Fractionation Trends and Variability of Rare Earth Elements and Selected Critical Metals in Pelagic Sediment from Abyssal Basin of NE Pacific (Clarion-Clipperton Fracture Zone)
Abstract
:1. Introduction
1.1. General Physical and Chemical Behavior of Rare Earth Elements
1.2. REE in Deep-Sea Sediments
1.3. REE in CCFZ
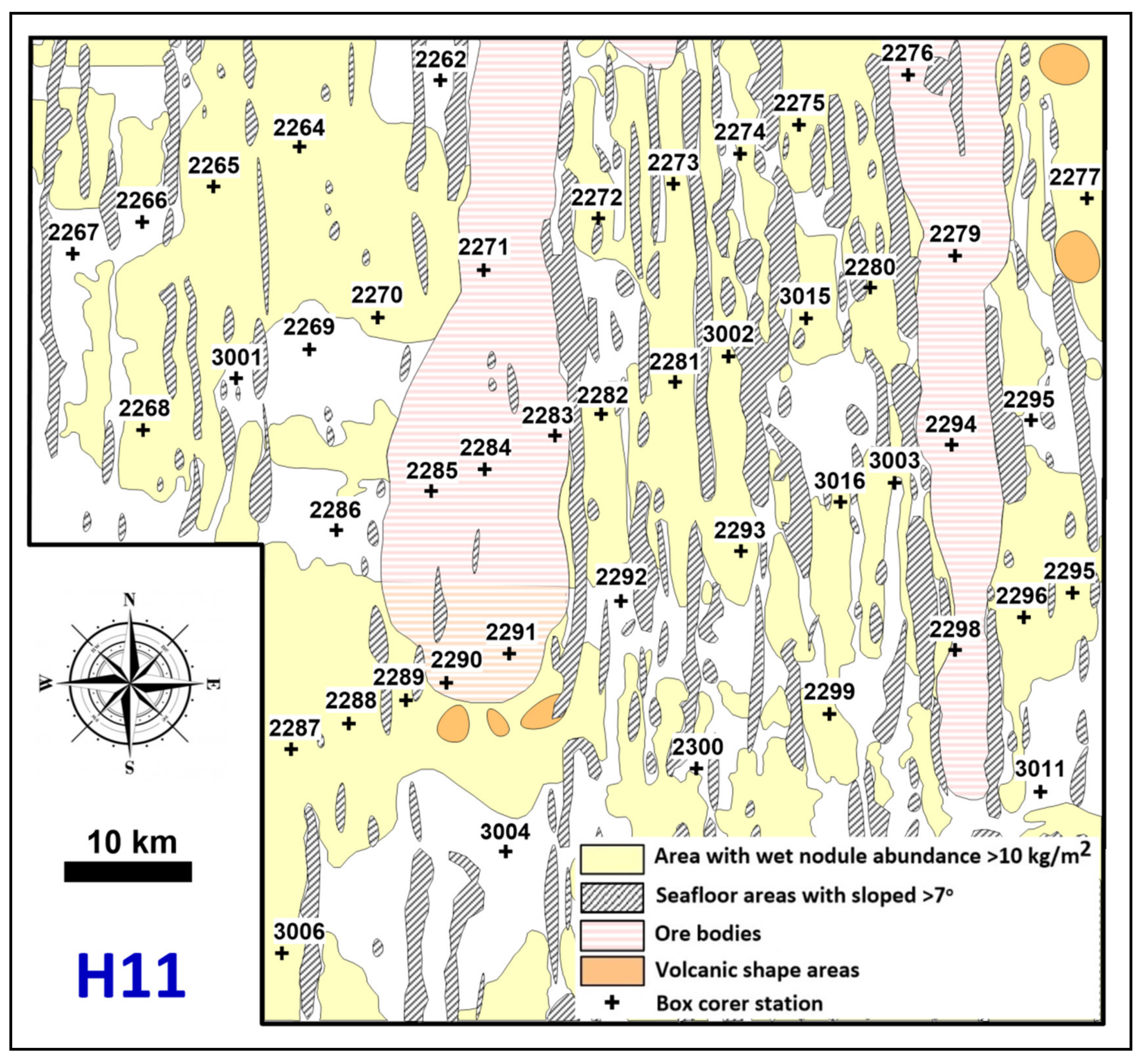
2. Geological Setting
3. Materials and Methods
3.1. Sample Collection and Processing
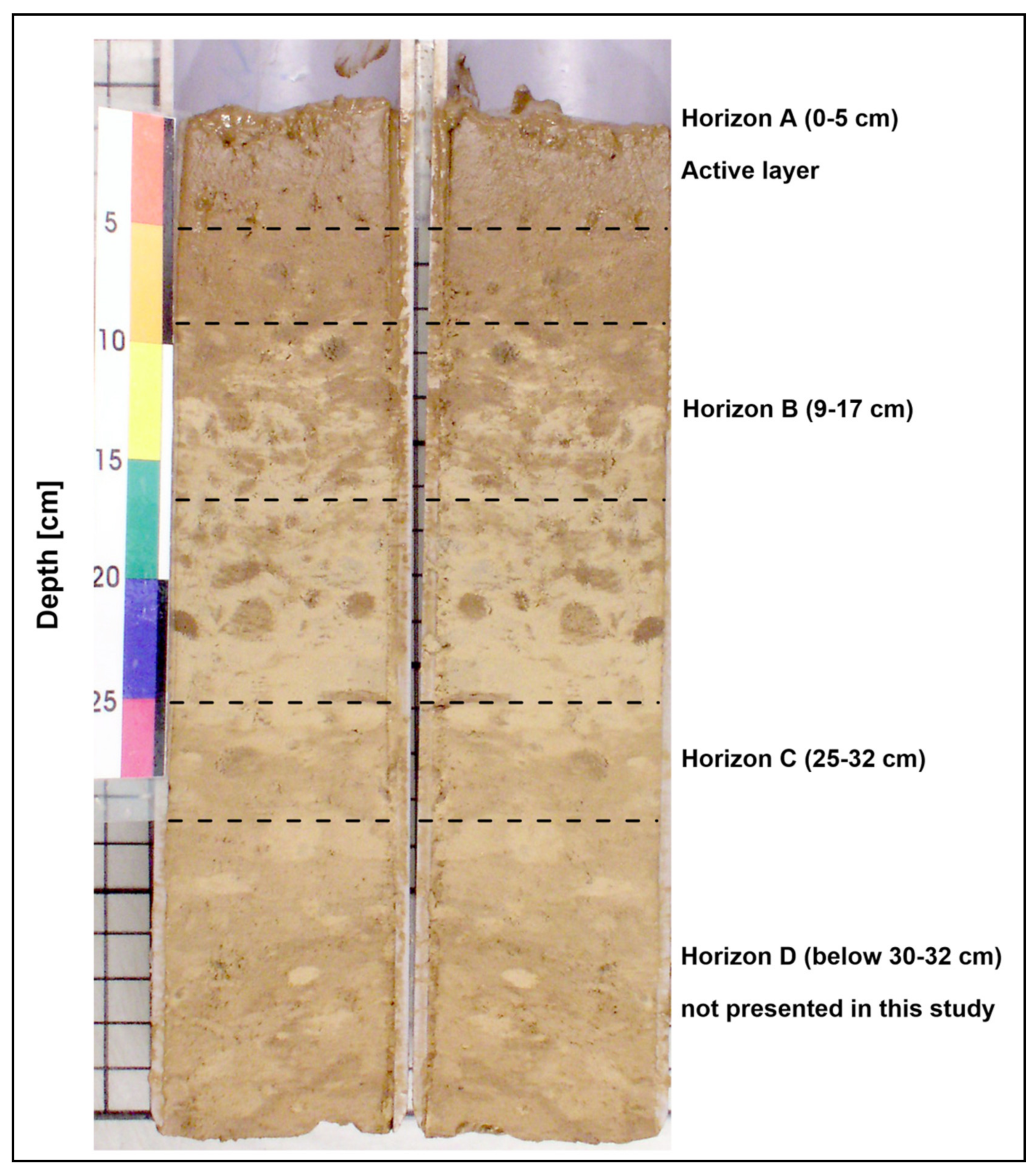
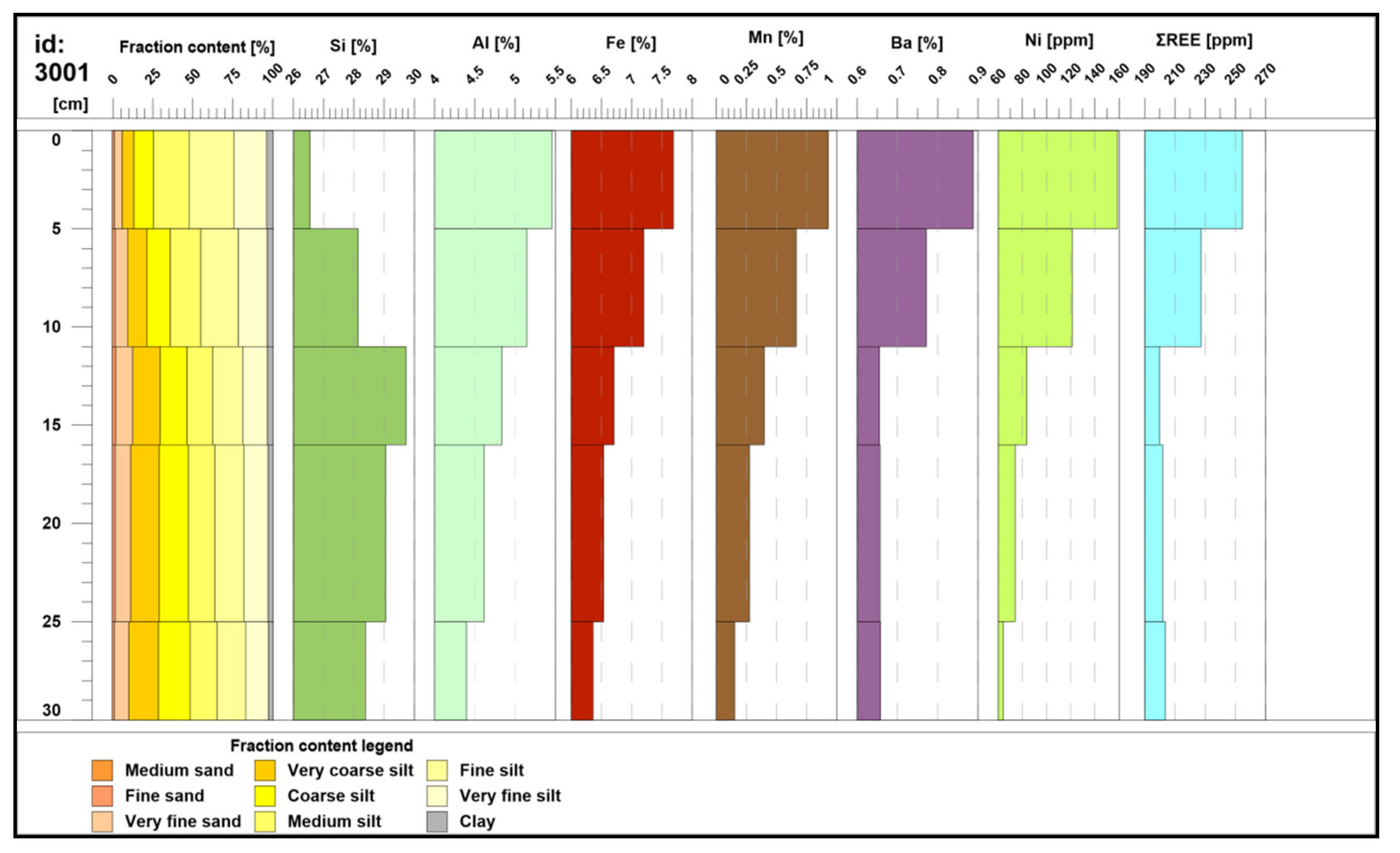
- horizon A—semiliquid geochemically active surface layer (0−5 cm; number of samples: 45), subsequently called “active layer” [43]. This horizon contains the highest amount of nonburied polymetallic nodules, which were removed from the samples before analysis. The horizon is characterized by a dark brown to brown color, the highest water content, and friable consistency.
- horizon B—the upper part of the middle section (9−17 cm; n = 45). This layer is transitional between the uppermost semiliquid horizon A and the denser horizon C below. This sediment is beige with brownish bioturbation traces, lower water content than the uppermost layer, and is also more firm.
- horizon C—the intermediate part of the middle section, (25−32 cm; n = 45). Sediment is dark beige with some brownish bioturbation traces, moderate water content, and greater firmness compared to horizons A and B.
- horizon D—the lower part of the middle section (below 30–32 cm; up to 42 cm, n = 2). The horizon with the lowest water content and greatest firmness.
3.2. Grain Size Distribution Analysis
3.3. Mineralogy
3.4. Geochemistry
3.5. Data Processing
4. Results
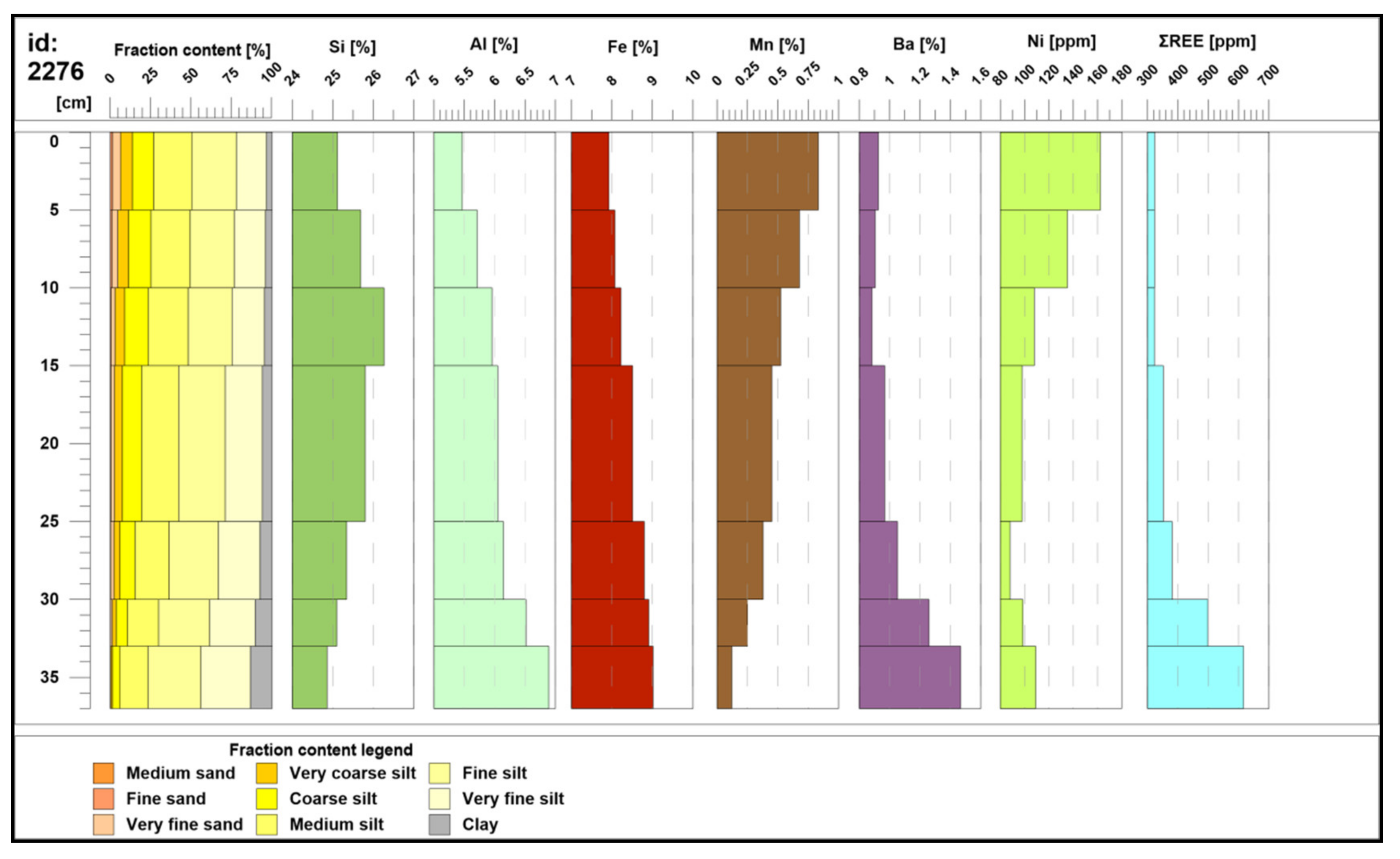
4.1. Grain Size Analysis
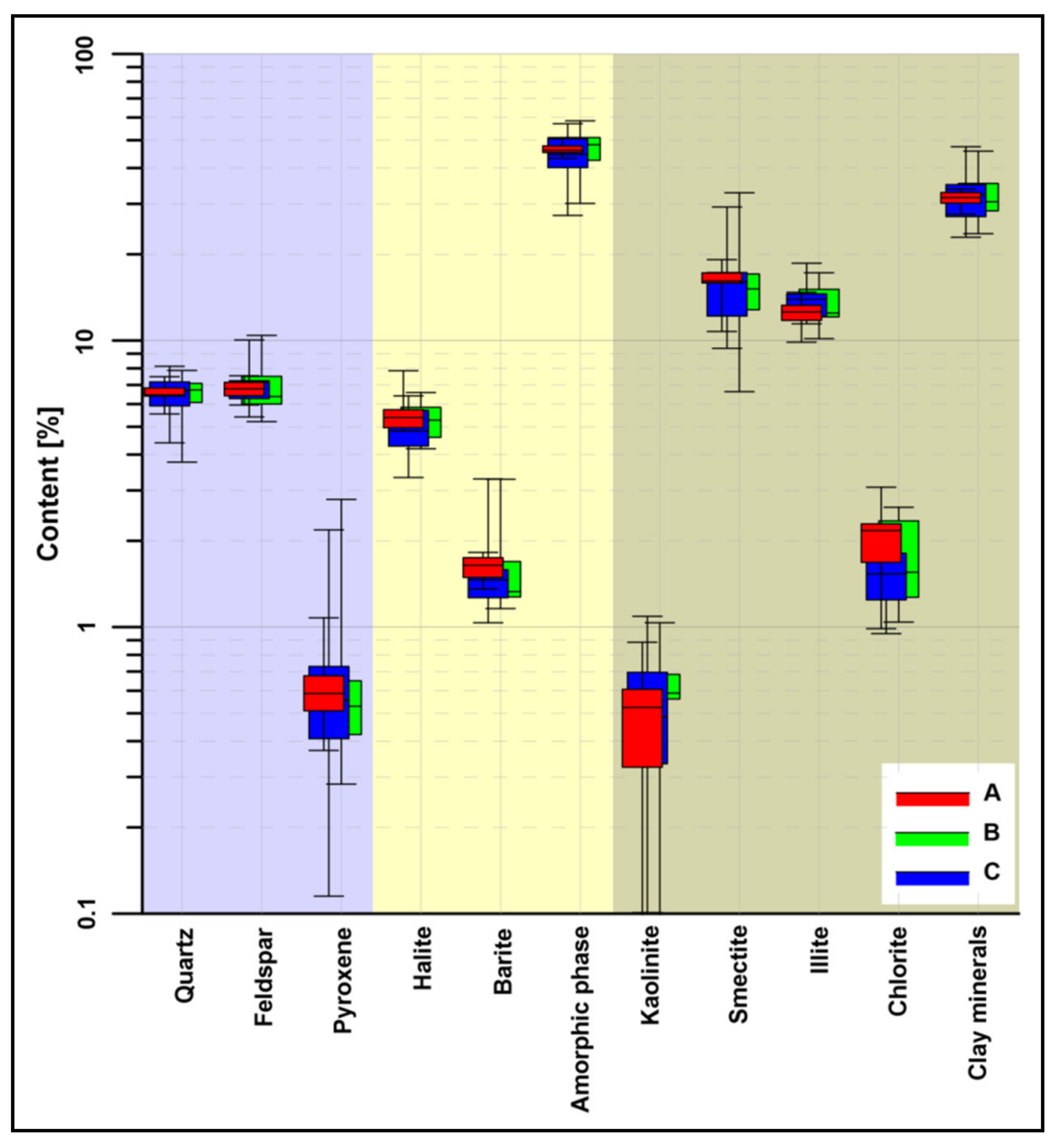
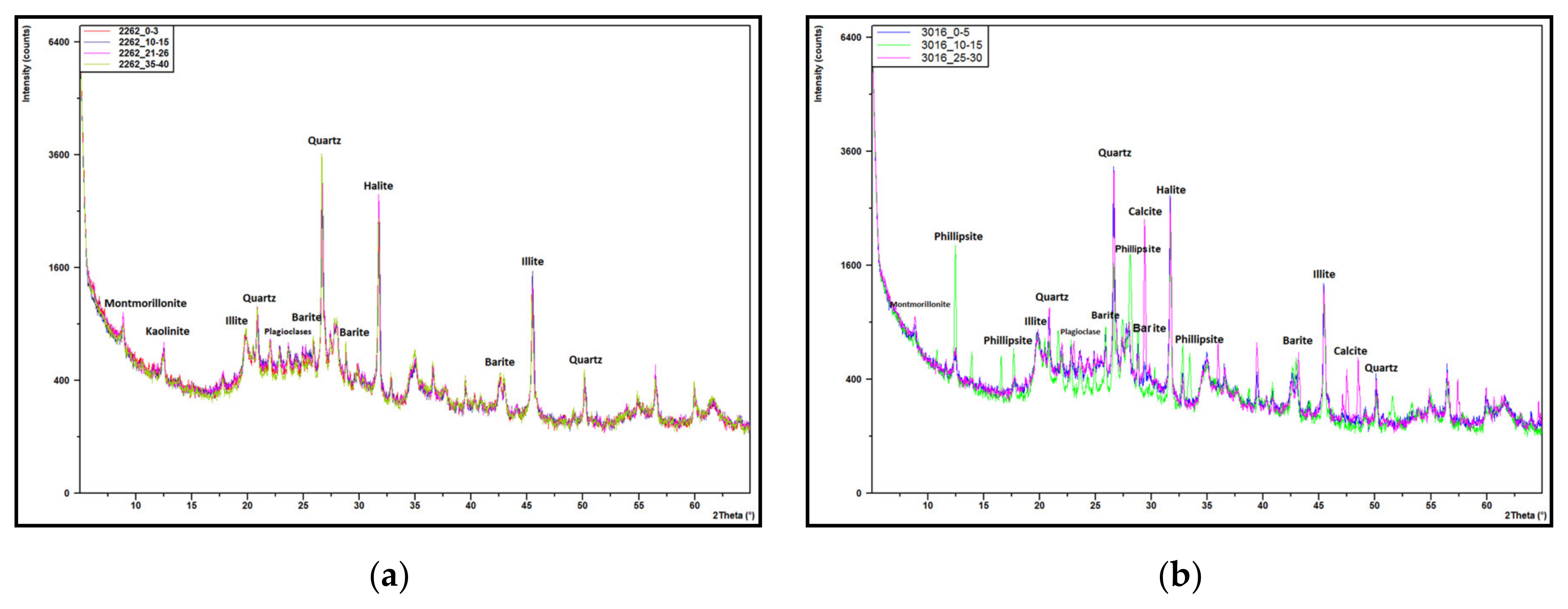
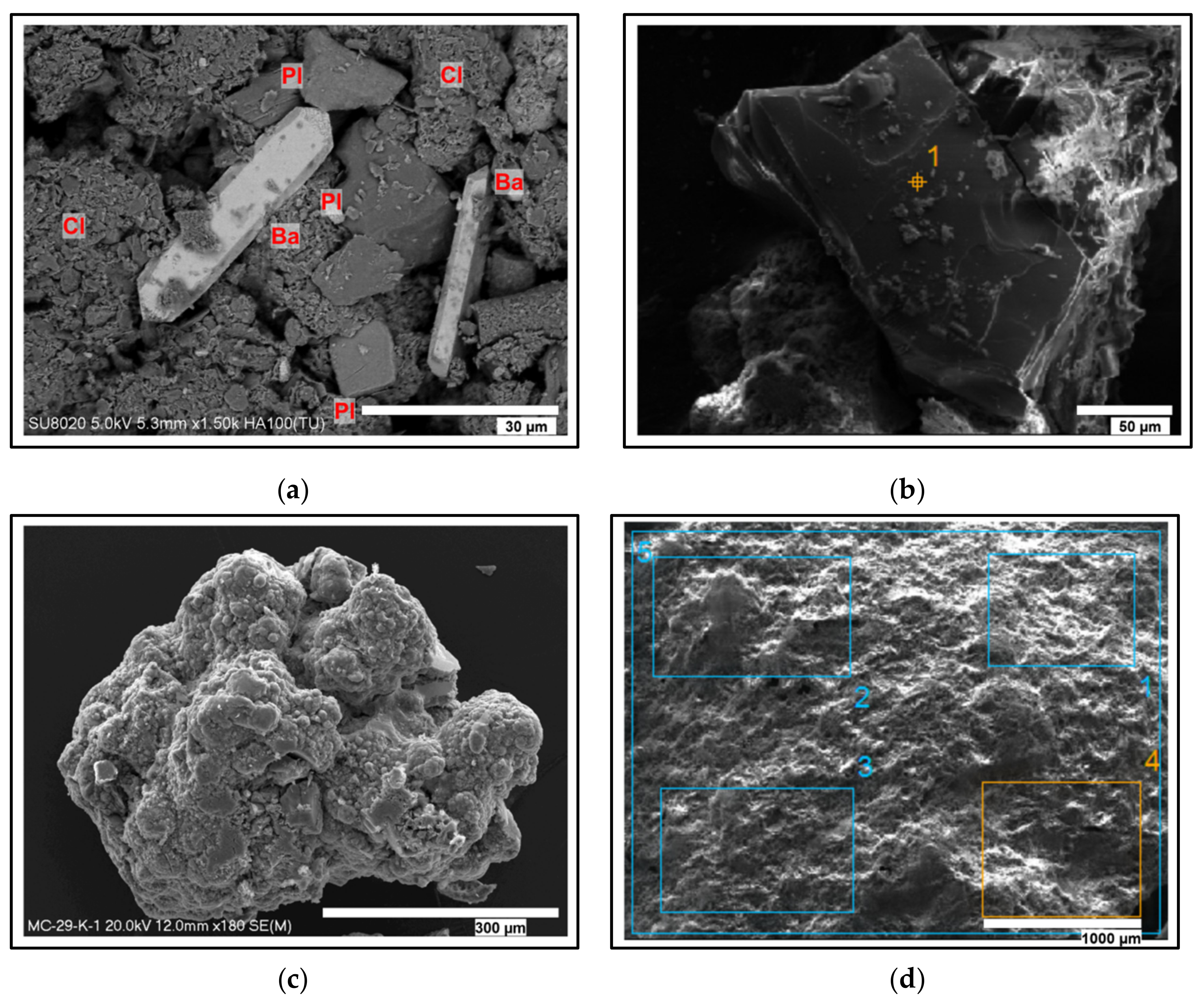
4.2. Bulk XRD Mineralogy
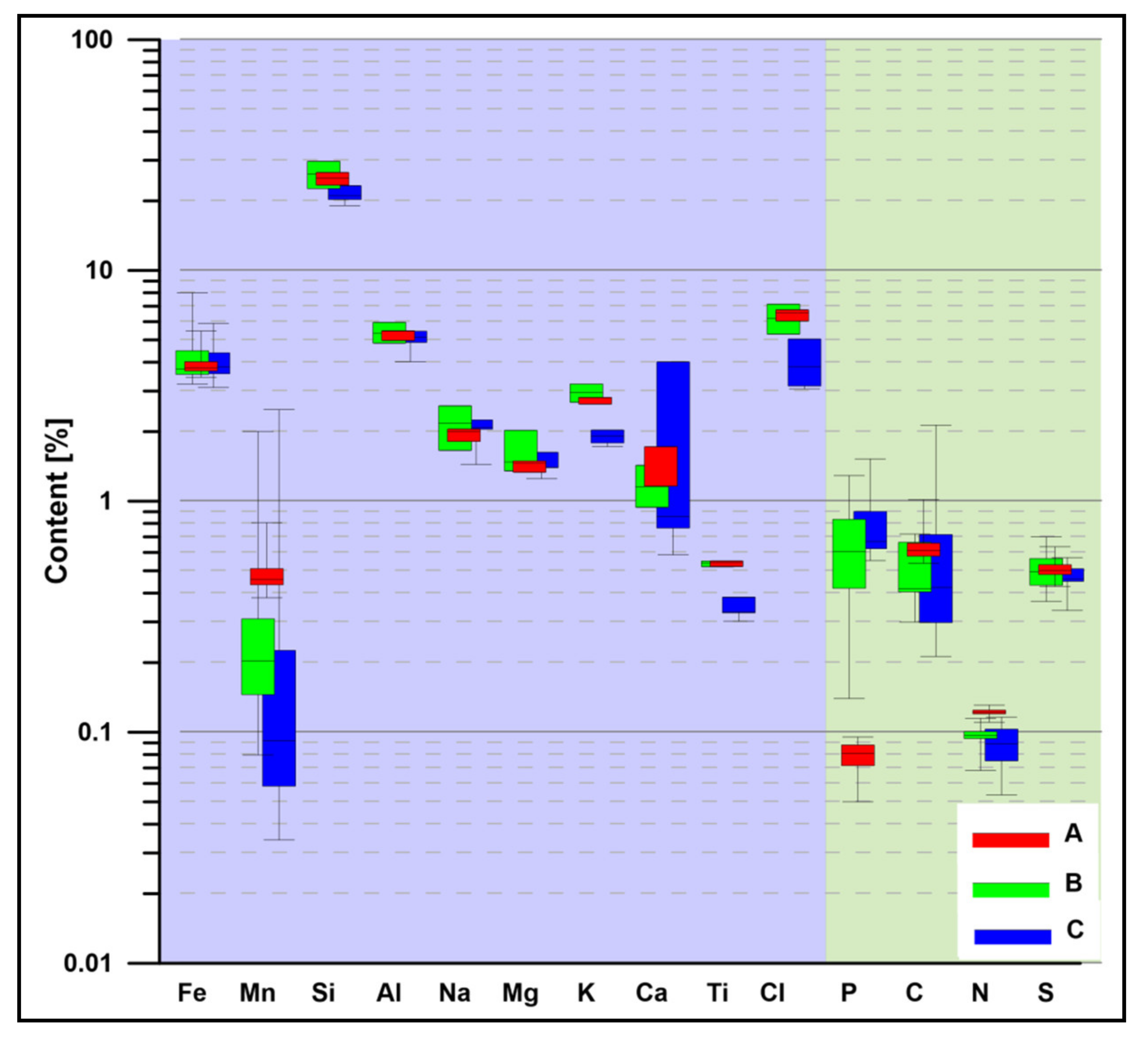
4.3. Geochemistry of Major Components, Trace Metals, and Biogenic Elements
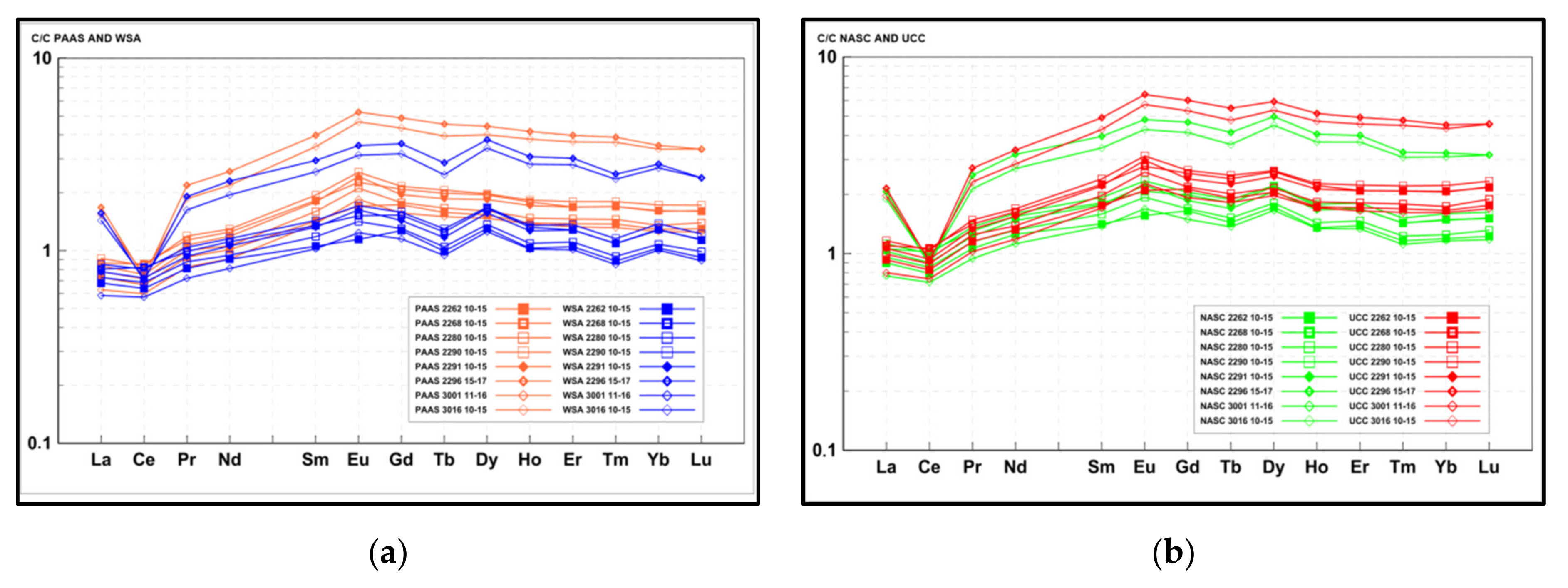
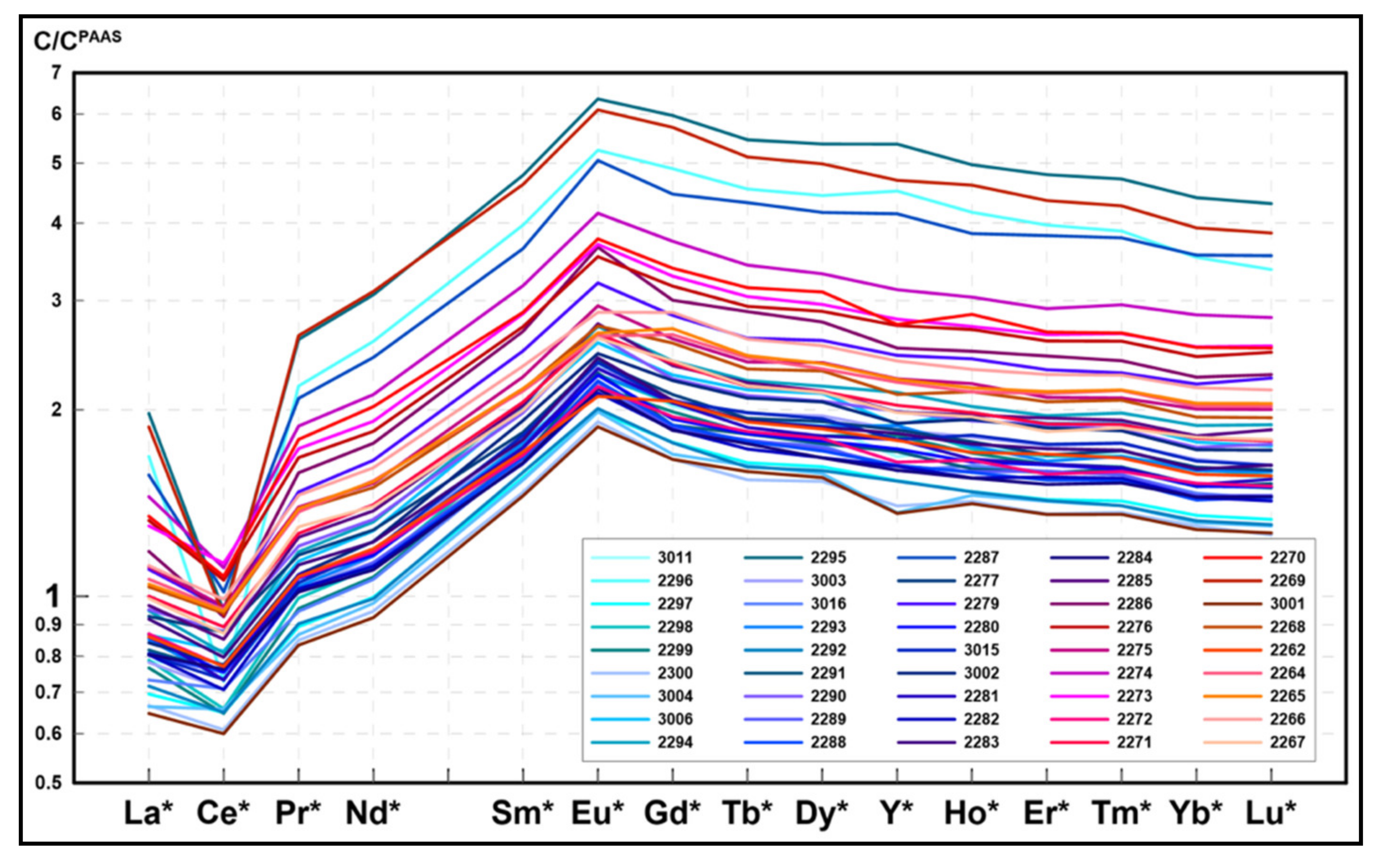
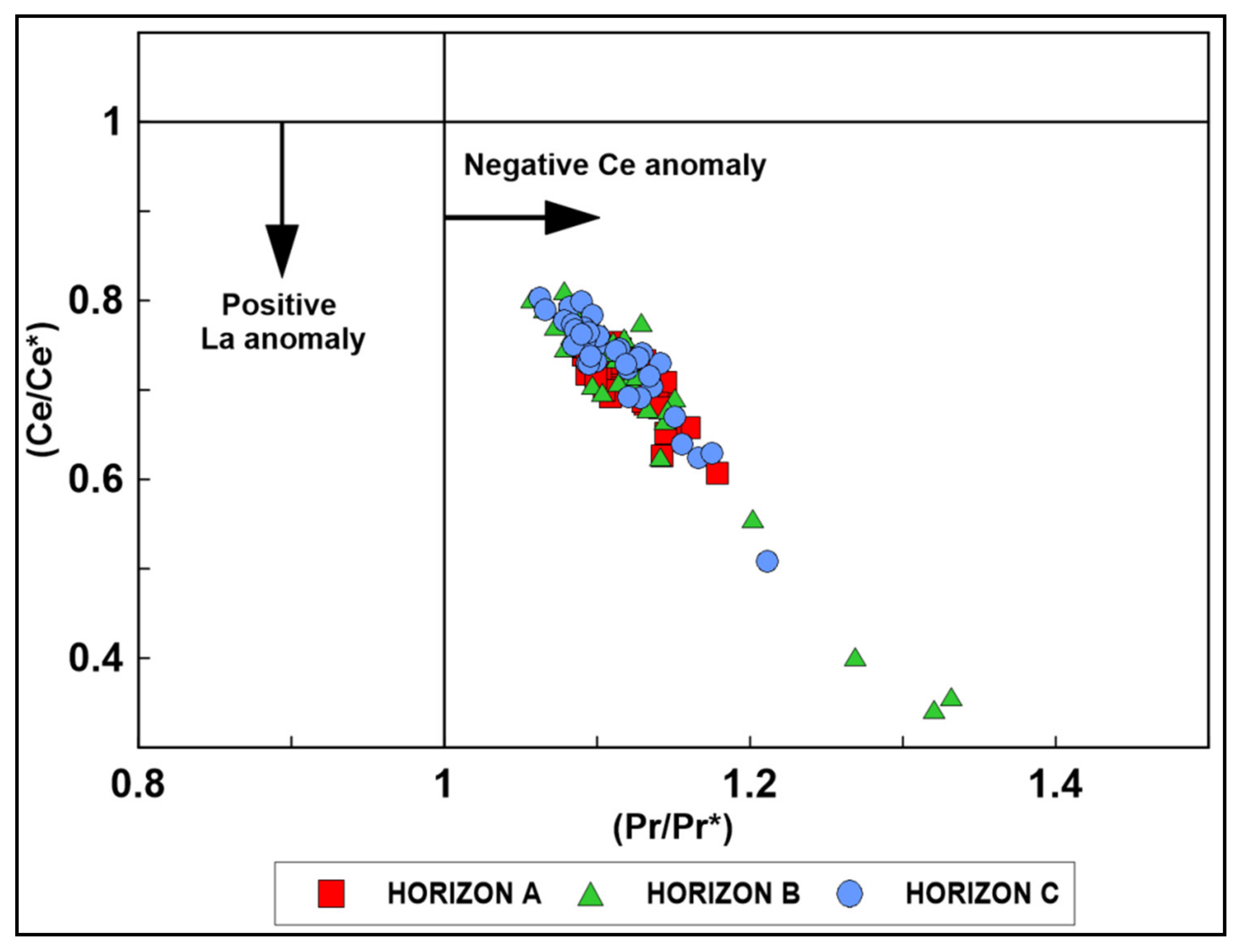
4.4. Rare Earh Elements
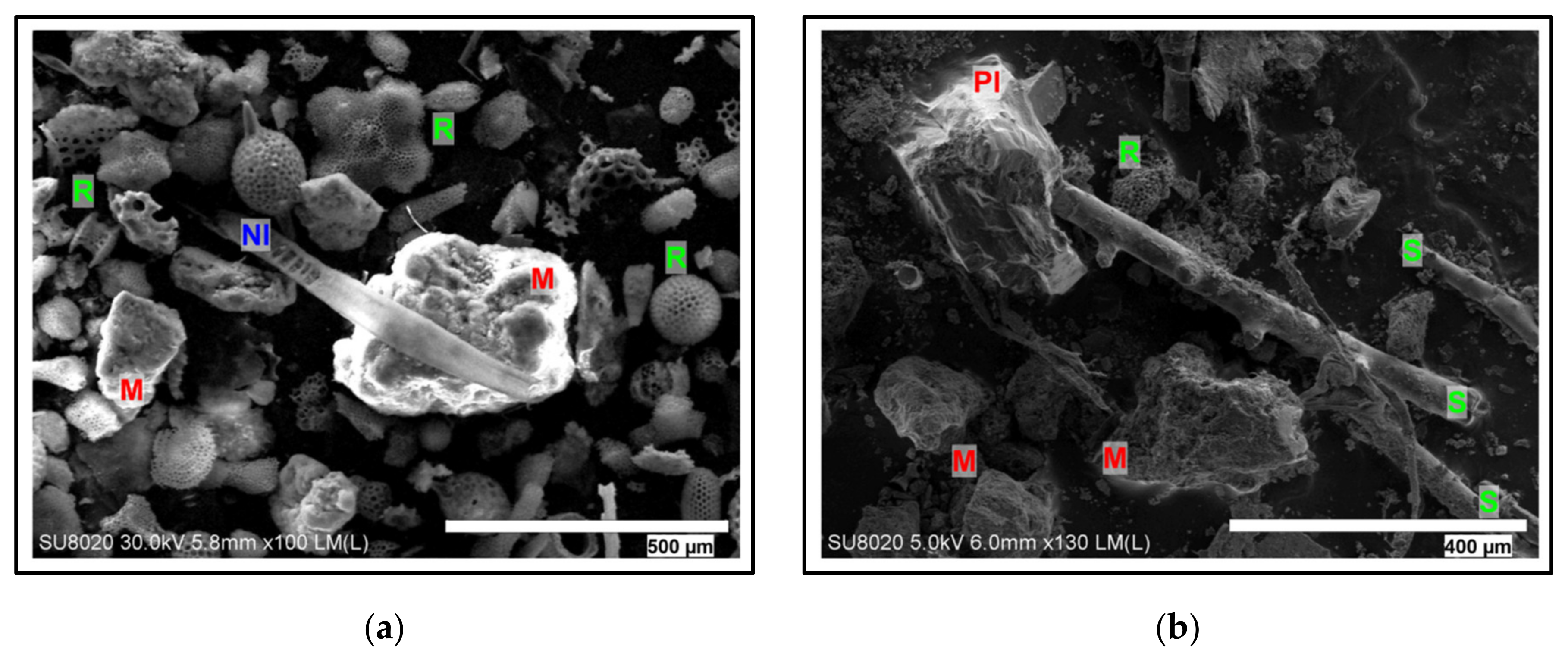
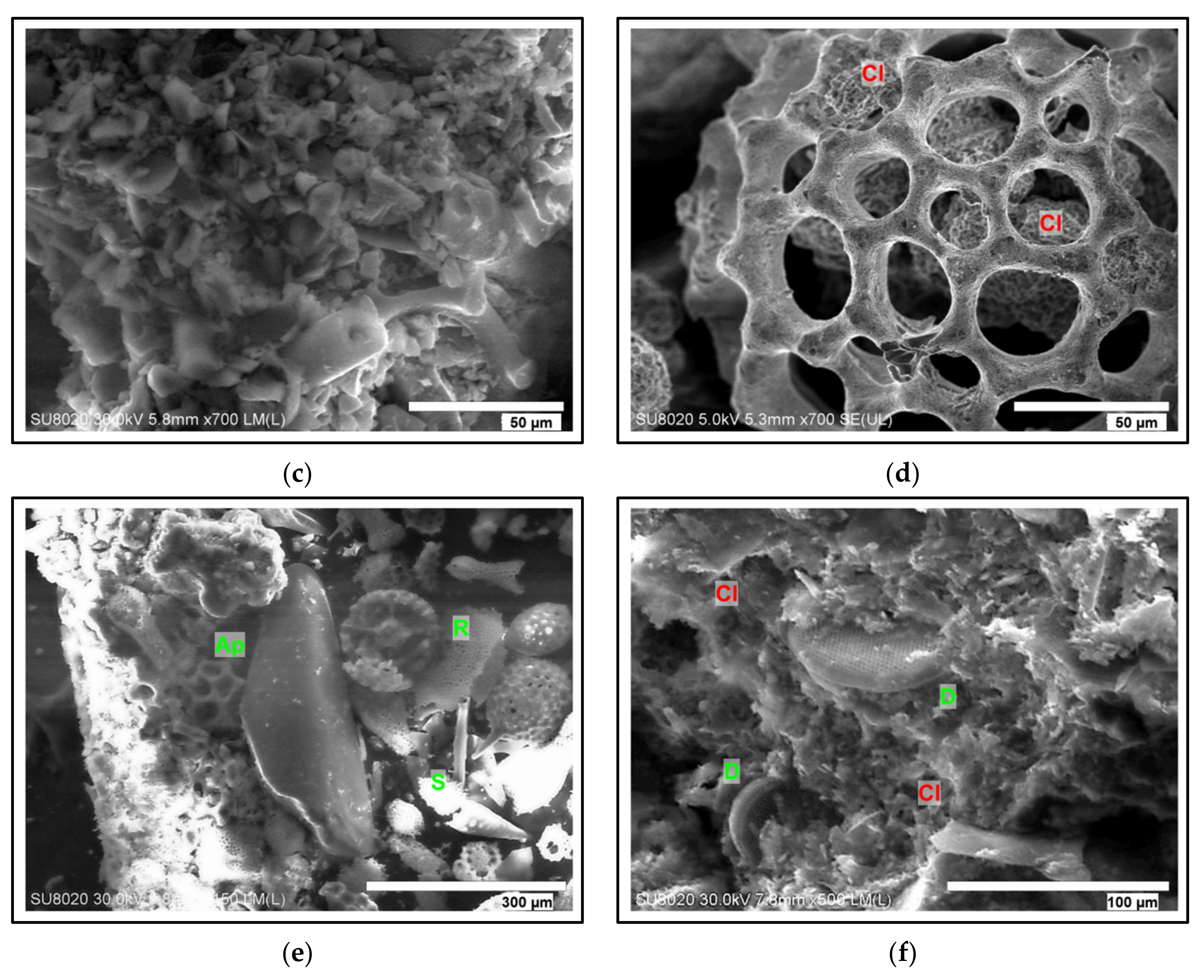
4.5. SEM-EDX
4.6. Correlations
4.6.1. Horizon A
4.6.2. Horizon B
4.6.3. Horizon C
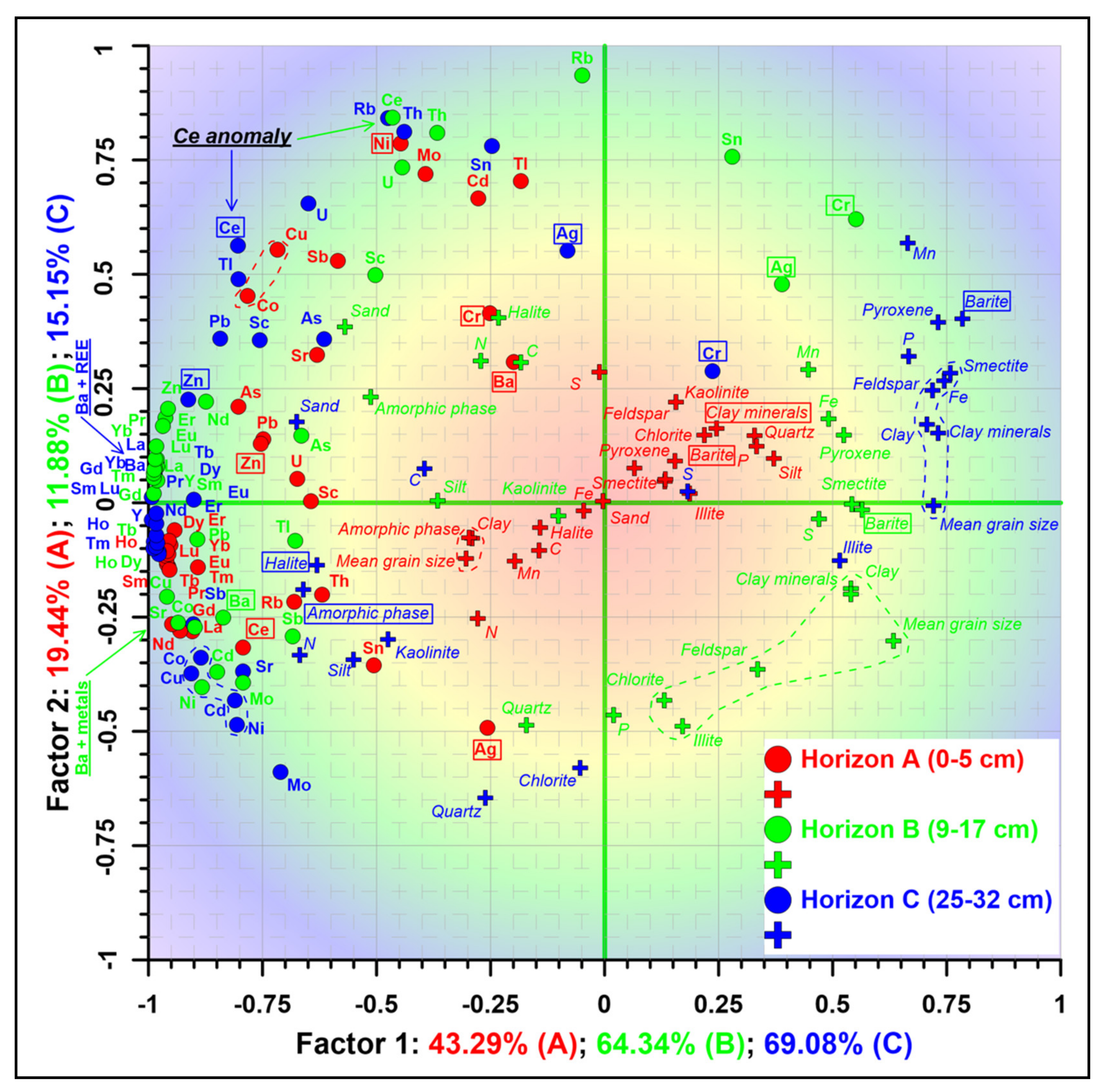
4.7. Principal Components Analysis (PCA)
4.8. Spatial Variability
5. Discussion
- moderate biological productivity of surface waters (higher in the eastern area and lower in the west);
- bathymetry and distinctive linear topography of the seafloor;
- low sedimentation rates;
- depth of CCD ranges from 4000 to 4500 m, which determines CaCO3 contents <10%;
- early diagenesis processes affecting neoformation and transformation of clay minerals;
- tectonic, volcanic and seismic activity [43].
6. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byrne, R.H.; Sholkovitz, E.R. Marine chemistry and geochemistry of the lanthanides. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 497–593. [Google Scholar]
- US Geological Survey Mineral Commodity. Summaries 2014. USGS, US Department of the Interior, Washington, DC. 2014. Available online: https://www.usgs.gov/centers/nmic/mineral-commodity-summaries (accessed on 6 March 2020).
- Deng, Y.; Ren, J.; Guo, Q.; Cao, J.; Wang, H.; Liu, C. Rare earth element geochemistry characteristics of seawater and pore water from deep sea in western Pacific. Sci. Rep. 2017, 7, 16539. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, A.V. Geochemistry of rare earth elements in the ocean. Lithol. Miner. Resour. 2004, 39, 289–307. [Google Scholar] [CrossRef]
- Wilde, P.; Quinby-Hunt, M.S.; Erdtmann, B.D. The whole-rock cerium anomaly: A potential indicator of eustatic sea-level changes in shales of the anoxic facies. Sediment. Geol. 1996, 101, 43–53. [Google Scholar] [CrossRef]
- Piper, D.Z.; Bau, M. Normalized rare earth elements in water, sediments, and wine: Identifying sources and environmental redox conditions. Am. J. Anal. Chem. 2013, 4, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, A.V.; Sval’nov, V.N. Geochemistry of rare earth elements in micro and macronodules from the Pacific bioproductive zone. Lithol. Miner. Resour. 2000, 35, 19. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Shatrov, V.A.; Sirotin, V.I.; Voitsekhovsky, G.V.; Belyavtseva, E.E. Rare earth elements as indicators of the tectonic activity of the basement. Dokl. Earth Sci. 2008, 423, 1467–1468. [Google Scholar] [CrossRef]
- Kato, Y.; Fujinaga, K.; Nakamura, K.; Takaya, Y.; Kitamura, K.; Ohta, J.; Toda, R.; Nakashima, T.; Iwamori, H. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat. Geosci. 2011, 4, 535–539. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Kotliński, R.; Maciąg, Ł.; Zawadzki, D. Potential and Recent Problems of the Possible Polymetallic Sources in the Oceanic Deposits. Geol. Miner. Resour. World Ocean 2015, 40, 65–80. [Google Scholar]
- Zawadzki, D.; Maciąg, Ł.; Kotliński, R.A. Osady eupelagiczne Pacyfiku jako potencjalne źródło pozyskiwania pierwiastków ziem rzadkich. Biul. Państwowego Inst. Geol. 2015, 465, 131–142. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, C.; Shi, X.; Li, H.; Huang, M.; Huang, D. Geochemical characteristics of REY-rich pelagic sediments from the GC02 in central Indian Ocean Basin. J. Rare Earths 2017, 35, 1047–1058. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Guo, Q.; Cao, J.; Wang, H.; Liu, C. Geochemistry characteristics of REY-rich sediment from deep sea in Western Pacific, and their indicative significance. Acta Petrol. Sin. 2018, 34, 733–747. [Google Scholar]
- Pak, S.-J.; Seo, I.; Lee, K.-Y.; Hyeong, K. Rare earth elements and other critical metals in deep seabed mineral deposits: Composition and implications for resource potential. Minerals 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Takaya, Y.; Yasukawa, K.; Kawasaki, T.; Fujinaga, K.; Ohta, J.; Usui, Y.; Nakamura, K.; Kimura, J.-I.; Chang, Q.; Hamada, M.; et al. The tremendous potential of deep-sea mud as a source of rare-earth elements. Sci. Rep. 2018, 8, 5763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obhođaš, J.; Sudac, D.; Meric, I.; Pettersen, H.E.S.; Uroić, M.; Nađ, K.; Valković, V. In-situ measurements of rare earth elements in deep sea sediments using nuclear methods. Sci. Rep. 2019, 8, 4925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasukawa, K.; Ohta, J.; Miyazaki, T.; Vaglarov, B.S.; Chang, Q.; Ueki, K.; Toyama, C.; Kimura, J.-I.; Tanaka, E.; Nakamura, K.; et al. Statistic and isotopic characterization of deep-sea sediments in the western North Pacific Ocean: Implications for genesis of the sediment extremely enriched in rare earth elements. Geochem. Geophys. Geosyst. 2019, 20, 3402–3430. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Ma, W.; Zhang, X.; Ren, J. Geochemical characteristics of sediments in southeast sea area of Minamitorishima Island and their indication of rare earth elements resources. Geol. Sci. Technol. Inf. 2019, 38, 205–214. [Google Scholar] [CrossRef]
- Sa, R.; Sun, X.; He, G.; Xu, L.; Pan, Q.; Liao, J.; Zhu, K.; Deng, X. Enrichment of rare earth elements in siliceous sediments under slow deposition: A case study of the central north Pacific. Ore Geol. Rev. 2018, 94, 12–23. [Google Scholar] [CrossRef]
- Liao, J.; Sun, X.; Wu, Z.; Sa, R.; Guan, Y.; Lu, Y.; Li, D.; Liu, Y.; Deng, Y.; Pan, Y. Fe-Mn (oxyhydr)oxides as an indicator of REY enrichment in the deep-sea sediments from the central North Pacific. Ore Geol. Rev. 2019, 112, 103044. [Google Scholar] [CrossRef]
- Sattarova, V.V.; Aksentov, K.I. Geochemistry of rare-earth elements in the surface bottom sediments of the northwestern Pacific. Russ. Geol. Geophys. 2019, 60, 150–162. [Google Scholar] [CrossRef]
- Sholkovitz, E.R. Rare-earth elements in marine sediments and geochemical standards. Chem. Geol. 1990, 88, 333–347. [Google Scholar] [CrossRef]
- Osborne, A.H.; Hathorne, E.C.; Schijf, J.; Plancherel, Y.; Böoning, P.; Frank, M. The potential of sedimentary foraminiferal rare earth element patterns to trace water masses in the past. Geochem. Geophys. Geosyst. 2017, 18, 1550–1568. [Google Scholar] [CrossRef]
- Li, Y.-H.; Schoonmaker, J.E. Chemical Composition and Mineralogy of Marine Sediments: Sediments, Diagenesis, and Sedimentary Rocks. In Treatise on Geochemistry, 1st ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier Ltd.: Oxford, UK, 2003; Volume 7, pp. 1–35. [Google Scholar]
- Elderfield, H.; Hawkesworth, C.J.; Greaves, M.J.; Calvert, S.E. Rare earth element geochemistry of oceanic ferromanganese nodules and associated sediments. Geochim. Cosmochim. Acta 1981, 45, 513–528. [Google Scholar] [CrossRef]
- Kon, Y.; Hoshino, M.; Sanematsu, K.; Morita, S.; Tsunematsu, M.; Okamoto, N.; Yano, N.; Tanaka, M.; Takagi, T. Geochemical characteristics of apatite in heavy REE rich deep-sea mud from Minami-Torishima area, Southeastern Japan. Resour. Geol. 2014, 64, 47–57. [Google Scholar] [CrossRef]
- Ijiri, A.; Okamura, K.; Ohta, J.; Nishio, Y.; Hamada, Y.; Iijima, K.; Inagaki, F. Uptake of porewater phosphate by REY-rich mud in the western north Pacific Ocean. Geochem. J. 2018, 52, 373–378. [Google Scholar] [CrossRef]
- Abbott, A.N.; Löhr, S.; Trethewy, M. Are clay minerals the primary control on the oceanic rare earth element budget? Front. Mar. Sci. 2019, 6, 504. [Google Scholar] [CrossRef] [Green Version]
- Uramoto, G.; Morono, Y.; Tomioka, N.; Wakaki, S.; Nakada, R.; Wagai, R.; Uesugi, K.; Takeuchi, A.; Hoshino, M.; Suzuki, Y.; et al. Significant contribution of subseafloor microparticles to the global manganese budget. Nat. Commun. 2019, 10, 400. [Google Scholar] [CrossRef]
- Mikhailik, P.E.; Mikhailik, E.V.; Zarubina, N.V.; Blokhin, M.G. Distribution of rare-earth elements and yttrium in hydrothermal sedimentary ferromanganese crusts of the Sea of Japan (from phase analysis results). Russ. Geol. Geophys. 2017, 58, 1530–1542. [Google Scholar] [CrossRef]
- Goldberg, E.D. The Oceans as a Chemical System. In Global Coastal Ocean. Multiscale Interdisciplinary Processes; Hill, M.N., Ed.; Harvard University Press: Cambridge, MA, USA, 2003; Volume 2, pp. 3–25. [Google Scholar] [CrossRef] [Green Version]
- Menendez, A.; James, R.H.; Lichtschlag, A.; Connelly, D.; Peel, K. Controls on the chemical composition of ferromanganese nodules in the Clarion-Clipperton Fracture Zone, eastern equatorial Pacific. Mar. Geol. 2019, 409, 1–14. [Google Scholar] [CrossRef]
- Ren, J.; Yao, H.; Zhu, K.; He, G.; Deng, X.; Wang, H.; Liu, J.; Fu, P.; Yang, S. Enrichment mechanism of rare earth elements and yttrium in deep-sea mud of Clarion-Clipperton Region. Earth Sci. Front. 2015, 22, 200–211. [Google Scholar] [CrossRef]
- Strekopytov, S.; Dubinin, A.V.; Volkov, I.I. General regularities in behavior of rare earth elements in pelagic sediments of the Pacific Ocean. Lithol. Miner. Resour. 1999, 34, 133–145. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1981; pp. 1–780. [Google Scholar]
- Kotliński, R.; Stoyanova, V. Control factors of Polymetallic Nodules Distribution within the Eastern Area of the Clarion-Clipperton Zone (CCZ). In Proceedings of the 6th ISOPE Ocean Mining Symposium, Changsha, China, 9–13 October 2005; Chung, J.S., Liu, S., Eds.; International Society of Offshore and Polar Engineers (ISOPE): Cupertino, CA, USA, 2005; pp. 1–11. [Google Scholar]
- Mewes, K.; Mogollón, J.M.; Picard, A.; Rühlemann, C.; Kuhn, T.; Nöthen, K.; Kasten, S. Impact of depositional and bio-geochemical processes on small scale variations in nodule abundance in the Clarion-Clipperton Fracture Zone. Deep-Sea Res. Part I 2014, 91, 125–141. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Wang, X.; Yin, X.; Zhu, B.; Guo, K.; Huang, X. The Geochemistry and bioturbation of clay sediments associated with amalgamated crusts at the Gagua Ridge. Minerals 2019, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- Volz, J.B.; Liu, B.; Köster, M.; Henkel, S.; Koschinsky, A.; Kasten, S. Post-depositional manganese mobilization during the last glacial period in sediments of the eastern Clarion-Clipperton Zone, Pacific Ocean. Earth Planet. Sci. Lett. 2020, 532, 116012. [Google Scholar] [CrossRef]
- A Geological Model of Polymetallic Nodule Deposits in the Clarion–Clipperton Fracture Zone; International Seabed Authority Technical Study No. 6; International Seabed Authority: Kingston, Jamaica, 2010; pp. 1–211. Available online: https://ran-s3.s3.amazonaws.com/isa.org.jm/s3fs-public/files/documents/tstudy6.pdf (accessed on 12 February 2020).
- Kotliński, R. Pole konkrecjonośne Clarion-Clipperton—Źródło surowców w przyszłości. Górnictwo I Geoinżynieria 2011, 35, 195–214. [Google Scholar]
- International Seabed Authority. Available online: https://www.isa.org.jm (accessed on 2 January 2020).
- Dreisetl, I. Deep Sea Exploration for Metal Reserves—Objectives, Methods and Look Into the Future. In Deep Sea Mining Value Chain: Organization, Technology and Development, 1st ed.; Abramowski, T., Ed.; IOM: Szczecin, Poland, 2016; pp. 105–118. [Google Scholar]
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. In Treatise on Geochemistry, 2nd ed.; Chapter 11; Turekian, K.K., Holland, H.D., Eds.; Elsevier Science: London, UK, 2013; Volume 13, pp. 273–291. [Google Scholar]
- Petersen, S.; Krätschell, A.; Augustin, N.; Jamieson, J.; Hein, J.; Hannington, M.D. News from the seabed—Geological characteristics and resource potential of deep-sea mineral resources. Mar. Policy 2016, 70, 175–187. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Von Stackelberg, U.; Beiersdorf, H. The formation of manganese nodules between the Clarion and Clipperton Fracture zones south east of Hawaii. Mar. Geol. 1991, 98, 411–423. [Google Scholar] [CrossRef]
- Morgan, C.L. Resource Estimates of the Clarion-Clipperton Manganese Nodule Deposits. In Handbook of Marine Mineral Deposits, 1st ed.; Cronan, D.S., Ed.; CRC Press: London, UK, 1999; pp. 145–170. [Google Scholar]
- Szamałek, K. Stan rozpoznania oceanicznych zasobów mineralnych. Przegląd Geol. 2018, 66, 189–194. [Google Scholar]
- Yubko, V.; Kotliński, R. Volcanic, Tectonic and Sedimentary Factors. In Prospectors Guide for the Clarion–Clipperton Zone Polymetallic Nodule Deposits, Development of a Geological Models for the Clarion-Clipperton Zone Polymetallic Nodule Deposit, 1st ed.; Morgan, C., Ed.; ISA: Kingston, Jamaica, 2009; Volume 1, pp. 11–34. [Google Scholar]
- Kotliński, R.; Rühle, E. Geneza i geologia oceanów. In Surowce Mineralne Mórz i Oceanów, 1st ed.; Kotliński, R., Szamałek, K., Eds.; Wydawnictwo Naukowe Scholar: Warszawa, Poland, 1999; pp. 66–101. [Google Scholar]
- Ocean Drilling Program. Leg 199 Preliminary Report. Available online: http://www-odp.tamu.edu/publications/prelim/199_prel/199PREL.PDF (accessed on 12 February 2020).
- Radziejewska, T. Characteristics of the Sub-equatorial North-Eastern Pacific Ocean’s Abyss, with a Particular Reference to the Clarion-Clipperton Fracture Zone. In Meiobenthos in the Sub-Equatorial Pacific Abyss, 1st ed.; Radziejewska, T., Ed.; Springer Briefs in Earth System Sciences: Berlin/Heidelberg, Germany, 2014; pp. 13–28. [Google Scholar] [CrossRef]
- de Baar, H.J.W.; Bruland, K.W.; Schijf, J.; van Heuven, S.M.A.C.; Behrens, M.K. Low cerium among the dissolved rare earth elements in the central North Pacific Ocean. Geochim. Cosmochim. Acta 2018, 236, 5–40. [Google Scholar] [CrossRef]
- Abramowski, T. Activities of the IOM within the scope of geological exploration for polymetallic nodule resources. Oral presentation at the ISA Workshop—Polymetallic Nodules Resource Classification, Goa, India, 3–17 October 2014. Available online: https://ran-s3.s3.amazonaws.com/isa.org.jm/s3fs-public/documents/EN/Workshops/2014a/IOM.pdf (accessed on 12 February 2010).
- Interoceanmetal Joint Organization. Available online: www.interoceanmetal.gov.pl (accessed on 27 December 2019).
- Ryżak, M.; Bartmiński, P.; Bieganowski, A. Metody wyznaczania rozkładu granulometrycznego gleb mineralnych. Acta Agrophys. 2009, 175, 1–84. [Google Scholar]
- Maciąg, Ł; Kotliński, R.; Borówka, R. Zmienność litologiczna osadów ilasto-krzemionkowych z obszaru IOM (Strefa Rozłamowa Clarion—Clipperton, E Pacyfik). Górnictwo I Geoinżynieria 2011, 35, 243–255. [Google Scholar]
- Folk, R.L.; Ward, W.C. Brazos River bar: A study in the significance of grain size parameters. J. Sediment. Petrol. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Eberl, D.D. User’s Guide To Rockjock—A Program For Determining Quantitative Mineralogy From Powder X-Ray Diffraction Data; U.S. Geological Survey, Open-File Report 03-78; U.S. Geological Survey: Boulder, CO, USA, 2003; pp. 1–48. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, S. Wyznaczanie zawartości substancji amorficznej w skałach metodą Rietvelda (XRD). Naft. -Gaz 2014, 10, 700–706. [Google Scholar]
- Brouwer, P. Theory of XRF: Getting Acquainted with the Principles, 3rd ed.; PANalytical: Almela, The Netherlands, 2010; pp. 1–62. [Google Scholar]
- Maciąg, Ł.; Zawadzki, D.; Kotliński, R.A.; Borówka, R.K. Zmienność wybranych metali ciężkich (Cr, Cu, Ni, Pb, Zn) w mułach ilastych krzemionkowych wschodniego Pacyfiku (strefa rozłamowa Clarion-Clipperton, IOM N11). In Współczesne Problemy Badań Geograficznych, 1st ed.; Borówka, R.K., Cedro, A., Kavetskyy, I., Eds.; PPH ZAPOL: Szczecin, Poland, 2013; pp. 93–100. [Google Scholar]
- Trojanowicz, M.; Kołacińska, K. Recent advances in flow injection analysis. Analyst 2016, 141, 2085–2139. [Google Scholar] [CrossRef]
- Sindern, S. Analysis of rare earth elements in rock and mineral samples by ICP-MS and LA-ICP-MS. Phys. Sci. Rev. 2017, 2, 20160066. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution, 1st ed.; Blackwell Scientific Publications: Oxford, UK, 1985; pp. 1–312. [Google Scholar]
- Piper, D.Z. Rare-earth elements in the sedimentary cycle: A summary. Chem. Geol. 1974, 14, 285–304. [Google Scholar] [CrossRef]
- Gromet, L.P.; Haskin, L.A.; Korotev, R.L.; Dymek, R.F. The “North American shale composite”: Its compilation, major and trace element characteristics. Geochim. Cosmochim. Acta 1984, 48, 2469–2482. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2, 1–24. [Google Scholar] [CrossRef]
- Schmidt, R.A.; Smith, R.H.; Lasch, J.E.; Mosen, A.W.; Olehy, D.A.; Vasilevshis, J. Abundances of fourteen rare earth elements, scandium, and yttrium in meteoritic and terrigenous matter. Geochim. Cosmochim. Acta 1963, 27, 577–622. [Google Scholar] [CrossRef]
- Davis, J.C. Statistics and Data Analysis in Geology, 3rd ed.; Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–638. [Google Scholar]
- Mucha, J.; Wasilewska-Błaszczyk, M.; Kotliński, R.; Maciąg, Ł. Variability and Accuracy of Polymetallic Nodules Abundance Estimations in the IOM Area–Statistical And Geostatistical Approach. In Proceedings of the Tenth ISOPE Ocean Mining and Gas Hydrates Symposium, Szczecin, Poland, 22–26 September 2013; pp. 27–31. [Google Scholar]
- Maciąg, Ł; Zawadzki, D. Spatial variability and resources estimation of selected critical metals and rare earth elements in surface sediments from the Clarion-Clipperton Fracture Zone, equatorial Pacific Ocean, Interoceanmetal claim area. In Proceedings of the IAMG 201—20th Annual Conference of the International Association for Mathematical Geosciences, State College, PA, USA, 10–16 August 2019; IAMG: Lawrence, KS, USA; pp. 174–178. [Google Scholar]
- Maciąg, Ł.; Harff, J. Application of multivariate geostatistics for local-scale lithological mapping—case study of pelagic surface sediments from the Clarion–Clipperton Fracture Zone, north–eastern equatorial Pacific (Interoceanmetal claim area). Comput. Geosci. 2020, 104474. [Google Scholar] [CrossRef]
- Dubinin, A.V.; Sval’nov, V.N. Micronodules in the Guatemalan Basin: Geochemistry of Rare Earth Elements. Lithol. Miner. Resour. 1995, 5, 428–436. [Google Scholar]
- Stoch, L. Minerały Ilaste, 1st ed.; Wydawnictwa Geologiczne: Warszawa, Poland, 1974; pp. 1–503. [Google Scholar]
- Meunier, A.; Velde, B. Illite. Origins, Evolution and Metamorphism, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–288. [Google Scholar] [CrossRef]
- Maciąg, Ł. Zmienność Mineralogiczna Osadów Pelagicznych Pacyfiku (Neogen–Czwartorzęd). Master’s Thesis, University of Szczecin, Poland, 21 September 2009. [Google Scholar]
- Burch Fischer, G.; Ryan, P.C. The smectite to disordered kaolinite transition in a tropical soil chronosequence, Pacific coast, Costa Rica. Clays Clay Miner. 2006, 54, 571–586. [Google Scholar] [CrossRef]
- Hillier, S. Chlorite in sediments. In Encyclopedia of Sediments and Sedimentary Rocks. Encyclopedia of Earth Sciences Series, 1st ed.; Middleton, G.V., Church, M.J., Coniglio, M., Hardie, L.A., Longstaffe, F.J., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Dubinin, A.V.; Uspenskaya, T.Y.; Rimskaya-Korsakova, M.N.; Demidova, T.P. Rare elements and Nd and Sr isotopic composition in micronodules from the Brazil Basin, Atlantic Ocean. Lithol. Miner. Resour. 2017, 52, 81–101. [Google Scholar] [CrossRef]
- Liao, S.; Tao, C.; Dias, A.A.; Su, X.; Yang, Z.; Ni, J.; Liang, J.; Yang, W.; Liu, J.; Li, W.; et al. Surface sediment composition and distribution of hydrothermal derived elements at the Duanqiao-1 hydrothermal field, Southwest Indian Ridge. Mar. Geol. 2019, 416, 1–11. [Google Scholar] [CrossRef]
- Bonatti, E.; Kraemer, T.; Rydell, H. Classification and genesis of submarine iron-manganese deposits. In Ferromanganese Deposits on the Ocean Floor, 1st ed.; Horn, D.R., Ed.; National Science Foundation: Washington, DC, USA, 1972; pp. 149–161. [Google Scholar]
- Volz, J.B.; Mogollón, J.M.; Geibert, W.; Arbizu, P.M.; Koschinsky, A.; Kasten, S. Natural spatial variability of depositional conditions, biogeochemical processes and element fluxes in sediments of the eastern Clarion-Clipperton Zone, Pacific Ocean. Deep Sea Res. Part I 2018, 140, 159–172. [Google Scholar] [CrossRef]
- Yuan, G.; Cao, Y.; Schulz, H.-S.; Hao, F.; Gluyas, J.; Liu, K.; Yang, T.; Wang, Y.; Xi, K.; Li, F. A review of feldspar alteration and its geological significance in sedimentary basins: From shallow aquifers to deep hydrocarbon reservoirs. Earth Sci. Rev. 2019, 191, 114–140. [Google Scholar] [CrossRef]
- Olivier, N.; Boyet, M. Rare earth and trace elements of microbialites in Upper Jurassic coral- and sponge microbialite reefs. Chem. Geol. 2006, 230, 105–123. [Google Scholar] [CrossRef]
- Matyszkiewicz, J.; Kochan, A.; Duś, A. Influence of local sedimentary conditions on development of microbialites in the Oxfordian carbonate buildups from the southern part of the Kraków–Częstochowa Upland (South Poland). Sediment. Geol. 2012, 263–264, 109–132. [Google Scholar] [CrossRef]
- Subha Anand, S.; Rahaman, W.; Lathika, N.; Thamban, M.; Patil, S.; Mohan, R. Trace elements and Sr, Nd isotope compositions of surface sediments in the Indian Ocean: An evaluation of sources and processes for sediment transport and dispersal. Geochem. Geophys. Geosyst. 2019, 20, 1–23. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Balashov, Y.A.; Sharkov, I.V.; Sherstennikov, O.G.; Ronov, A.B. Rare Earth Elements in the Major Lithologic Rock Types in the Sedimentary Cover of the Russian Platform. Geokhimiya 1994, 32, 789–803. [Google Scholar]
- Pattan, J.N.; Pearce, N.J.G.; Mislankar, P.G. Constraints in using Cerium-anomaly of bulk sediments as an indicator of paleo bottom water redox environment: A case study from the Central Indian Ocean Basin. Chem. Geol. 2005, 221, 260–278. [Google Scholar] [CrossRef]
- Duliu, O.G.; Alexe, V.; Moutte, J.; Szobotca, S.A. Major and trace element distributions in manganese nodules and micronodules as well as abyssal clay from the Clarion-Clipperton abyssal plain, Northeast Pacific. Geo-Mar. Lett. 2009, 29, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Widmann, P.; Kuhn, T.; Schultz, H. Enrichment of mobilizable manganese in deep sea sediments in relation to Mn nodules abundance. In Proceedings of the EGU General Assembly, Vienna, Austria, 27 April–2 May 2014. [Google Scholar]
- Paul, S.A.L.; Haeckel, M.; Bau, M.; Bajracharya, R.; Koschinsky, A. Small-scale heterogeneity of trace metals including rare earth elements and yttrium in deep-sea sediments and pore waters of the Peru Basin, southeastern equatorial Pacific. Biogeosciences 2019, 16, 4829–4849. [Google Scholar] [CrossRef] [Green Version]
- Usui, A.; Bau, M.; Toshitsugu, Y. Manganese microchimneys buried in the Central Pacific pelagic sediments: Evidence of intraplate water circulation? Mar. Geol. 2017, 141, 269–285. [Google Scholar] [CrossRef]
- Molina-Kescher, M.; Hathorne, E.C.; Osborne, A.H.; Behrens, M.K.; Kölling, M.; Pahnke, K.; Frank, M. The influence of basaltic islands on the oceanic REE distribution: A case study from the tropical South Pacific. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Crocket, K.C.; Hill, E.; Abell, R.E.; Johnson, C.; Gary, S.F.; Brand, T.; Hathorne, E.C. Rare earth element distribution in the NE Atlantic: Evidence for benthic sources, longevity of the seawater signal, and biogeochemical cycling. Front. Mar. Sci. 2018, 5, 147. [Google Scholar] [CrossRef] [Green Version]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The last glacial maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, D.Z. Rare earth elements in ferromanganese nodules and other marine phases. Geochim. Cosmochim. Acta 1974, 38, 1007–1022. [Google Scholar] [CrossRef]
- Fadel, A.; Bloma, H.; Pérez-Huertac, A.; Jeffries, T.; Märsse, T.; Ahlberga, P.E. Rare earth elements (REEs) in vertebrate microremains from the Upper Pridoli Ohesaare beds of Saaremaa Island, Estonia: Geochemical clues to palaeoenvironment. Est. J. Earth Sci. 2015, 64, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Nakamura, K.; Fujinaga, K.; Iwamori, H.; Kato, Y. Tracking the spatiotemporal variations of statistically independent components involving enrichment of rare-earth elements in deep-sea sediments. Sci. Rep. 2016, 6, 29603. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A. Barite in the ocean—occurrence, geochemistry and paleoceanographic applications. Sedimentology 2012, 59, 1817–1835. [Google Scholar] [CrossRef]
- Maciąg, Ł; Zawadzki, D.; Kotliński, R.A.; Borówka, R.K.; Wróbel, R. Authigenic barite crystals from the eupelagic sediments of CCFZ (IOM H22 area, Clarion-Clipperton Fracture Zone, Pacific). In Proceedings of the Joint 5th Central-European Mineralogical Conference and 7th Mineral Sciences in the Carpathians Conference. Book of Contributions and Abstracts, Comenius University, Bratislava, Slovakia, 26–30 June 2018; pp. 122–124. [Google Scholar]
- Halbach, P.; Scherhag, C.; Hebisch, U.; Marchig, V. Geochemical and mineralogical control of genetic types of deep-sea nodules from the Pacific Ocean. Miner. Depos. 1981, 16, 59–84. [Google Scholar] [CrossRef]
- Paytan, A.; Mearon, S.; Cobb, K.; Kastner, M. Origin of marine barite deposits: Sr and S isotope characterization. Geology 2002, 30, 747–750. [Google Scholar] [CrossRef]
- Liguori, B.T.P.; de Almeida, M.G.; de Rezende, C.E. Barium and its importance as an indicator of (Paleo)productivity. An. Da Acad. Bras. De Ciências 2016, 88, 2093–2103. [Google Scholar] [CrossRef] [Green Version]
- Guichard, F.; Church, T.M.; Treuil, M.; Jaffrezic, H. Rare earths in barites: Distribution and effects on aqueous partitioning. Geochim. Cosmochim. Acta 1979, 43, 983–997. [Google Scholar] [CrossRef]
- Koski, R.A.; Hein, J.R. Stratiform Barite Deposits in the Roberts Mountains Allochthon, Nevada: A Review of Potential Analogs in Modern Sea-Floor Environments. Chapter H of contributions to Industrial-Minerals Research. Bulletin 2209-H, USGS. 2003. Available online: https://pubs.usgs.gov/bul/b2209-h (accessed on 30 March 2020).
- Popoola, S.O.; Han, X.; Wang, Y.; Qiu, Z.; Ye, Y.; Cai, Y. Mineralogical and geochemical signatures of metalliferous sediments in wocan-1 and wocan-2 hydrothermal sites on the Carlsberg Ridge, Indian Ocean. Minerals 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzki, D.; Maciąg, Ł.; Abramowski, T.; McCartney, K. Fractionation Trends and Variability of Rare Earth Elements and Selected Critical Metals in Pelagic Sediment from Abyssal Basin of NE Pacific (Clarion-Clipperton Fracture Zone). Minerals 2020, 10, 320. https://doi.org/10.3390/min10040320
Zawadzki D, Maciąg Ł, Abramowski T, McCartney K. Fractionation Trends and Variability of Rare Earth Elements and Selected Critical Metals in Pelagic Sediment from Abyssal Basin of NE Pacific (Clarion-Clipperton Fracture Zone). Minerals. 2020; 10(4):320. https://doi.org/10.3390/min10040320
Chicago/Turabian StyleZawadzki, Dominik, Łukasz Maciąg, Tomasz Abramowski, and Kevin McCartney. 2020. "Fractionation Trends and Variability of Rare Earth Elements and Selected Critical Metals in Pelagic Sediment from Abyssal Basin of NE Pacific (Clarion-Clipperton Fracture Zone)" Minerals 10, no. 4: 320. https://doi.org/10.3390/min10040320