Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba
Abstract
:1. Introduction
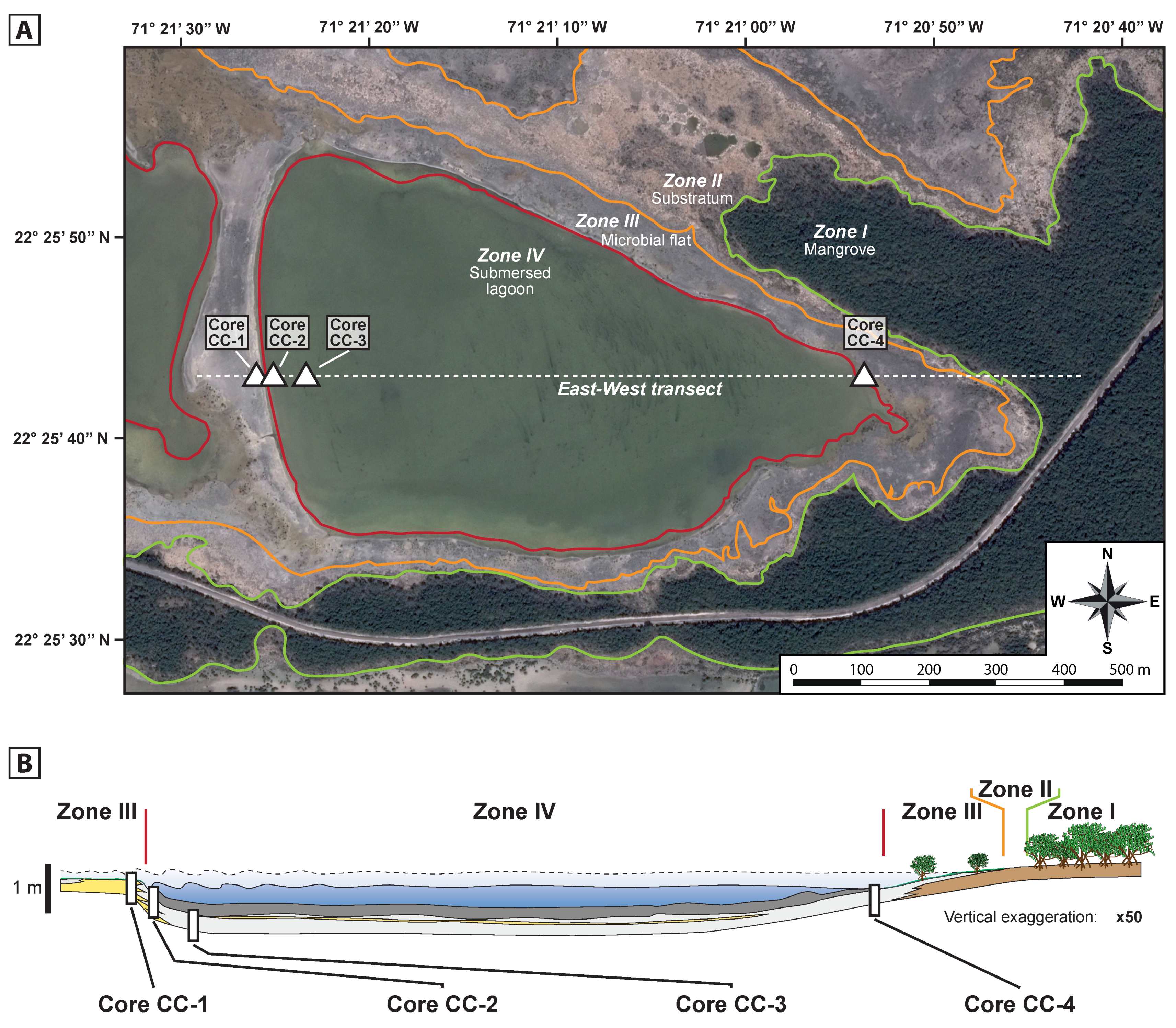
2. Setting
3. Materials and Methods
3.1. Sampling and Preparation
3.2. Microscopy and Mineralogy
3.3. Elemental and Stable Isotope Analyzes
3.4. Data Processing
4. Results
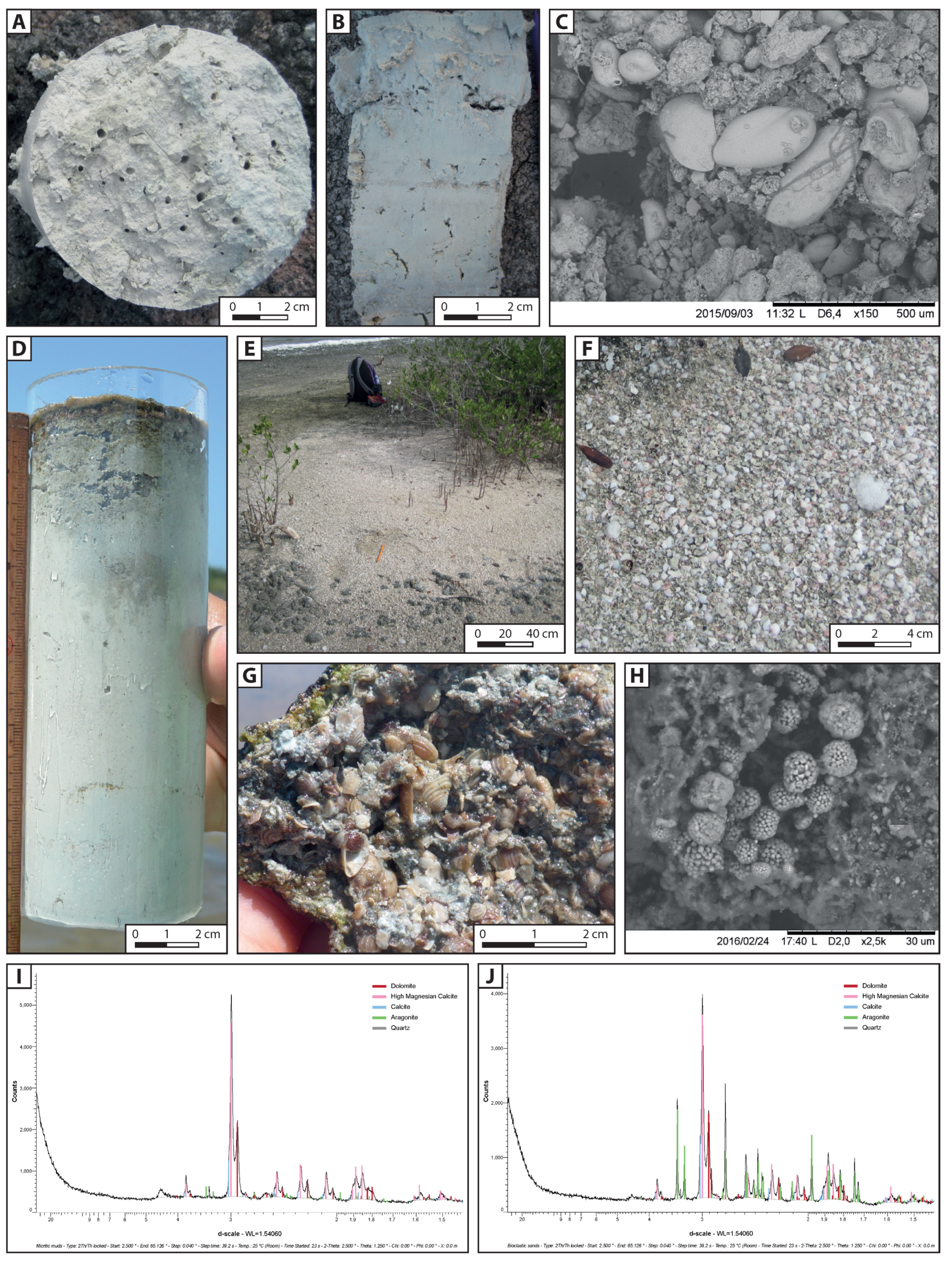
4.1. Sediment Characterisation
4.1.1. Micritic Muds
4.1.2. Bioclastic Sands
4.1.3. Microbial Deposits
4.2. Depositional Environments
4.2.1. Zone I: Mangrove
4.2.2. Zone II: Substratum Area
4.2.3. Zone III: Microbial Flat
4.2.4. Zone IV: Submersed Lagoon
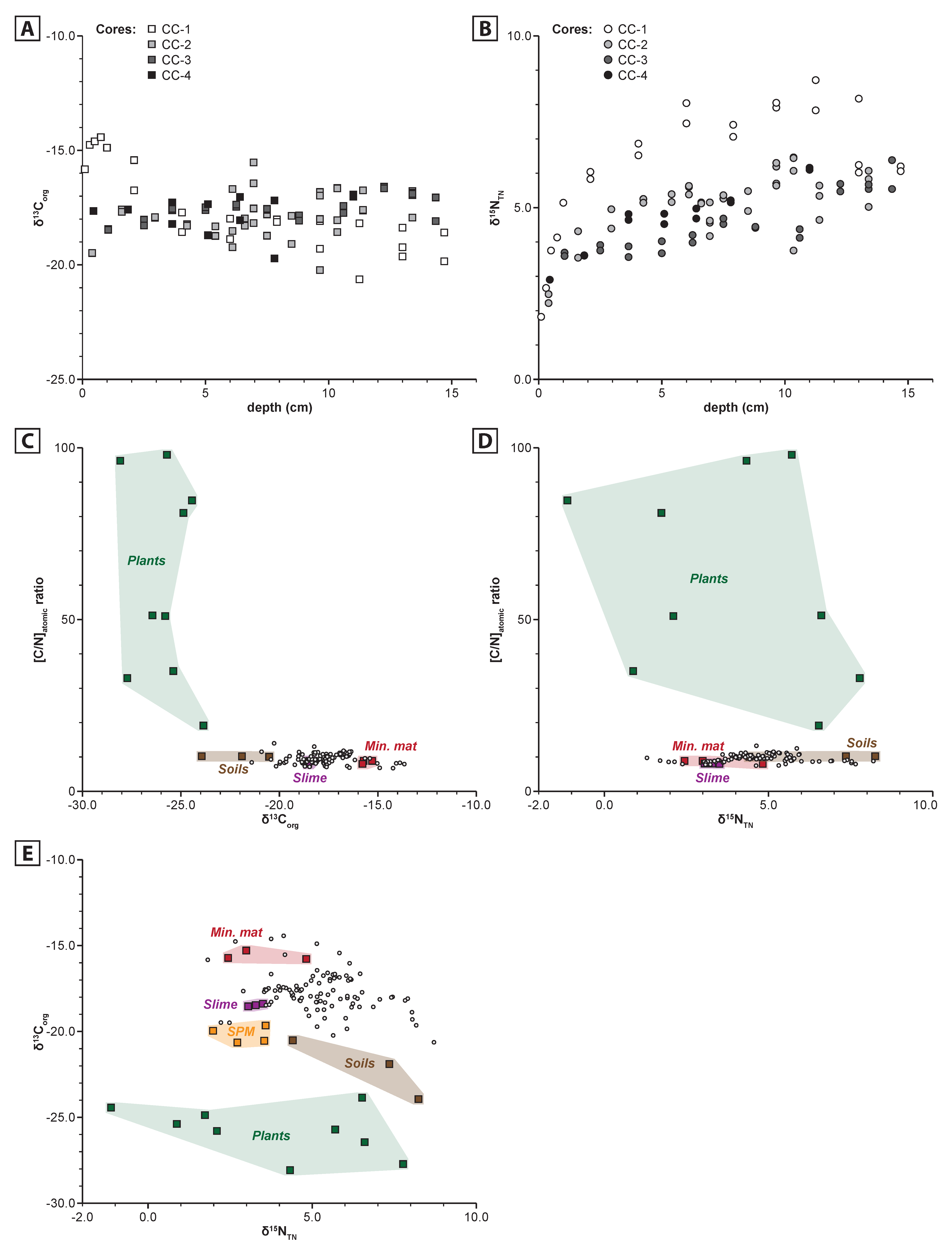
4.3. Organic Matter Sources
4.4. Sedimentary Organic Matter
- Core CC-1 (15 cm-long) was collected at the western border of the lagoon, in the microbial flat (Zone III). The upper part of the core consists of a 1.1 cm thick mineralising microbial mat (Figure 5). In this part, the TOC and TN contents slightly decreased from the top to the bottom of the microbial mat (Table 1). The δ13Corg remains quite stable within the mats, except for the uppermost interval (0–0.2 cm). The δ15N gradually increases downcore in the microbial mat. The [C/N]atomic ratio of this mineralising microbial mat decreased toward the base of the mat (0.9–1.1 cm). Below 1.1 cm depth, the remainder of the core was mainly composed of micritic muds including <10% content of bivalves (A. auberiana), foraminifera (Miliolina sp.), ostracods and a few gastropods (C. eburneum). The upper part of the micritic muds (interval 1.1–3.1 cm) was dark grey, contrasting the light colour observed underneath. In the 1.1–3.1 cm interval, the TOC and TN record had lower values that in the upper microbial mat. In the light-coloured micritic muds, the TOC and TN remained below 1% downcore. The [C/N]atomic ratio slightly increased downcore in the micritic muds (from the top to the bottom of the core). The δ13Corg remained high in the 1.1–3.1 cm interval, with a value close to that in the overlying mineralising microbial mat. The δ13Corg sharply decreased below, down to 10.5–12.0 cm depth, especially in the 200–2000 µm fraction. Below 12 cm, the δ13Corg displayed a slight decrease. The δ15NTN values followed an increasing trend downcore (to 11 cm), below which it decreased. The measurements of the TOC, TN and δ15NTN revealed a minor change in the trend seen above 12.0 cm. The 12.0–14.0 cm depth interval was contained a cavity covered inside by a cryptic microbial mat with TOC and TN values slightly higher with respect to the surrounding micritic muds.
- Core CC-2 (14 cm-long) was taken about 10 m further from the shore than Core CC-1, just beyond the boundary between Zones III and IV (Figure 2). The uppermost 0.8 cm was a nonmineralised slime comprising a high content of carbonate particles (calcimetry of 94.17% and 96.06% for the 0–200 µm and 200–2000 µm fractions, respectively), either micritic or bioclastic (fragmented bivalves shells). The slime had a TOC and TN content compared to other mineralised microbial mats, the values of which were lower in the 200–2000 µm fraction than in the 0–200 µm fraction. The [C/N]atomic ratio and δ13Corg values were similar in both fractions. The δ15NTN values were slightly higher for the 0–200 µm fraction than for the 200–2000 µm one. The underlying 0.8–2.4 cm interval consisted of a micritic muds layer rich in disarticulated and fragmented valves of A. auberiana (ca. 15%). When comparing the bioclastic layer with the slime, the organic matter content was lower, the [C/N]atomic ratio was similar and the δ13Corg and δ15NTN values higher in the bioclastic interval.
- The remaining 12.1 cm of the core mostly comprised micritic muds. However, two levels of fossil mineralising microbial mats could be identified between 5.8 cm and 7.1 cm (Figure 5). Both are discontinuous, probably cut by erosional processes. The uppermost of these mats (ca. 6 mm thick) was found between 5.8 and 6.4 cm. It was composed of five successive sequences of green and yellowish to greyish laminae. Each sequence represented a relic mat. The lower relic microbial mat, found a depth of ca. 7.0 cm, was only 1.5 mm thick, and was composed only of a green/yellowish-grey sequence. The δ13Corg of both fossil mineralising microbial mats were more positive (ca. +2–3‰) than the surrounding sediments and similar to the surficial mineralising microbial mats in CC-1. These microbial mats were surrounded by micritic muds. However, some darker-coloured parts contrasted with the usual light grey colouration of this sediment. The darker parts were discontinuous and located below the relic mineralising microbial mats. Regardless of the fraction and the sampling depth, the TOC and TN values of the darker parts were nearly two times lower than the lighter parts (all fractions considered). Pyrite was more abundant in the darker parts than in the light parts. The [C/N]atomic ratio, however, did not indicate major differences between the light and dark-coloured muds. The δ13Corg diverges, with more positive values for dark parts than for the light parts (all fractions considered). The δ15NTN values were not significantly different between the two parts, but, in both cases, tend to increase downcore.
- Core CC-3 (14 cm-thick) was sampled in the deepest flat-bottom part of the lagoon, where the depth averaged 75 cm at dynamic equilibrium (Figure 2). A nonmineralised slime thrived at the surface of the sediment with a thickness varying between 1 and 25 cm. In Core CC-3, the slime layer was 2.1 cm and overlaid micritic muds with variable content of bioclasts (bivalves, foraminifera, ostracods and few gastropods). The dry wt. % TOC and TN content of the slime was higher for the 0–200 µm fraction than for the 200–2000 µm fraction. The carbonate content reached 48.9% for 0–200 µm fraction, and 63.23% for the 200–2000 µm fraction. The [C/N]atomic ratio, the δ13Corg and δ15NTN values of Core CC-3 slime were similar to those measured in the slime of the lagoon. The [C/N]atomic ratio of the 0–200 µm fraction was slightly higher than that of the 200–2000 µm fraction. Near the bottom of the slime, a small interval of micritic mud (2.1–2.9 cm) with a low organic matter content was observed. In this depth horizon, the TOC and TN decreased sharply. The [C/N]atomic ratio of this interval was of the same order of magnitude as the slime, with a lower value for the 200–2000 µm fraction. The δ13Corg and the δ15NTN of this horizon were similar to those of the overlying slime, with no significant change between both size fractions. Downcore, in the micritic mud interval, the TOC and the TN remained constant with a systematically higher value for the 200–2000 µm fraction. Two discontinuous intervals of bioclastic sands (9.5–11.7 cm and 14.0–14.7 cm), rich in micritic mud between grains, contained a smaller amount of organic carbon for both fractions. The nitrogen content, TN decreased in the 9.5–11.7 cm and 14.0–14.7 cm intervals, respectively. These lower TOC and TN contents suggest a lower organic matter content within the bioclastic rich intervals. The [C/N]atomic ratio slightly increased downcore, from 8.73 just below the slime to 11.76 at the bottom of the core (all fractions included). Variations in TOC content were found between different facies; however, the δ13Corg values were comparable. The δ15NTN values did not change among facies, but, as observed in Cores CC-1 and CC-2, displayed an increasing trend of this proxy downcore.
- Core CC-4 (13 cm-thick) was collected from Zone IV at the eastern side of the lagoon (Figure 2), a few meters from the boundary with Zone III and approximately 75 m away from the mangrove and evergreen forest (Zone I). This sampling site is at a transition between zones with different potential sources of organic matter. The uppermost 2.8 cm of the core consisted of an nonmineralised slime similar to that observed in Core CC-2, i.e., depicting a very high carbonate content (either bioclasts or mud-sized grains), with a calcimetry of 89.9% for the 0–200 µm fraction, and up to 99.3% for the 200–2000 µm fraction. The TOC and TN were low, especially for the 200–2000 µm fraction. The following depth interval (between 4.5 and 8.5 cm) consisted of micritic muds with low TOC and TN values. The [C/N]atomic ratio increased downcore from 10.07 below the slime to 11.90 at the bottom (all fractions comprised). This micritic interval was underlain by bioclastic sand in which the δ13Corg remains relatively constant downcore in the 0–200 µm fraction but decreased in the 200–2000 µm fraction. The δ15NTN increased for both fractions, with the higher value measured in the deeper interval. Below 8.5 cm, the remainder of the core consisted of a bioclastic sand interval, mainly composed of disarticulated to slightly/moderately fragmented valves of A. auberiana, and had a low content of micritic mud. The dark coloration could be attributed to an abundance of organic matter. However, the TOC and TN values indicated a low organic matter content. The [C/N]atomic ratio was lower in this bioclastic interval than the upper micritic ones. This bioclastic interval had a similar δ13Corg value as the sediment above, and the highest δ15NTN value measured in this core.
5. Discussion
5.1. Dynamics of Organic Matter Sources
5.2. Preserved Organic Matter, Quantitative Aspect
5.3. Preserved Organic Matter, Qualitative Aspect
5.3.1. TOC and TN
5.3.2. [C/N]atomic ratio
5.3.3. δ13Corg
- δ13CorgSed = −17.86‰ (average δ13Corg signature of the sediments)
- δ13CorgSlime = −18.47‰ (average δ13Corg signature of the slime)
- x = relative contribution of slime in organic matter content
- δ13CorgMM = −15.60‰ (average δ13Corg signature of the mineralising microbial mat)
- (1 − x) = relative contribution of mineralising microbial mat in sedimentary organic matter content
5.3.4. δ15NTN
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carreira, R.S.; Araújo, M.P.; Costa, T.L.F.; Spörl, G.; Knoppers, B.A. Lipids in the sedimentary record as markers of the sources and deposition of organic matter in a tropical Brazilian estuarine–lagoon system. Mar. Chem. 2011, 127, 1–11. [Google Scholar] [CrossRef]
- Adams, R.; Arafat, Y.; Eate, V.; Grace, M.R.; Saffarpour, S.; Weatherley, A.J.; Western, A.W. A catchment study of sources and sinks of nutrients and sediments in south-east Australia. J. Hydrol. 2014, 515, 166–179. [Google Scholar] [CrossRef]
- Keith, M.K.; Sobieszczyk, S.; Goldman, J.H.; Rounds, S.A. Investigating organic matter in Fanno Creek, Oregon, Part 2 of 3: Sources, sinks, and transport of organic matter with fine sediment. J. Hydrol. 2014, 519, 3010–3027. [Google Scholar] [CrossRef]
- Meyers, P.A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Silliman, J.E.; Meyers, P.A.; Ostrom, P.H.; Ostrom, N.E.; Eadie, B.J. Insights into the origin of perylene from isotopic analyses of sediments from Saanich Inlet, British Columbia. Org. Geochem. 2000, 31, 1133–1142. [Google Scholar] [CrossRef]
- Gontharet, S.; Mathieu, O.; Lévêque, J.; Milloux, M.J.; Lesourd, S.; Philippe, S.; Caillaud, J.; Gardel, A.; Sarrazin, M.; Proisy, C. Distribution and sources of bulk organic matter (OM) on a tropical intertidal mud bank in French Guiana from elemental and isotopic proxies. Chem. Geol. 2014, 376, 1–10. [Google Scholar] [CrossRef]
- Degens, E.T.; Ittekkot, V. The carbon cycle—Tracking the path of organic particles from sea to sediment. Geol. Soc. Lond. Spec. Publ. 1987, 26, 121–135. [Google Scholar] [CrossRef]
- Calvert, S.E. Oceanographic controls on the accumulation of organic matter in marine sediments. Geol. Soc. Lond. Spec. Publ. 1987, 26, 137–151. [Google Scholar] [CrossRef]
- Morris, R.J. The formation of organic-rich deposits in two deep-water marine environments. Geol. Soc. Lond. Spec. Publ. 1987, 26, 153–166. [Google Scholar] [CrossRef]
- Hedges, J.I.; Keil, R.G. Sedimentary organic matter preservation: An assessment and speculative synthesis. Mar. Chem. 1995, 49, 81–115. [Google Scholar] [CrossRef]
- Meyers, P.A. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol. 1994, 114, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wu, Y.; Jennerjahn, T.C.; Ittekkot, V.; He, Q. Distribution of organic matter in the Changjiang (Yangtze River) Estuary and their stable carbon and nitrogen isotopic ratios: Implications for source discrimination and sedimentary dynamics. Mar. Chem. 2007, 106, 111–126. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Hillaire-Marcel, C.; Ghaleb, B.; Soto-Jiménez, M.; Páez-Osuna, F. Recent sedimentary history of anthropogenic impacts on the Culiacan River Estuary, northwestern Mexico: Geochemical evidence from organic matter and nutrients. Environ. Pollut. 2002, 118, 365–377. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Hillaire-Marcel, C.; Páez-Osuna, F.; Ghaleb, B.; Soto-Jiménez, M. Historical trends of metal pollution recorded in the sediments of the Culiacan River Estuary, Northwestern Mexico. Appl. Geochem. 2003, 18, 577–588. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Páez-Osuna, F.; Soto-Jiménez, M.; Hillaire-Marcel, C.; Ghaleb, B. The loading history of trace metals and nutrients in Altata-Ensenada del Pabellón, lagoon complex, northwestern Mexico. J. Environ. Radioact. 2003, 69, 129–143. [Google Scholar] [CrossRef]
- Alonso Hernandez, C.; Santanaa, S.P.; Brunorib, C.; Morabitoc, R.; Delfantid, R.; Papuccid, C. Historical trend in heavy metal pollution in the sediments of Cienfuegos Bay (Cuba), defined by 210Pb and 137Cs geochronology. Environ. Stud. 2006, 39, 424. [Google Scholar]
- Alonso-Hernandez, C.M.; Diaz-Asencio, M.; Munoz-Caravaca, A.; Delfanti, R.; Papucci, C.; Ferretti, O.; Crovato, C. Recent changes in sedimentation regime in Cienfuegos Bay, Cuba, as inferred from 210Pb and 137Cs vertical profiles. Cont. Shelf Res. 2006, 26, 153–167. [Google Scholar] [CrossRef]
- Díaz-Asencio, M.; Alonso-Hernández, C.M.; Bolanos-Álvarez, Y.; Gómez-Batista, M.; Pinto, V.; Morabito, R.; Hernández-Albernas, J.I.; Eriksson, M.; Sanchez-Cabeza, J.A. One century sedimentary record of Hg and Pb pollution in the Sagua estuary (Cuba) derived from 210Pb and 137Cs chronology. Mar. Pollut. Bull. 2009, 59, 108–115. [Google Scholar] [CrossRef]
- Díaz-Asencio, M.; Alvarado, J.A.C.; Alonso-Hernández, C.; Quejido-Cabezas, A.; Ruiz-Fernández, A.C.; Sanchez-Sanchez, M.; Gómez-Mancebo, M.B.; Froidevaux, P.; Sanchez-Cabeza, J.A. Reconstruction of metal pollution and recent sedimentation processes in Havana Bay (Cuba): A tool for coastal ecosystem management. J. Hazard. Mater. 2011, 196, 402–411. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Hillaire-Marcel, C. 210Pb-derived ages for the reconstruction of terrestrial contaminant history into the Mexican Pacific coast: Potential and limitations. Mar. Pollut. Bull. 2009, 59, 134–145. [Google Scholar] [CrossRef]
- Alonso-Hernández, C.M.; Garcia-Moya, A.; Tolosa, I.; Diaz-Asencio, M.; Corcho-Alvarado, J.A.; Morera-Gomez, Y.; Fanelli, E. Tracing organic matter sources in a tropical lagoon of the Caribbean Sea. Cont. Shelf Res. 2017, 148, 53–63. [Google Scholar] [CrossRef]
- Cifuentes, L.A.; Coffin, R.B.; Solorzano, L.; Cardenas, W.; Espinoza, J.; Twilley, R.R. Isotopic and Elemental Variations of Carbon and Nitrogen in a Mangrove Estuary. Estuar. Coast. Shelf Sci. 1996, 43, 781–800. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, H.; Gacia, E.; Kennedy, D.P.; Papadimitriou, S.; Duarte, C.M. Organic carbon sources to SE Asian coastal sediments. Estuar. Coast. Shelf Sci. 2004, 60, 59–68. [Google Scholar] [CrossRef]
- Showers, W.J.; Angle, D.G. Stable isotopic characterization of organic carbon accumulation on the Amazon continental shelf. Cont. Shelf Res. 1986, 6, 227–244. [Google Scholar] [CrossRef]
- Aller, R.C.; Blair, N.E.; Xia, Q.; Rude, P.D. Remineralization rates, recycling, and storage of carbon in Amazon shelf sediments. Cont. Shelf Res. 1996, 16, 753–786. [Google Scholar] [CrossRef]
- Aller, R.C.; Heilbrun, C.; Panzeca, C.; Zhu, Z.; Baltzer, F. Coupling between sedimentary dynamics, early diagenetic processes, and biogeochemical cycling in the Amazon–Guianas mobile mud belt: Coastal French Guiana. Mar. Geol. 2004, 208, 331–360. [Google Scholar] [CrossRef]
- Zhu, Z.; Aller, R.C.; Mak, J. Stable carbon isotope cycling in mobile coastal muds of Amapá, Brazil. Cont. Shelf Res. 2002, 22, 2065–2079. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F. The composition of sedimentary organic matter in relation to the dynamic features of a mangrove-fringed coast in French Guiana. Estuar. Coast. Shelf Sci. 2003, 56, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Bouton, A.; Vennin, E.; Pace, A.; Bourillot, R.; Dupraz, C.; Thomazo, C.; Brayard, A.; Désaubliaux, G.; Visscher, P.T. External controls on the distribution, fabrics and mineralization of modern microbial mats in a coastal hypersaline lagoon, Cayo Coco (Cuba). Sedimentology 2016. n/a-n/a. [Google Scholar] [CrossRef]
- Pace, A.; Bourillot, R.; Bouton, A.; Vennin, E.; Braissant, O.; Dupraz, C.; Duteil, T.; Bundeleva, I.; Patrier, P.; Galaup, S.; et al. Formation of stromatolite lamina at the interface of oxygenic–anoxygenic photosynthesis. Geobiology 2018, 16, 378–398. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Seong-Joo, L.; Browne, K.; Golubic, S. On Stromatolite Lamination. In Microbial Sediments; Riding, R., Awramik, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 16–24. [Google Scholar]
- Bowlin, E.M.; Klaus, J.S.; Foster, J.S.; Andres, M.S.; Custals, L.; Reid, R.P. Environmental controls on microbial community cycling in modern marine stromatolites. Sediment. Geol. 2012, 263–264, 45–55. [Google Scholar] [CrossRef]
- Dupraz, C.; Fowler, A.; Tobias, C.; Visscher, P.T. Stromatolitic knobs in Storr’s Lake (San Salvador, Bahamas): A model system for formation and alteration of laminae. Geobiology 2013, 11, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Harwood Theisen, C.; Sumner, D.Y.; Mackey, T.J.; Lim, D.S.S.; Brady, A.L.; Slater, G.F. Carbonate fabrics in the modern microbialites of Pavilion Lake: Two suites of microfabrics that reflect variation in microbial community morphology, growth habit, and lithification. Geobiology 2015, 13, 357–372. [Google Scholar] [CrossRef]
- Alcolado, P.M.; Menedez, F.; Garcia-Parrado, P.; Zuniga, D.; Martinez-Darana, B.; Losa, M.; Gomez, R. Cayo Coco, Sabana-Camaguey Archipelago, Cuba. In Caribbean Coral Reef, Seagrass and Mangrove Sites, Coastal Region and Small Island Papers No. 3; Kjerfve, B., Ed.; UNESCO: Paris, France, 1998; pp. 221–228. [Google Scholar]
- IGEO/ICGC/IGN. Nuevo Atlas Nacional de Cuba; Instituto de Geografía de la Academia de Ciencias de Cuba, Instituto Cubano de Geodesia y Cartografía/Instituto Geográfico Nacional: La Habana, Cuba, 1989. [Google Scholar]
- Borhidi, A. Phytogeography and Vegetation Ecology of Cuba, 2nd ed.; Akadémiai Kiadó: Budapest, Hungary, 1996. [Google Scholar]
- G’meiner, A.A. Holocene Environmental Change Inferred from Fossil Pollen and Microcharcoal at Cenote Jennifer, Cayo Coco, Cuba; McGill University: Montreal, QB, Canada, 2016. [Google Scholar]
- Peros, M.C.; Reinhardt, E.G.; Davis, A.M. A 6000-year record of ecological and hydrological changes from Laguna de la Leche, north coastal Cuba. Quat. Res. 2007, 67, 69–82. [Google Scholar] [CrossRef]
- Menéndez-Perez, H.; Díaz-Martínez, R.; González-de Zayas, R.; González-Fernández, J.A. Caracterización hidroquímica del Gran Humedal del Norte, Ciego de Ávila, Cuba. Min. Y Geol. 2011, 27, 15–41. [Google Scholar]
- González, J.A.; Menéndez, H.; González, R.; Días, G.B.; Borroto, P. Contribución de las variaciones naturales y antrópicas a los valores de temperatura y salinidad de las aguas de la bahía de Perros. Ser. Oceanol. 2013, 12, 36–47. [Google Scholar]
- González-Fernández, J.A.; Menéndez-Pérez, H.; González-Santana, R.; Fonseca-Roselló, J.A.; González-Abreu Fernández, R.; Vidal-Olivera, V. Papel regulador de las zonas inundables del humedal en el intercambio salino subterráneo entre las bahías interiores-acuíferos en el gran humedal del norte de Ciego de Avila. Cienc. En Su Pc 2008, 2, 66–74. [Google Scholar]
- Craig, H. The geochemistry of stable carbon isotopes. Geochim. Cosmochim. Acta 1953, 3, 53–92. [Google Scholar] [CrossRef]
- Mariotti, A. Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 1983, 303, 685–687. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Stal, L.J.; van Gemerden, H.; Krumbein, W.E. Structure and development of a benthic marine microbial mat. Fems Microbiol. Lett. 1985, 31, 111–125. [Google Scholar] [CrossRef]
- Van Gemerden, H. Microbial mats: A joint venture. Mar. Geol. 1993, 113, 3–25. [Google Scholar] [CrossRef]
- Decho, A. Exopolymer Microdomains as a Structuring Agent for Heterogeneity Within Microbial Biofilms. In Microbial Sediments; Riding, R., Awramik, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 9–15. [Google Scholar]
- Visscher, P.T.; van Gemerden, H. Sulfur Cycling in Laminated Marine Microbial Ecosystems. In Biogeochemistry of Global Change; Oremland, R., Ed.; Springer: Boston, MA, USA, 1993; pp. 672–690. [Google Scholar]
- Arp, G.; Reimer, A.; Reitner, J. Microbialite Formation in Seawater of Increased Alkalinity, Satonda Crater Lake, Indonesia. J. Sediment. Res. 2003, 73, 105–127. [Google Scholar] [CrossRef]
- Visscher, P.T.; van Gemerden, H. Production and Consumption of Dimethylsulfoniopropionate in Marine Microbial Mats. Appl. Environ. Microbiol. 1991, 57, 3237–3242. [Google Scholar] [CrossRef] [Green Version]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M.; Hoeft, S.E.; Macintyre, I.G.; Thompson, J.A. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas); the role of sulfur cycling. Am. Mineral. 1998, 83, 1482–1493. [Google Scholar] [CrossRef]
- Schneider, D.; Reimer, A.; Reitner, J.; Daniel, R. Phylogenetic Analysis of a Microbialite-Forming Microbial Mat from a Hypersaline Lake of the Kiritimati Atoll, Central Pacific. PLoS ONE 2013, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Carreras, E.; Vázquez-Montes De Oca, R.; Martín-Palacios, R. Restauración ecológica de algunos ecosistemas boscosos y arbustivos costeros de Ciego de Ávila, según mecanismos funcionales. Rev. For. Baracoa 2012, 31, 23–33. [Google Scholar]
- Bianchi, T.S.; Canuel, E.A. Chemical Biomarkers in Aquatic Ecosystems; Princeton University Press: Princeton, NJ, USA, 2011; p. 392. [Google Scholar]
- Gallagher, K.; Dupraz, C.; Braissant, O.; Norman, R.; Decho, A.; Visscher, P. Mineralization of sedimentary biofilms: Modern mechanistic insights. In Biofilm: Formation, Development and Properties; Bailey, W.C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2000; pp. 227–258. [Google Scholar]
- Visscher, P.T.; Stolz, J.F. Microbial mats as bioreactors: Populations, processes, and products. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 87–100. [Google Scholar] [CrossRef]
- Sand-jensen, K. Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw. Biol. 1998, 39, 663–679. [Google Scholar] [CrossRef]
- Tyson, R.V. Distribution of the Amorphous Group. In Sedimentary Organic Matter: Organic facies and Palynofacies; Springer: Dordrecht, The Netherlands, 1995; pp. 249–259. [Google Scholar]
- Burdige, D.J. Preservation of Organic Matter in Marine Sediments: Controls, Mechanisms, and an Imbalance in Sediment Organic Carbon Budgets? Chem. Rev. 2007, 107, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.C.; Land, L.S. Lime mud deposition and calcareous algae in the Bight of Abaco, Bahamas: A budget. J. Sed. Petrol. 1975, 45, 763–786. [Google Scholar]
- Jørgensen, B.B. Processes at the sediment-water interface. In The Major Biochemical Cycles and Their Interactions; Bolin, B., Cook, R.B., Eds.; John Wiley: New York, NY, USA, 1983; pp. 477–515. [Google Scholar]
- Reeburgh, W.S. Rates of biogeochemical processes in anoxic sediments. Annu. Rev. Earth Planet. Sci. 1983, 11, 269–298. [Google Scholar] [CrossRef] [Green Version]
- Mitterer, R.M.; Dzou, I.P.; Miranda, R.M.; Caughey, M.E. Extractable and pyrolyzed hydrocarbons in shallow-water carbonate sediments, Florida Bay, Florida. Org. Geochem. 1988, 13, 283–294. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Molluscan shell proteins. Comptes Rendus Palevol. 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Hare, P. Amino acid composition of some calcified proteins. Carnegie Inst. Wash. Yearbk. 1965, 64, 223–232. [Google Scholar]
- Winterwerp, J.C.; Van Kesteren, W.G. Introduction to the Physics of Cohesive Sediment Dynamics in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2004; Volume 56. [Google Scholar]
- Bauersachs, T.; Schouten, S.; Schwark, L. Characterization of the sedimentary organic matter preserved in Messel oil shale by bulk geochemistry and stable isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 410, 390–400. [Google Scholar] [CrossRef]
- Lokier, S.; Fiorini, F.; Odeh, W.A. Carbonate facies distribution within arid mangrove systems. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 1 April 2018; p. 1414. [Google Scholar]
- Fiorini, F.; Lokier, S.W. Recent Miliolidae from lagoonal environments of the UAE. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 1 April 2019. [Google Scholar]
- Bordenave, M.L. Applied Petroleum Geochemistry; Technip: Paris, France, 1993; p. 524. [Google Scholar]
- Isla, F.I. Chapter 9 Coastal Lagoons. In Geomorphology and Sedimentology of Estuaries. Developments in Sedimentology; Perillo, G.M.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 53, pp. 241–272. [Google Scholar]
- De Falco, G.; Magni, P.; Teräsvuori, L.M.H.; Matteucci, G. Sediment grain size and organic carbon distribution in the Cabras lagoon (Sardinia, Western Mediterranean). Chem. Ecol. 2004, 20, 367–377. [Google Scholar] [CrossRef]
- Kristensen, E. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 2000, 426, 1–24. [Google Scholar] [CrossRef]
- Arndt, S.; Jørgensen, B.B.; LaRowe, D.E.; Middelburg, J.J.; Pancost, R.D.; Regnier, P. Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth-Sci. Rev. 2013, 123, 53–86. [Google Scholar] [CrossRef]
- Jessen, G.L.; Lichtschlag, A.; Ramette, A.; Pantoja, S.; Rossel, P.E.; Schubert, C.J.; Struck, U.; Boetius, A. Hypoxia causes preservation of labile organic matter and changes seafloor microbial community composition (Black Sea). Sci. Adv. 2017, 3, e1601897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvert, S.E.; Pedersen, T.F. Geochemistry of Recent oxic and anoxic marine sediments: Implications for the geological record. Mar. Geol. 1993, 113, 67–88. [Google Scholar] [CrossRef]
- Cowie, G.L.; Hedges, J.I. The role of anoxia in organic matter preservation in coastal sediments: Relative stabilities of the major biochemicals under oxic and anoxic depositional conditions. Org. Geochem. 1992, 19, 229–234. [Google Scholar] [CrossRef]
- Tribovillard, N.; Caulet, J.; Vergnaud-Grazzini, C.; Moureau, N.; Tremblay, P. Geochemical study of a glacial–interglacial transition in the upwelling influenced Somalia margin, NW Indian Ocean: An unexpected lack of organic matter accumulation. Mar. Geol. 1996, 133, 157–182. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Vlug, T.; Jaco, F.; van der Nat, W.A. Organic matter mineralization in marine systems. Glob. Planet. Chang. 1993, 8, 47–58. [Google Scholar] [CrossRef]
- Ibach, L.E.J. Relationship Between Sedimentation Rate and Total Organic Carbon Content in Ancient Marine Sediments. Aapg Bull. 1982, 66, 170–188. [Google Scholar] [CrossRef]
- Thamban, M.; Rao, V.P.; Raju, S.V. Controls on organic carbon distribution in sediments from the eastern Arabian Sea Margin. Geo-Mar. Lett. 1997, 17, 220–227. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Pevear, D.R.; Hill, R.J. Mineral Surface Control of Organic Carbon in Black Shale. Science 2002, 295, 657–660. [Google Scholar] [CrossRef] [Green Version]
- Van Der Loeff, R.M.M.; Meadows, P.S.; Allen, J.A. Oxygen in Pore Waters of Deep-Sea Sediments [and Discussion]. Philos. Trans. R. Soc. London. Ser. Amathematical Phys. Sci. 1990, 331, 69–84. [Google Scholar]
- Huc, A.Y.; Le Fournier, J.; Vandenbroucke, M.; Bessereau, G.; Katz, B.J. Northern Lake Tanganyika—An Example of Organic Sedimentation in an Anoxic Rift Lake. In Lacustrine Basin Exploration: Case Studies and Modern Analogs; American Association of Petroleum Geologists: Tusla, OK, USA, 1990; Volume 50. [Google Scholar]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—an overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef] [Green Version]
- Barros, G.V.; Martinelli, L.A.; Oliveira Novais, T.M.; Ometto, J.P.H.B.; Zuppi, G.M. Stable isotopes of bulk organic matter to trace carbon and nitrogen dynamics in an estuarine ecosystem in Babitonga Bay (Santa Catarina, Brazil). Sci. Total Environ. 2010, 408, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, M.; Kamiya, H. Elemental (C, N, P) and isotopic (δ13C, δ15N) signature of primary producers and their contribution to the organic matter in coastal lagoon sediment. Landsc. Ecol. Eng. 2014, 10, 65–75. [Google Scholar] [CrossRef]
- Van Mooy, B.A.S.; Keil, R.G.; Devol, A.H. Impact of suboxia on sinking particulate organic carbon: Enhanced carbon flux and preferential degradation of amino acids via denitrification. Geochim. Cosmochim. Acta 2002, 66, 457–465. [Google Scholar] [CrossRef]
- Thomazo, C.; Ader, M.; Farquhar, J.; Philippot, P. Methanotrophs regulated atmospheric sulfur isotope anomalies during the Mesoarchean (Tumbiana Formation, Western Australia). Earth Planet. Sci. Lett. 2009, 279, 65–75. [Google Scholar] [CrossRef]
- Lehmann, M.F.; Bernasconi, S.M.; Barbieri, A.; McKenzie, J.A. Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochim. Cosmochim. Acta 2002, 66, 3573–3584. [Google Scholar] [CrossRef]
- Gälman, V.; Rydberg, J.; de-Luna, S.S.; Bindler, R.; Renberg, I. Carbon and nitrogen loss rates during aging of lake sediment: Changes over 27 years studied in varved lake sediment. Limnol. Oceanogr. 2008, 53, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Fry, B.; Wainright, S.C. Diatom sources of 13 C-rich carbon in marine food webs. Mar. Ecol. Prog. Ser. 1991, 76, 149–157. [Google Scholar] [CrossRef]
- Pascal, P.-Y.; Gros, O.; Boschker, H.T.S. Temporal fluctuations in the trophic role of large benthic sulfur bacteria in mangrove sediment. Food Webs 2016, 7, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Deming, J.W.; Baross, J.A. The Early Diagenesis of Organic Matter: Bacterial Activity. In Organic Geochemistry: Principles and Applications; Engel, M.H., Macko, S.A., Eds.; Springer: Boston, MA, USA, 1993; pp. 119–144. [Google Scholar]
- Sansjofre, P.; Ader, M.; Trindade, R.I.F.; Elie, M.; Lyons, J.; Cartigny, P.; Nogueira, A.C.R. A carbon isotope challenge to the snowball Earth. Nature 2011, 478, 93–96. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Matson, P.A.; Vitousek, P. Principles of Terrestrial Ecosystem Ecology; Springer Science & Business Media: New York, NY, USA, 2011; p. 447. [Google Scholar]
- Fasching, C.; Behounek, B.; Singer, G.A.; Battin, T.J. Microbial degradation of terrigenous dissolved organic matter and potential consequences for carbon cycling in brown-water streams. Sci. Rep. 2014, 4, 4981. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Komatsu, K.; Kohzu, A.; Tomioka, N.; Shinohara, R.; Satou, T.; Watanabe, F.N.; Tada, Y.; Hamasaki, K.; Kushairi, M. Bacterial contribution to dissolved organic matter in eutrophic Lake Kasumigaura, Japan. Appl. Environ. Microbiol. 2013, 79, 7160–7168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Fernández, A.C.; Hillaire-Marcel, C.; de Vernal, A.; Machain-Castillo, M.L.; Vásquez, L.; Ghaleb, B.; Aspiazu-Fabián, J.A.; Páez-Osuna, F. Changes of coastal sedimentation in the Gulf of Tehuantepec, South Pacific Mexico, over the last 100 years from short-lived radionuclide measurements. Estuar. Coast. Shelf Sci. 2009, 82, 525–536. [Google Scholar] [CrossRef]
- Rumolo, P.; Barra, M.; Gherardi, S.; Marsella, E.; Sprovieri, M. Stable isotopes and C/N ratios in marine sediments as a tool for discriminating anthropogenic impact. J. Environ. Monit. 2011, 13, 3399–3408. [Google Scholar] [CrossRef]
- Brahney, J.; Ballantyne, A.P.; Turner, B.L.; Spaulding, S.A.; Otu, M.; Neff, J.C. Separating the influences of diagenesis, productivity and anthropogenic nitrogen deposition on sedimentary δ15N variations. Org. Geochem. 2014, 75, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Ader, M.; Thomazo, C.; Sansjofre, P.; Busigny, V.; Papineau, D.; Laffont, R.; Cartigny, P.; Halverson, G.P. Interpretation of the nitrogen isotopic composition of Precambrian sedimentary rocks: Assumptions and perspectives. Chem. Geol. 2016, 429, 93–110. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Pinti, D.L.; Hashizume, K. Biogeochemical Cycles of Sulfur and Nitrogen in the Archean Ocean and Atmosphere. In Archean Geodynamics and Environments; Benn, K., Mareschal, J.-C., Condie, K.C., Eds.; American Geophysical Union: Washington, DC, USA, 2006; Volume 164, pp. 305–320. [Google Scholar]
- Casciotti, K.L. Inverse kinetic isotope fractionation during bacterial nitrite oxidation. Geochim. Cosmochim. Acta 2009, 73, 2061–2076. [Google Scholar] [CrossRef] [Green Version]
- Sigman, D.M.; Karsh, K.; Casciotti, K. Ocean process tracers: Nitrogen isotopes in the ocean. In Encyclopedia of Ocean Sciences; Steele, J.H., Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: Oxford, UK, 2009; pp. 40–54. [Google Scholar]
- Freudenthal, T.; Wagner, T.; Wenzhöfer, F.; Zabel, M.; Wefer, G. Early diagenesis of organic matter from sediments of the eastern subtropical Atlantic: Evidence from stable nitrogen and carbon isotopes. Geochim. Cosmochim. Acta 2001, 65, 1795–1808. [Google Scholar] [CrossRef]
- Holmes, E.; Lavik, G.; Fischer, G.; Segl, M.; Ruhland, G.; Wefer, G. Seasonal variability of δ15N in sinking particles in the Benguela upwelling region. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 377–394. [Google Scholar] [CrossRef]
- Robinson, R.S.; Kienast, M.; Luiza Albuquerque, A.; Altabet, M.; Contreras, S.; De Pol Holz, R.; Dubois, N.; Francois, R.; Galbraith, E.; Hsu, T.-C.; et al. A review of nitrogen isotopic alteration in marine sediments. Paleoceanography 2012, 27, 1–13. [Google Scholar] [CrossRef]
- Kienast, M.; Higginson, M.J.; Mollenhauer, G.; Eglinton, T.I.; Chen, M.-T.; Calvert, S.E. On the sedimentological origin of down-core variations of bulk sedimentary nitrogen isotope ratios. Paleoceanography 2005, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]




| Description | Zone | Characteristics | Calcimetry | TOC (wt. %) | TN (wt. %) | [TOC/TN]atomic Ratio | δ13Corg (‰, vs. VPDB) | δ15NTN (‰, vs. Atmospheric N2) | |
|---|---|---|---|---|---|---|---|---|---|
| Microbial mats | Mineralising microbial mat | III | green layer | 81.84% | 7.13 ± 0.03 | 0.94 ± 0.00 | 8.85 ± 0.02 | −15.72 ± 0.03 | 2.44 ± 0.15 |
| III | pink layer | 72.77% | 8.73 ± 0.1 | 1.15 ± 0.02 | 8.86 ± 0.05 | −15.29 ± 0.01 | 2.99 ± 0.1 | ||
| III | black layer | 76.58% | 4.78 ± 0.13 | 0.7 ± 0.01 | 7.97 ± 0.07 | −15.78 ± 0.02 | 4.82 ± 0.06 | ||
| III | sediment | 81.32% | 2.01 ± 0.01 | 0.31 ± 0.00 | 7.56 ± 0.02 | −18.62 ± 0.02 | 6.38 ± 0.16 | ||
| Slime | IV | Top | 86.27% | 4.49 ± 0.21 | 0.65 ± 0.04 | 8.05 ± 0.12 | −18.47 ± 0.09 | 3.28 ± 0.25 | |
| IV | Middle | 84.97% | 4.67 ± 0.05 | 0.68 ± 0.01 | 8.01 ± 0.01 | −18.39 ± 0.03 | 3.5 ± 0.07 | ||
| IV | Bottom | 88.02% | 4.3 ± 0.08 | 0.62 ± 0.01 | 8.09 ± 0.16 | −18.54 ± 0.06 | 3.05 ± 0.05 | ||
| Soil | Soil 1 | I-II | Plant fragments | 51.39% | 4.72 ± 0.07 | 0.54 ± 0.01 | 10.2 ± 0.06 | −23.94 ± 0.02 | 8.24 ± 0.19 |
| Soil 2 | I-II | Plant fragments | 48.56% | 12.43 ± 0.05 | 1.42 ± 0.01 | 10.21 ± 0.11 | −21.9 ± 0.05 | 7.35 ± 0.17 | |
| Soil 3 | I-II | Microb. mat fragments | 43.60% | 20.4 ± 0.16 | 2.37 ± 0.19 | 10.04 ± 0.82 | −20.52 ± 0.02 | 4.41 ± 0.15 | |
| Plants | Avicennia germinans | I-II (III) | Thin branches | - | 53.49 ± 0.16 | 0.77 ± 0.00 | 81.04 ± 0.25 | −24.87 ± 0.01 | 1.74 ± 0.06 |
| I-II (III) | Branches | - | 53.12 ± 0.3 | 0.73 ± 0.09 | 84.66 ± 9.82 | −24.43 ± 0.42 | –1.13 ± 0.38 | ||
| I-II (III) | Leaves | - | 54.64 ± 0.01 | 1.25 ± 0.00 | 51 ± 0.01 | −25.8 ± 0.02 | 2.1 ± 0.04 | ||
| I-II (III) | Buds | - | 48.3 ± 0.05 | 1.61 ± 0.01 | 35 ± 0.25 | −25.38 ± 0.05 | 0.88 ± 0.06 | ||
| Sarcocornia perennis | I-II (III) | Brown branches | - | 47.01 ± 1.01 | 0.57 ± 0.03 | 96.21 ± 2.71 | −28.08 ± 0.01 | 4.33 ± 0.22 | |
| I-II (III) | Green branches | - | 50.26 ± 0.4 | 1.78 ± 0.1 | 32.94 ± 1.57 | −27.72 ± 0.06 | 7.77 ± 0.25 | ||
| Batis maritima | I-II (III) | Roots | - | 50.76 ± 1.68 | 1.16 ± 0.14 | 51.2 ± 5.12 | −26.44 ± 0.32 | 6.6 ± 0.24 | |
| I-II (III) | Branches | - | 50.79 ± 0.08 | 0.61 ± 0.01 | 97.94 ± 0.98 | −25.71 ± 0.03 | 5.7 ± 0.13 | ||
| I-II (III) | Buds | - | 21.41 ± 0.4 | 1.3 ± 0.17 | 19.15 ± 2.11 | −23.85 ± 0.5 | 6.52 ± 0.27 | ||
| SPM* | SPM 1 | IV | - | - | - | - | - | −20.54 | 3.54 |
| SPM 2 | IV | - | - | - | - | - | −20.64 | 2.72 | |
| SPM 3 | IV | - | - | - | - | - | −19.96 | 1.98 | |
| SPM 4 | IV | - | - | - | - | - | −19.65 | 3.58 | |
| Core | Zone | Interval (cm) | Facies | Calcimetry | Fraction | TOC (wt. %) | TN (wt. %) | [TOC/TN]atomic Ratio | δ13Corg (‰, vs. VPDB) | δ15NTN (‰, vs. Atmospheric N2) |
|---|---|---|---|---|---|---|---|---|---|---|
| CC-1 | III | 0–0.2 | Mineralising microbial mat | 75.79% | <2000 µm | 8.85 ± 0.38 | 1.06 ± 0.04 | 9.74 ± 0.13 | −15.83 ± 0.08 | 1.82 ± 0.07 |
| 0..2–0.4 | Mineralising microbial mat | 74.34% | <2000 µm | 8.82 ± 0.1 | 1.2 ± 0.01 | 8.60 ± 0.03 | −14.76 ± 0.07 | 2.66 ± 0.06 | ||
| 0.4–0.6 | Mineralising microbial mat | 71.78% | <2000 µm | 7.5 ± 0.24 | 1 ± 0.03 | 8.71 ± 0.03 | −14.61 ± 0.03 | 3.75 ± 0.04 | ||
| 0.6–0.9 | Mineralising microbial mat | 82.91% | <2000 µm | 4.53 ± 0.11 | 0.63 ± 0.01 | 8.41 ± 0.07 | −14.43 ± 0.06 | 4.13 ± 0.05 | ||
| 0.9–1.1 | Mineralising microbial mat | 85.54% | <2000 µm | 4.1 ± 0.06 | 0.64 ± 0.01 | 7.47 ± 0.02 | −14.89 ± 0.06 | 5.14 ± 0.13 | ||
| 1.1–3.1 | Micritic mud | 92.34% | 0–200 µm | 1.21 ± 0.01 | 0.18 ± 0.00 | 7.94 ± 0.23 | −16.75 ± 0.05 | 6.04 ± 0.12 | ||
| 88.38% | 200–2000 µm | 2.9 ± 0.03 | 0.45 ± 0.01 | 7.53 ± 0.05 | −15.43 ± 0.07 | 5.83 ± 0.04 | ||||
| 3.1–5.0 | Micritic mud | 91.67% | 0–200 µm | 0.7 ± 0.01 | 0.09 ± 0.00 | 8.73 ± 0.10 | −17.72 ± 0.01 | 6.52 ± 0.06 | ||
| 88.44% | 200–2000 µm | 1.87 ± 0.02 | 0.25 ± 0.00 | 8.63 ± 0.08 | −18.57 ± 0.08 | 6.86 ± 0.09 | ||||
| 5.0–7.0 | Micritic mud | 90.77% | 0–200 µm | 0.54 ± 0.01 | 0.07 ± 0.00 | 8.85 ± 0.12 | −17.99 ± 0.06 | 7.45 ± 0.08 | ||
| 93.81% | 200–2000 µm | 0.79 ± 0.02 | 0.11 ± 0.00 | 8.24 ± 0.08 | −18.88 ± 0.14 | 8.04 ± 0.06 | ||||
| 7.0–8.8 | Micritic mud | 87.56% | 0–200 µm | 0.61 ± 0.01 | 0.08 ± 0.00 | 8.80 ± 0.21 | −18.02 ± 0.07 | 7.41 ± 0.02 | ||
| 93.45% | 200–2000 µm | 0.56 ± 0.03 | 0.07 ± 0.00 | 8.71 ± 0.34 | −18.13 ± 0.44 | 7.06 ± 0.10 | ||||
| 8.8–10.5 | Micritic mud | 89.46% | 0–200 µm | 0.46 ± 0.00 | 0.06 ± 0.00 | 9.14 ± 0.06 | −18.09 ± 0.10 | 7.91 ± 0.04 | ||
| 96.35% | 200–2000 µm | 0.33 ± 0.00 | 0.04 ± 0.00 | 8.72 ± 0.10 | −19.30 ± 0.07 | 8.05 ± 0.08 | ||||
| 10.5–12.0 | Micritic mud | 89.09% | 0–200 µm | 0.42 ± 0.00 | 0.06 ± 0.00 | 8.89 ± 0.12 | −18.19 ± 0.04 | 7.83 ± 0.13 | ||
| 97.18% | 200–2000 µm | 0.39 ± 0.00 | 0.05 ± 0.00 | 8.80 ± 0.18 | −20.63 ± 0.20 | 8.71 ± 0.06 | ||||
| 12.0–14.0 | Micritic mud | 85.69% | 0–200 µm | 0.62 ± 0.02 | 0.08 ± 0.00 | 8.98 ± 0.09 | −18.38 ± 0.10 | 6.24 ± 0.11 | ||
| 87.49% | 200–2000 µm | 0.83 ± 0.01 | 0.11 ± 0.00 | 8.95 ± 0.10 | −19.23 ± 0.08 | 6.02 ± 0.16 | ||||
| Cryptic microbial mat | 87.30% | <2000 µm | 0.89 ± 0.09 | 0.13 ± 0.01 | 7.92 ± 0.21 | −19.64 ± 0.22 | 8.17 ± 0.72 | |||
| 14.0–15.4 | Micritic mud | 85.63% | 0–200 µm | 0.71 ± 0.02 | 0.09 ± 0.00 | 8.90 ± 0.11 | −18.59 ± 0.03 | 6.20 ± 0.06 | ||
| 83.85% | 200–2000 µm | 1.31 ± 0.02 | 0.16 ± 0.00 | 9.50 ± 0.30 | −19.85 ± 0.15 | 6.06 ± 0.08 | ||||
| CC-2 | IV | 0–0.8 | Microbial slime | 94.17% | 0–200 µm | 1.54 ± 0.00 | 0.21 ± 0.00 | 8.56 ± 0.35 | −19.49 ± 0.01 | 2.48 ± 0.17 |
| 96.06% | 200–2000 µm | 0.91 ± 0.62 | 0.12 ± 0.08 | 8.93 ± 0.35 | −19.48 ± 0.15 | 2.22 ± 0.51 | ||||
| 0.8–2.4 | Bioclastic sand | 96.00% | 0–200 µm | 0.79 ± 0.00 | 0.1 ± 0.00 | 9.07 ± 0.09 | −17.58 ± 0.02 | 4.31 ± 0.14 | ||
| 97.53% | 200–2000 µm | 0.69 ± 0.01 | 0.09 ± 0.00 | 8.51 ± 0.26 | −17.69 ± 0.34 | 3.54 ± 0.05 | ||||
| 2.4–3.5 | Micritic mud | 90.30% | 0–200 µm | 1.19 ± 0.01 | 0.15 ± 0.00 | 9.38 ± 0.09 | −17.95 ± 0.05 | 4.95 ± 0.16 | ||
| 89.21% | 200–2000 µm | 1.77 ± 0.03 | 0.22 ± 0.00 | 9.39 ± 0.14 | −17.92 ± 0.02 | 4.39 ± 0.04 | ||||
| 3.5–5.0 | Micritic mud | 84.52% | 0–200 µm | 1.76 ± 0.06 | 0.22 ± 0.01 | 9.47 ± 0.20 | −18.23 ± 0.08 | 5.25 ± 0.16 | ||
| 83.57% | 200–2000 µm | 2.4 ± 0.05 | 0.29 ± 0.00 | 9.59 ± 0.10 | −18.29 ± 0.12 | 5.14 ± 0.15 | ||||
| 5.0–5.8 | Micritic mud | 88.22% | 0–200 µm | 1.45 ± 0.02 | 0.18 ± 0.00 | 9.50 ± 0.15 | −18.32 ± 0.07 | 5.39 ± 0.14 | ||
| 83.38% | 200–2000 µm | 2.45 ± 0.07 | 0.3 ± 0.01 | 9.48 ± 0.20 | −18.74 ± 0.03 | 5.16 ± 0.04 | ||||
| CC-2 | IV | 5.8–6.4 | Micritic mud | 89.34% | 0–200 µm | 1.32 ± 0.04 | 0.16 ± 0.01 | 9.55 ± 0.03 | −18.52 ± 0.06 | 5.60 ± 0.28 |
| 88.89% | 200–2000 µm | 1.76 ± 0.03 | 0.21 ± 0.01 | 9.65 ± 0.40 | −19.23 ± 0.02 | 5.38 ± 0.17 | ||||
| Mineralising microbial mat | - | <2000 µm | - | - | - | −16.69 ± 0.03 | 5.63 ± 0.75 | |||
| 6.4–6.8 | Micritic mud | 91.53% | 0–200 µm | 1.02 ± 0.01 | 0.13 ± 0.00 | 9.44 ± 0.04 | −17.98 ± 0.02 | 5.15 ± 0.27 | ||
| 91.25% | 200–2000 µm | 1.58 ± 0.02 | 0.19 ± 0.00 | 9.60 ± 0.07 | −18.29 ± 0.10 | 5.12 ± 0.23 | ||||
| 6.8–7.1 | Micritic mud | 89.50% | 0–200 µm | 1.19 ± 0.02 | 0.14 ± 0.00 | 9.70 ± 0.06 | −17.54 ± 0.03 | 4.56 ± 0.37 | ||
| 91.80% | 200–2000 µm | 1.36 ± 0.00 | 0.17 ± 0.00 | 9.56 ± 0.24 | −18.18 ± 0.00 | 4.62 ± 0.65 | ||||
| Mineralising microbial mat | 92.72% | 0–200 µm | 0.87 ± 0.01 | 0.11 ± 0.00 | 9.37 ± 0.33 | −16.44 ± 0.01 | 5.15 ± 0.34 | |||
| 90.48% | 200–2000 µm | 2.21 ± 0.01 | 0.3 ± 0.01 | 8.58 ± 0.25 | −15.53 ± 0.01 | 4.17 ± 0.60 | ||||
| 7.1–7.9 | Micritic mud | 87.61% | 0–200 µm | 1.26 ± 0.02 | 0.15 ± 0.00 | 9.60 ± 0.22 | −17.80 ± 0.43 | 5.26 ± 0.35 | ||
| 85.09% | 200–2000 µm | 2.16 ± 0.02 | 0.25 ± 0.00 | 10.25 ± 0.13 | −18.73 ± 0.14 | 5.36 ± 0.07 | ||||
| (dark) | 87.65% | <2000 µm | 1.63 ± 0.02 | 0.19 ± 0.00 | 9.75 ± 0.23 | −16.03 ± 0.04 | 6.14 ± 0.42 | |||
| 7.9–9.1 | Micritic mud | 86.89% | 0–200 µm | 1.12 ± 0.00 | 0.05 ± 0.07 | 9.60 ± 0.15 | −17.86 ± 0.02 | 5.48 ± 0.22 | ||
| 86.89% | 200–2000 µm | 1.97 ± 0.05 | 0.22 ± 0.00 | 10.25 ± 0.13 | −19.09 ± 0.05 | 4.90 ± 0.31 | ||||
| 9.1–10.2 | Micritic mud | 75.98% | 0–200 µm | 3.49 ± 0.02 | 0.4 ± 0.01 | 10.21 ± 0.22 | −16.82 ± 0.04 | 5.70 ± 0.39 | ||
| 68.28% | 200–2000 µm | 5.33 ± 0.04 | 0.6 ± 0.04 | 10.45 ± 0.63 | −16.98 ± 0.01 | 6.19 ± 1.15 | ||||
| 84.22% | 0–200 µm | 1.15 ± 0.04 | 0.14 ± 0.00 | 9.56 ± 0.27 | −18.00 ± 0.05 | 6.30 ± 0.21 | ||||
| 80.71% | 200–2000 µm | 2.51 ± 0.02 | 0.26 ± 0.00 | 11.30 ± 0.19 | −20.23 ± 0.07 | 5.64 ± 0.16 | ||||
| 10.2–10.5 | Micritic mud | 71.54% | 0–200 µm | 4.44 ± 0.06 | 0.48 ± 0.00 | 10.80 ± 0.08 | −16.64 ± 0.01 | 6.46 ± 0.19 | ||
| 85.90% | 200–2000 µm | 3.75 ± 0.00 | 0.58 ± 0.00 | 7.58 ± 0.03 | −16.66 ± 0.01 | 3.75 ± 0.28 | ||||
| (dark) | 82.41% | 0–200 µm | 1.46 ± 0.01 | 0.18 ± 0.00 | 9.48 ± 0.20 | −18.05 ± 0.06 | 6.07 ± 0.16 | |||
| (dark) | 76.90% | 200–2000 µm | 2.54 ± 0.08 | 0.28 ± 0.00 | 10.65 ± 0.26 | −18.57 ± 0.05 | 6.44 ± 0.16 | |||
| (mid dark) | 81.15% | <2000 µm | 2.82 ± 0.02 | 0.3 ± 0.00 | 11.10 ± 0.16 | −18.11 ± 0.17 | 5.86 ± 0.04 | |||
| 10.5–12.3 | Micritic mud | 75.82% | <2000 µm | 3.9 ± 0.02 | 0.44 ± 0.01 | 10.22 ± 0.10 | −16.75 ± 0.04 | 5.34 ± 0.21 | ||
| 81.08% | <2000 µm | 2.4 ± 0.01 | 0.29 ± 0.00 | 9.67 ± 0.03 | −17.63 ± 0.04 | 4.64 ± 0.16 | ||||
| (dark) | 85.25% | <2000 µm | 2.12 ± 0.01 | 0.23 ± 0.00 | 10.75 ± 0.05 | −17.60 ± 0.03 | 5.64 ± 0.37 | |||
| 12.3–14.5 | Micritic mud | 75.57% | 0–200 µm | 3.87 ± 0.01 | 0.44 ± 0.00 | 10.16 ± 0.03 | −16.78 ± 0.02 | 5.02 ± 0.10 | ||
| 67.22% | 200–2000 µm | 5.38 ± 0.04 | 0.56 ± 0.01 | 11.26 ± 0.20 | −16.85 ± 0.16 | 5.83 ± 0.06 | ||||
| (dark) | 77.92% | <2000 µm | 1.91 ± 0.03 | 0.2 ± 0.01 | 11.18 ± 0.94 | −17.94 ± 0.21 | 6.07 ± 0.52 | |||
| CC-3 | IV | 0–2.1 | Slime | 48.89% | 0–200 µm | 16.06 ± 0.02 | 2.34 ± 0.01 | 8.00 ± 0.03 | −18.44 ± 0.01 | 3.69 ± 0.03 |
| 63.23% | 200–2000 µm | 11.59 ± 0.4 | 1.71 ± 0.05 | 7.89 ± 0.07 | −18.47 ± 0.01 | 3.59 ± 0.02 | ||||
| 2.1–2.9 | Micritic mud | 95.96% | 0–200 µm | 1.03 ± 0.01 | 0.14 ± 0.00 | 8.56 ± 0.05 | −18.03 ± 0.02 | 3.91 ± 0.05 | ||
| 96.39% | 200–2000 µm | 1.09 ± 0.02 | 0.16 ± 0.00 | 7.79 ± 0.17 | −18.28 ± 0.06 | 3.75 ± 0.04 | ||||
| 2.9–4.4 | Micritic mud | 89.87% | 0–200 µm | 2.47 ± 0.06 | 0.33 ± 0.01 | 8.80 ± 0.13 | −17.62 ± 0.01 | 3.87 ± 0.04 | ||
| 80.16% | 200–2000 µm | 5.41 ± 0.07 | 0.73 ± 0.01 | 8.65 ± 0.02 | −17.56 ± 0.02 | 3.56 ± 0.09 | ||||
| CC-3 | IV | 4.4–5.6 | Micritic mud | 89.91% | 0–200 µm | 2.4 ± 0.00 | 0.31 ± 0.00 | 9.03 ± 0.00 | −17.61 ± 0.03 | 4.02 ± 0.05 |
| 82.71% | 200–2000 µm | 4.93 ± 0.03 | 0.63 ± 0.01 | 8.69 ± 0.02 | −17.49 ± 0.04 | 3.67 ± 0.05 | ||||
| 5.6–6.9 | Micritic mud | 91.28% | 0–200 µm | 1.78 ± 0.02 | 0.22 ± 0.00 | 9.46 ± 0.13 | −17.47 ± 0.02 | 4.20 ± 0.16 | ||
| 82.46% | 200–2000 µm | 4.16 ± 0.03 | 0.53 ± 0.00 | 9.10 ± 0.09 | −17.37 ± 0.03 | 3.98 ± 0.07 | ||||
| 6.9–8.1 | Micritic mud | 90.15% | 0–200 µm | 1.76 ± 0.01 | 0.2 ± 0.00 | 10.25 ± 0.03 | −17.60 ± 0.10 | 4.52 ± 0.16 | ||
| 83.24% | 200–2000 µm | 3.15 ± 0.01 | 0.36 ± 0.00 | 10.12 ± 0.13 | −17.56 ± 0.02 | 4.68 ± 0.29 | ||||
| 8.1–9.5 | Micritic mud | 88.25% | 0–200 µm | 2.1 ± 0.01 | 0.24 ± 0.00 | 10.34 ± 0.28 | −17.84 ± 0.01 | 4.41 ± 0.14 | ||
| 80.20% | 200–2000 µm | 3.72 ± 0.08 | 0.41 ± 0.01 | 10.68 ± 0.06 | −18.07 ± 0.03 | 4.44 ± 0.18 | ||||
| 9.5–11.7 | Bioclastic sand | 88.82% | 0–200 µm | 1.81 ± 0.02 | 0.19 ± 0.01 | 10.72 ± 0.25 | −17.43 ± 0.06 | 4.12 ± 0.35 | ||
| 90.47% | 200–2000 µm | 1.77 ± 0.01 | 0.19 ± 0.00 | 10.71 ± 0.29 | −17.74 ± 0.04 | 4.37 ± 0.16 | ||||
| 11.7–12.8 | Micritic mud | 82.52% | 0–200 µm | 2.59 ± 0.07 | 0.27 ± 0.01 | 11.33 ± 0.07 | −16.59 ± 0.06 | 5.47 ± 0.06 | ||
| 76.85% | 200–2000 µm | 3.64 ± 0.05 | 0.38 ± 0.01 | 11.28 ± 0.03 | −16.66 ± 0.12 | 5.69 ± 0.09 | ||||
| 12.8–14.0 | Micritic mud | 85.86% | 0–200 µm | 2.25 ± 0.02 | 0.24 ± 0.01 | 11.11 ± 0.22 | −16.96 ± 0.03 | 5.54 ± 0.20 | ||
| 78.00% | 200–2000 µm | 3.52 ± 0.06 | 0.36 ± 0.01 | 11.51 ± 0.07 | −16.90 ± 0.08 | 5.67 ± 0.04 | ||||
| 14.0–14.7 | Bioclastic sand | 88.96% | 0–200 µm | 1.8 ± 0.03 | 0.19 ± 0.00 | 11.03 ± 0.15 | −17.06 ± 0.02 | 5.54 ± 0.14 | ||
| 98.56% | 200–2000 µm | 0.37 ± 0.01 | 0.04 ± 0.00 | 12.49 ± 1.99 | −18.09 ± 0.49 | 6.38 ± 0.31 | ||||
| CC-4 | IV | 0–0.9 | Slime | 89.89% | 0–2000 µm | 0.45 ± 0.00 | 0.07 ± 0.00 | 7.95 ± 0.07 | −17.65 ± 0.03 | 2.90 ± 0.09 |
| 0.9–2.8 | 87.07% | 0–200 µm | 0.74 ± 0.00 | 0.1 ± 0.00 | 8.20 ± 0.06 | −17.58 ± 0.06 | 3.61 ± 0.06 | |||
| 98.64% | 200–2000 µm | 0.37 ± 0.00 | 0.06 ± 0.00 | 7.75 ± 0.28 | −17.59 ± 0.01 | 3.60 ± 0.13 | ||||
| 2.8–4.5 | Micritic mud | 88.86% | 0–200 µm | 1.22 ± 0.01 | 0.14 ± 0.00 | 9.93 ± 0.12 | −17.28 ± 0.02 | 4.81 ± 0.07 | ||
| 92.76% | 200–2000 µm | 1.38 ± 0.01 | 0.16 ± 0.00 | 10.21 ± 0.15 | −18.22 ± 0.04 | 4.64 ± 0.25 | ||||
| 4.5–5.7 | Micritic mud | 88.11% | 0–200 µm | 1.28 ± 0.01 | 0.15 ± 0.00 | 9.89 ± 0.09 | −17.35 ± 0.03 | 4.52 ± 0.20 | ||
| 91.91% | 200–2000 µm | 1.77 ± 0.02 | 0.18 ± 0.00 | 11.23 ± 0.05 | −18.71 ± 0.04 | 4.82 ± 0.44 | ||||
| 5.7–7.1 | Micritic mud | 86.71% | 0–200 µm | 1.84 ± 0.01 | 0.2 ± 0.00 | 10.51 ± 0.13 | −17.04 ± 0.04 | 4.97 ± 0.11 | ||
| 91.40% | 200–2000 µm | 1.6 ± 0.01 | 0.17 ± 0.00 | 11.14 ± 0.26 | −18.05 ± 0.05 | 4.68 ± 0.19 | ||||
| 7.1–8.5 | Micritic mud | 88.17% | 0–200 µm | 1.66 ± 0.04 | 0.18 ± 0.01 | 10.55 ± 0.14 | −17.19 ± 0.05 | 5.21 ± 0.21 | ||
| 94.77% | 200–2000 µm | 1.15 ± 0.02 | 0.1 ± 0.00 | 13.25 ± 0.40 | −19.72 ± 0.11 | 5.15 ± 0.12 | ||||
| 8.5–13.5 | Bioclastic sand | 90.58% | 0–200 µm | 1.13 ± 0.03 | 0.12 ± 0.00 | 10.78 ± 0.19 | −17.03 ± 0.08 | 6.10 ± 0.13 | ||
| 98.46% | 200–2000 µm | 0.21 ± 0.00 | 0.03 ± 0.00 | 9.87 ± 0.05 | −16.93 ± 0.06 | 6.16 ± 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouton, A.; Vennin, E.; Thomazo, C.; Mathieu, O.; Garcia, F.; Jaubert, M.; Visscher, P.T. Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba. Minerals 2020, 10, 143. https://doi.org/10.3390/min10020143
Bouton A, Vennin E, Thomazo C, Mathieu O, Garcia F, Jaubert M, Visscher PT. Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba. Minerals. 2020; 10(2):143. https://doi.org/10.3390/min10020143
Chicago/Turabian StyleBouton, Anthony, Emmanuelle Vennin, Christophe Thomazo, Olivier Mathieu, Fabien Garcia, Maxime Jaubert, and Pieter T. Visscher. 2020. "Microbial Origin of the Organic Matter Preserved in the Cayo Coco Lagoonal Network, Cuba" Minerals 10, no. 2: 143. https://doi.org/10.3390/min10020143






