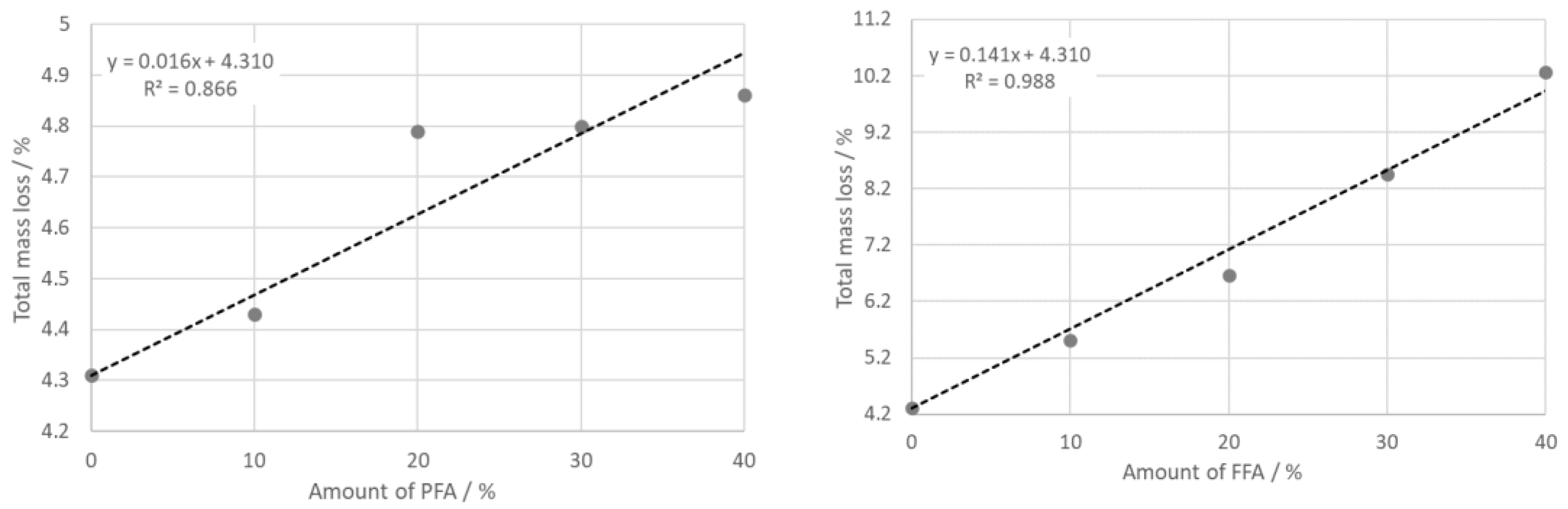
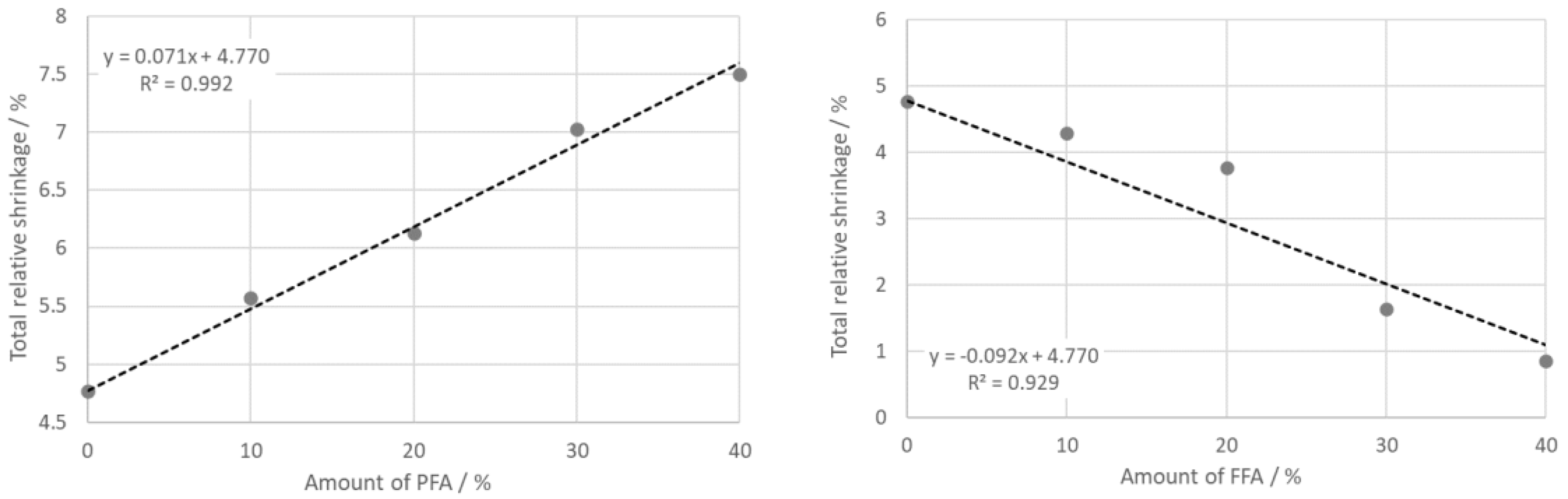
- The TDA shows expansion of all samples with a step between 500 °C and 600 °C as a consequence of dehydroxylation and the α → β transition of quartz. Beginning at 900 °C, intensive sintering took place, the rate of which increased with the part of PFA. Total shrinking after firing depended linearly on the amount of PFA and FFA. The reason was a high glassy phase content in PFA and FFA.
- Bulk density showed a decreasing trend up to 900 °C because of the mass loss, which was more intensive than thermal expansion. Above 900 °C, a steep increase in the bulk density up to 1100 °C was observed. Then, during cooling, the bulk density slightly increased down to the room temperature.
- Young’s modulus increased significantly during heating up to ~300 °C when water was removed from pores. Crystals set closer to each other and stronger contacts were created.
- Dehydroxylation was almost not reflected in Young’s modulus. At temperatures higher than 800 °C, Young’s modulus began to increase for all mixtures. This was caused by the solid-state sintering and sintering in the liquid phase at the highest temperatures.
- During cooling, until the glass transformation at ~750 °C was reached, Young’s modulus slightly increased. Under this temperature, Young’s modulus began to decrease slightly due to microcracking between phases with different thermal expansion coefficients.
- At around the β → α quartz transition, quartz grains shrank steeply and radial mechanical stresses on the quartz grain surfaces altered from compressive to tensile, creating micro-cracks in their close vicinity. At temperatures below 560 °C, the radial stress remained tensile, and consequently microcracking around the quartz grains continued, which caused decrease in Young’s modulus down to the room temperature.
- The lower the amount of PFA and FFA, the higher Young’s modulus was reached after sintering.
Author Contributions
Funding
Conflicts of Interest
References
- Velasco, P.M.; Ortiz, M.P.M.; Giró, M.A.M.; Velasco, L.M. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Queralt, I.; Querol, X.; López-Soler, A.; Plana, F. Use of coal fly ash for ceramics: A case study for a large Spanish power station. Fuel 1997, 76, 787–791. [Google Scholar] [CrossRef]
- Olgun, A.; Erdogan, Y.; Ayhan, Y.; Zeybek, B. Development of ceramic tiles from coal fly ash and tincal ore waste. Ceram. Int. 2005, 31, 153–158. [Google Scholar] [CrossRef]
- Sokolar, R.; Smetanova, L. Dry pressed ceramic tiles based on fly ash-clay body: Influence of fly ash granulometry and pentasodium triphosphate addition. Ceram. Int. 2010, 36, 215–221. [Google Scholar] [CrossRef]
- Knapek, M.; Húlan, T.; Dobroň, P.; Chmelík, F.; Trník, A.; Štubňa, I. Acoustic emission during firing of the illite-based ceramics with fly ash addition. Acta Phys. Pol. A 2015, 128, 783–786. [Google Scholar] [CrossRef]
- Kováč, J.; Trník, A.; Medveď, I.; Štubňa, I.; Vozár, L. Influence of fly ash added to a ceramic body on its thermophysical properties. Therm. Sci. 2016, 20, 603–612. [Google Scholar] [CrossRef]
- Húlan, T.; Trník, A.; Medveď, I.; Štubňa, I.; Kaljuvee, T. Building ceramics with an addition of pulverized combustion fly ash from the thermal power plant Nováky. AIP Conf. Proc. 2016, 1752, 040009. [Google Scholar] [CrossRef]
- Sokolar, R.; Vodova, L. The effect of fluidized fly ash on the properties of dry pressed ceramic tiles based on fly ash-clay body. Ceram. Int. 2011, 37, 2879–2885. [Google Scholar] [CrossRef]
- Zimmer, A.; Bergmann, C.P. Fly ash of mineral coal as ceramic tiles raw material. Waste Manag. 2007, 27, 59–68. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E. Fly ash addition in clayey materials to improve the quality of solid bricks. Constr. Build. Mater. 2009, 23, 1178–1184. [Google Scholar] [CrossRef]
- Chandra, N.; Sharma, P.; Pashkov, G.L.; Voskresenskaya, E.N.; Amritphale, S.S.; Baghel, N.S. Coal fly ash utilization: Low temperature sintering of wall tiles. Waste Manag. 2008, 28, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Guo, W.; Wang, T.; Yang, N.R. Study on fired bricks with replacing clay by fly ash in high volume ratio. Constr. Build. Mater. 2005, 19, 243–247. [Google Scholar] [CrossRef]
- Mukherji, S.K.; Machhoya, B.B.; Savsani, R.M.; Vyas, D.R.; Dan, T.K. The utilization of fly ash in the preparation of ceramic tableware and artware. Br. Ceram. Trans. 1993, 92, 254–257. [Google Scholar]
- Sola, O.C.; Yayla, M.; Sayin, B.; Atis, C.D. The effects of different types of fly ash on the compressive strength properties of briquettes. Adv. Mater. Sci. Eng. 2011, 2011, 430604. [Google Scholar] [CrossRef] [Green Version]
- Húlan, T.; Trník, A.; Kaljuvee, T.; Uibu, M.; Štubňa, I.; Kallavus, U.; Traksmaa, R. The study of firing of a ceramic body made from illite and fluidized bed combustion fly ash. J. Therm. Anal. Calorim. 2017, 127, 79–89. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhao, Y.C.; Qi, J.Y. Study on use of MSWI fly ash in ceramic tile. J. Hazard. Mater. 2007, 141, 106–114. [Google Scholar] [CrossRef]
- Erol, M.; Kuçukbayrak, S.; Ersoy-Meriçboyu, A. Comparison of the properties of glass, glass-ceramic and ceramic materials produced from coal fly ash. J. Hazard. Mater. 2008, 153, 418–425. [Google Scholar] [CrossRef]
- Knapek, M.; Húlan, T.; Minárik, P.; Dobroň, P.; Štubňa, I.; Stráská, J.; Chmelík, F. Study of microcracking in illite-based ceramics during firing. J. Eur. Ceram. Soc. 2016, 36, 221–226. [Google Scholar] [CrossRef]
- Štubňa, I.; Húlan, T.; Trník, A.; Vozár, L. Uncertainty in the determination of Young’s modulus of ceramics using the impulse excitation technique at elevated temperatures. Acta Acust. United Acust. 2018, 104, 269–276. [Google Scholar] [CrossRef]
- ASTM International. ASTM C 1259-15. Standard Test Method for Dynamic Young’s Modulus, Shear Modulus and Poisson’s Ratio for Advanced Ceramics by Impulse Excitation of Vibration; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Štubňa, I.; Sin, P.; Trník, A.; Vozár, L. Measuring the flexural strength of ceramics at elevated temperatures—An uncertainty analysis. Meas. Sci. Rev. 2014, 14, 35–40. [Google Scholar] [CrossRef]
- ASTM International. ASTM C 1161-18. Standard Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Tydlitát, V.; Trník, A.; Scheinherrová, L.; Podoba, R.; Černý, R. Application of isothermal calorimetry and thermal analysis for the investigation of calcined gypsum–lime–metakaolin–water system. J. Therm. Anal. Calorim. 2015, 122, 115–122. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Andrade, C. Calcium carbonate decomposition. J. Therm. Anal. Calorim. 2013, 111, 1197–1202. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navrro, C.; Sebastian, E.; Cazalla, O.; De La Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Miner. 2001, 13, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Jankula, M.; Húlan, T.; Štubňa, I.; Ondruška, J.; Podoba, R.; Šín, P.; Bačík, P.; Trník, A. The influence of heat on elastic properties of illitic clay Radobica. J. Ceram. Soc. Jpn. 2015, 123, 874–879. [Google Scholar] [CrossRef]
- Štubňa, I.; Šín, P.; Trník, A.; Podoba, R.; Vozár, L. Development of Young’s modulus of the green alumina porcelain raw mixture. J. Aust. Ceram. Soc. 2014, 50, 36–42. [Google Scholar]
- Štubňa, I.; Mánik, M.; Húlan, T.; Trník, A. Development of stress on quartz grain in illite ceramics during cooling stage of firing. J. Ceram. Soc. Jpn. 2020, 128, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Chmelík, F.; Trník, A.; Pešička, J.; Štubňa, I. Creation of microcracks in porcelain during firing. J. Eur. Ceram. Soc. 2011, 31, 2205–2209. [Google Scholar] [CrossRef]
- Bureau of Indian Standards. IS 1077. Common Burnt Clay Building Bricks—Specification; Bureau of Indian Standards: New Delhi, India, 1992.
- Griffith, M.C.; Vaculik, J. Out-of-plane flexural strength of unreinforced clay brick masonry walls. TMS J. 2007, 25, 53–68. [Google Scholar]
- The Brick Industry Association. Technical Notes 3A. Brick Masonry Material Properties; The Brick Industry Association: Reston, VA, USA, 1992. [Google Scholar]







| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | L.O.I. | |
|---|---|---|---|---|---|---|---|---|---|
| clay | 58.00 | 24.00 | 0.60 | 0.05 | 0.38 | 1.70 | 7.85 | 0.10 | 7.30 |
| PFA | 55.90 | 19.70 | 10.60 | 0.59 | 4.80 | 1.89 | 2.00 | 0.80 | 1.30 |
| FFA | 35.50 | 12.20 | 6.10 | 0.32 | 29.2 | 2.90 | 1.20 | 0.69 | 1.80 |
| Mineral | Clay | Grog | FFA | PFA |
|---|---|---|---|---|
| illite | 80.0 | - | - | - |
| montmorillonite | 4.0 | - | - | - |
| quartz | 12.0 | 10.0 | 17.5 | 3.0 |
| mullite | - | 11.0 | - | - |
| calcite | - | - | 11.0 | 0.5 |
| orthoclase | 4.0 | - | - | - |
| anorthite | - | - | 10.0 | 11.0 |
| magnetite | - | - | - | 5.5 |
| gypsum | - | - | 1.5 | - |
| amorphous (undefined) | - | 79.0 | 60.0 | 80.0 |
| Sample | Clay | Grog | PFA | Sample | Clay | Grog | FFA |
|---|---|---|---|---|---|---|---|
| F0 | 60 | 40 | 0 | F0 | 60 | 40 | 0 |
| PF10 | 60 | 30 | 10 | FF10 | 60 | 30 | 10 |
| PF20 | 60 | 20 | 20 | FF20 | 60 | 20 | 20 |
| PF30 | 60 | 10 | 30 | FF30 | 60 | 10 | 30 |
| PF40 | 60 | 0 | 40 | FF40 | 60 | 0 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Húlan, T.; Štubňa, I.; Ondruška, J.; Trník, A. The Influence of Fly Ash on Mechanical Properties of Clay-Based Ceramics. Minerals 2020, 10, 930. https://doi.org/10.3390/min10100930
Húlan T, Štubňa I, Ondruška J, Trník A. The Influence of Fly Ash on Mechanical Properties of Clay-Based Ceramics. Minerals. 2020; 10(10):930. https://doi.org/10.3390/min10100930
Chicago/Turabian StyleHúlan, Tomáš, Igor Štubňa, Ján Ondruška, and Anton Trník. 2020. "The Influence of Fly Ash on Mechanical Properties of Clay-Based Ceramics" Minerals 10, no. 10: 930. https://doi.org/10.3390/min10100930





