Mineralogical Characterization of Slags from the Oiola Site (Biscay, Spain) to Assess the Development in Bloomery Iron Smelting Technology from the Roman Period to the Middle Ages
Abstract
1. Introduction
2. Oiola Site
3. Materials and Methods
3.1. Sampling
3.2. Optical Microscopy
3.3. X-ray Powder Diffraction
3.4. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy
3.5. Raman Microspectroscopy
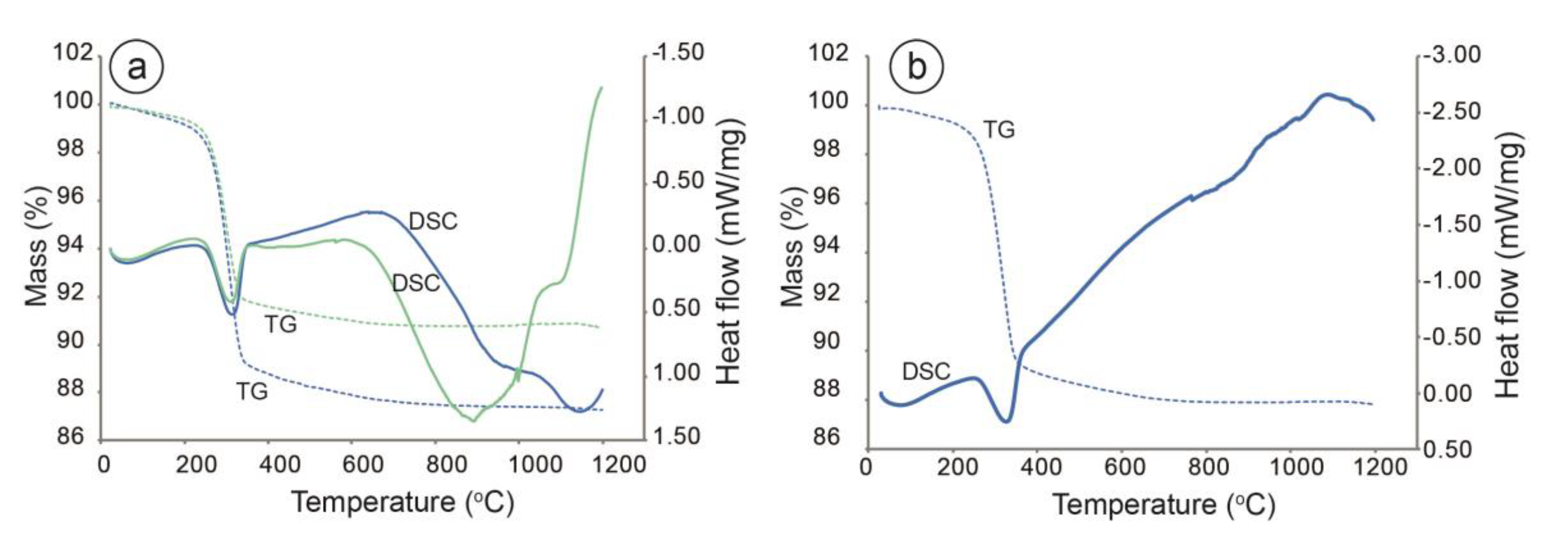
3.6. Thermo-Gravimetric Analysis (TGA)
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pleiner, R. Iron in Archaeology: The European Bloomery Smelters; Archeologický Ústav Avčr: Prague, Czech Republic, 2000. [Google Scholar]
- Serneels, V.; Perret, S. Quantification of smithing activities based on the investigation of slag and other material remains. Archaeometall. Eur. 2003, 1, 469–478. [Google Scholar]
- McDonnell, J.G. The Classification of Early Ironworking Slags. Ph.D. Thesis, University of Aston, Birmingham, UK, 1986. [Google Scholar]
- Selskienė, A. Examination of smelting and smithing slags formed in bloomery iron-making process. Chemija 2007, 18, 22–28. [Google Scholar]
- Senn, M.; Gfeller, U.; Guénette-Beck, B.; Lienemann, P.; Ulrich, A. Tools to qualify experiments with bloomery furnaces. Archaeometry 2010, 52, 131–145. [Google Scholar] [CrossRef]
- Charlton, M.F.; Blakelock, E.; Martinón-Torres, M.; Young, T. Investigating the production provenance of iron artifacts with multivariate methods. J. Archaeol. Sci. 2012, 39, 2280–2293. [Google Scholar] [CrossRef]
- Charlton, M.F.; Crew, P.; Rehren, T.; Shennan, S.J. Explaining the evolution of ironmaking recipes–An example from northwest Wales. J. Anthropol. Archaeol. 2010, 29, 352–367. [Google Scholar] [CrossRef]
- Parreño, C.M.; Martín, A.M.; Ferrer Eres, M.Á. Iron, Fuel and Slags: Reconstructing the Ironworking Process in Iberian Iron Age (Valencian Region). Pyrenae 2009, 40, 105–127. [Google Scholar]
- Tylecote, R.F. Furnaces, Crucibles and Slags, in the Coming of the Age of Iron; Wertime, T.A., Muhly, J.D., Eds.; Yale University Press: New Haven, CT, USA, 1980; pp. 183–226. [Google Scholar]
- Buchwald, V.F.; Wivel, H. Slag analysis as a method for the characterization and provenancing of ancient iron objects. Mater. Charact. 1998, 40, 73–96. [Google Scholar] [CrossRef]
- Bayley, J.; Dughworth, D.; Paynter, S. Centre for Archaeology Guidelines: Archaeometallurgy; English Heritage: London, UK, 2001. [Google Scholar]
- Ros-Latienda, L.; Fernández Carrasquilla, J. Caracterización de escorias metalúrgicas procedentes de yacimientos arqueológicos de Navarra (siglos II a.C.–IV d.C.). Rev. Metal. 2013, 49. [Google Scholar] [CrossRef]
- Eekelers, K.; Degryse, P.; Muchez, P. Petrographic investigation of smithing slag of the Hellenistic to Byzantine city of Sagalassos (SW-Turkey). Am. Mineral. 2016, 101, 1072–1083. [Google Scholar] [CrossRef][Green Version]
- Blakelock, E.; Martinon-Torres, M.; Veldhuijzen, H.A.; Young, T. Slag inclusions in iron objects and the quest for provenance: An experiment and a case study. J. Archaeol. Sci. 2009, 36, 1745–1757. [Google Scholar] [CrossRef]
- Cleere, H. Some operating parameters for Roman ironworks. Bull. Inst. Archaeol. 1976, 13, 233–246. [Google Scholar]
- Crew, P. The influence of clay and charcoal ash on bloomery slags. In Il Ferro nelle Alpi, Giacimenti Miniere e Metallurgia dall’Antichità al XVI Secolo, Atti del Convegno, Proceedings of The Conference, Bienno, Italy, 2–4 Ottobre 1998; Comune di Bienno: Bienno, Italy, 2000; pp. 38–48. [Google Scholar]
- Paynter, S. Regional variations in bloomery smelting slag of the Iron Age and Romano-British periods. Archaeometry 2006, 48, 271–292. [Google Scholar] [CrossRef]
- Gordon, R.B. Process deduced from ironmaking wastes and artefacts. J. Archaeol. Sci. 1997, 24, 9–18. [Google Scholar] [CrossRef]
- Benvenuti, M.; Mascaro, I.; Costagliola, P.; Tanelli, G.; Romualdi, A. Iron, copper and tin at Baratti, Populonia: Smelting processes and metal provenances. His. Metal. 2000, 34, 67–76. [Google Scholar]
- Török, B.; Gallina, Z.; Kovacs, A.; Kristaly, F. Early medieval iron bloomery centre at Zamárdi (Hungary) Complex archaeometrical examinations of the slags. Archeologické Rozhledy 2018, 70, 404–420. [Google Scholar]
- Tylecote, R.F.; Austin, J.N.; Wraith, A.B. The mechanism of the bloomery process in shaft furnaces. J. Iron Steel Inst. 1971, 209, 342–363. [Google Scholar]
- Pereda García, I. La metalurgia prehidráulica del hierro en Bizkaia: El caso de los alrededores del pantano de Oiola (Trapagarán, Bizkaia). Kobie 1992, 20, 109–122. [Google Scholar]
- Gil-Crespo, P.P. Introducción a la geología y mineralogía de los yacimientos de hierro de Bilbao. In Historia del Hierro en Bizkaia y su Entorno; Orue-Etxebarria, X., Apellaniz, E., Gil-Crespo, P.P., Eds.; Real Sociendad Bascongada de los Amigos del País: Bilbao, Spain, 2015; pp. 19–52. [Google Scholar]
- Kaczorek, D.; Sommer, M. Micromorphology, chemistry, and mineralogy of bog iron ores from Poland. Catena 2003, 54, 393–402. [Google Scholar] [CrossRef]
- Kaczorek, D.; Sommer, M.; Andruschkewitsch, I.; Oktaba, L.; Czerwinski, Z.; Stahr, K. A comparative micromorphological and chemical study of “Raseneisenstein” (bog iron ore) and “Ortstein”. Geoderma 2004, 121, 83–94. [Google Scholar] [CrossRef]
- Banning, A. Bog iron ores and their potential role in arsenic dynamics: An overview and a “Paleo Example”. Eng. Life Sci. 2008, 8, 641–649. [Google Scholar] [CrossRef]
- Thelemann, H.; Bebermeier, W.; Hoelzmann, P.; Lehnhardt, E. Bog iron ore as a resource for prehistoric iron production in Central Europe—A case study of the Widawa catchment area in eastern Silesia, Poland. Catena 2017, 149, 474–490. [Google Scholar] [CrossRef]
- Kaczorek, D.; Brümmer, G.; Sommer, M. Content and binding forms of heavy metals. Aluminium and phosphorus in bog iron ores from Poland. J. Environ. Qual. 2005, 38, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- García-Mondéjar, J.; Fernández-Mendiola, P.A.; Agirrezabala, L.M.; Aranburu, A.; López-Horgue, M.A.; Iriarte, E.; Martínez de Rituerto, S. El Aptiense-Albiense de la Cuenca Vasco-Cantábrica. In Geología de España; Vera, J.A., Ed.; SGE-IGME: Madrid, Spain, 2004; pp. 291–296. [Google Scholar]
- Gil Crespo, P.P. Las Mineralizaciones de Hierro en el Anticlinal de Bilbao: Mineralogía, Geoquímica y Metalogenia. Ph.D. Thesis, University of the Basque Country, Leioa, Spain, 1991. [Google Scholar]
- Portillo, H.; Zuluaga, M.C.; Ortega, L.A.; Alonso-Olazabal, A.; Murelaga, X.; Martinez-Salcedo, A. XRD, SEM/EDX and micro-Raman spectroscopy for mineralogical and chemical characterization of iron slags from the Roman archaeological site of Forua (Biscay, North Spain). Microchem. J. 2018, 138, 246–254. [Google Scholar] [CrossRef]
- Beukes, N.J.; Gutzmer, J.; Mukhopadhyay, J. The geology and genesis of high-grade hematite iron ore deposits. Appl. Earth Sci. 2003, 112, 18–25. [Google Scholar] [CrossRef]
- Ixer, R.A. Atlas of Opaque and Ore Minerals in Their Associations; Osprey Books: Oxford, UK, 1990. [Google Scholar]
- Ramdohr, P. The Ore Minerals and Their Intergrowths; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Park, J.S.; Rehren, T. Large-scale 2nd to 3rd century AD bloomery iron smelting in Korea. J. Archaeol. Sci. 2011, 38, 1180–1190. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Vernon, R.H. A Practical Guide to Rack Microstructure; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Kolesov, B.A.; Geiger, C.A. A Raman spectroscopic study of Fe–Mg olivines. Phys. Chem. Miner. 2004, 31, 142–154. [Google Scholar] [CrossRef]
- Mouri, T.; Enami, M. Raman spectroscopic study of olivine-group minerals. J. Miner. Petrol. Sci. 2008, 103, 100–104. [Google Scholar] [CrossRef]
- Ackerman, K.J.; Killick, D.J.; Herbert, E.W.; Kriger, C. A study of iron smelting at Lopanzo, Equateur Province, Zaıre. J. Archaeol. Sci. 1999, 26, 1135–1143. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Shebanova, O.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Strezov, V.; Ziolkowski, A.; Evans, T.; Nelson, P. Assessment of evolution of loss on ignition matter during heating of iron ores. J. Therm. Anal. Calorim. 2009, 100, 901–907. [Google Scholar] [CrossRef]
- Kawigraha, A.; Soedarsono, J.W.; Harjanto, S. Thermogravimetric Analysis of the Reduction of Iron Ore with Hydroxyl Content. J. Adv. Mater. Res. 2013, 774, 682–686. [Google Scholar] [CrossRef]
- Romero Gómez, P.; González, J.C.; Bustamante, A.; Ruiz Conde, A.; Sánchez Soto, P.J. Estudio in-situ de la transformación térmica de limonita utilizada como pigmento procedente de Perú. Bol. Soc. Esp. Cerám. Vidr. 2013, 52, 127–131. [Google Scholar] [CrossRef]
- Castro-Dorado, A. Petrografía de Rocas Ígneas y Metamórfica; Paraninfo: Madrid, Spain, 2015. [Google Scholar]
- Donaldson, C.H. An experimental investigation of olivine morphology. Contrib. Miner. Petr. 1976, 57, 187–213. [Google Scholar] [CrossRef]
- Manasse, A.; Mellini, M. Chemical and textural characterisation of medieval slags from the Massa Marittima smelting sites (Tuscany, Italy). J. Cult. Herit. 2002, 3, 187–198. [Google Scholar] [CrossRef]
- Gómez Ramos, P. Análisis de escorias férreas: Nuevas aportaciones al conocimiento de la siderurgia prerromana en España. Trab. Prehist. 1996, 53, 145–155. [Google Scholar] [CrossRef][Green Version]


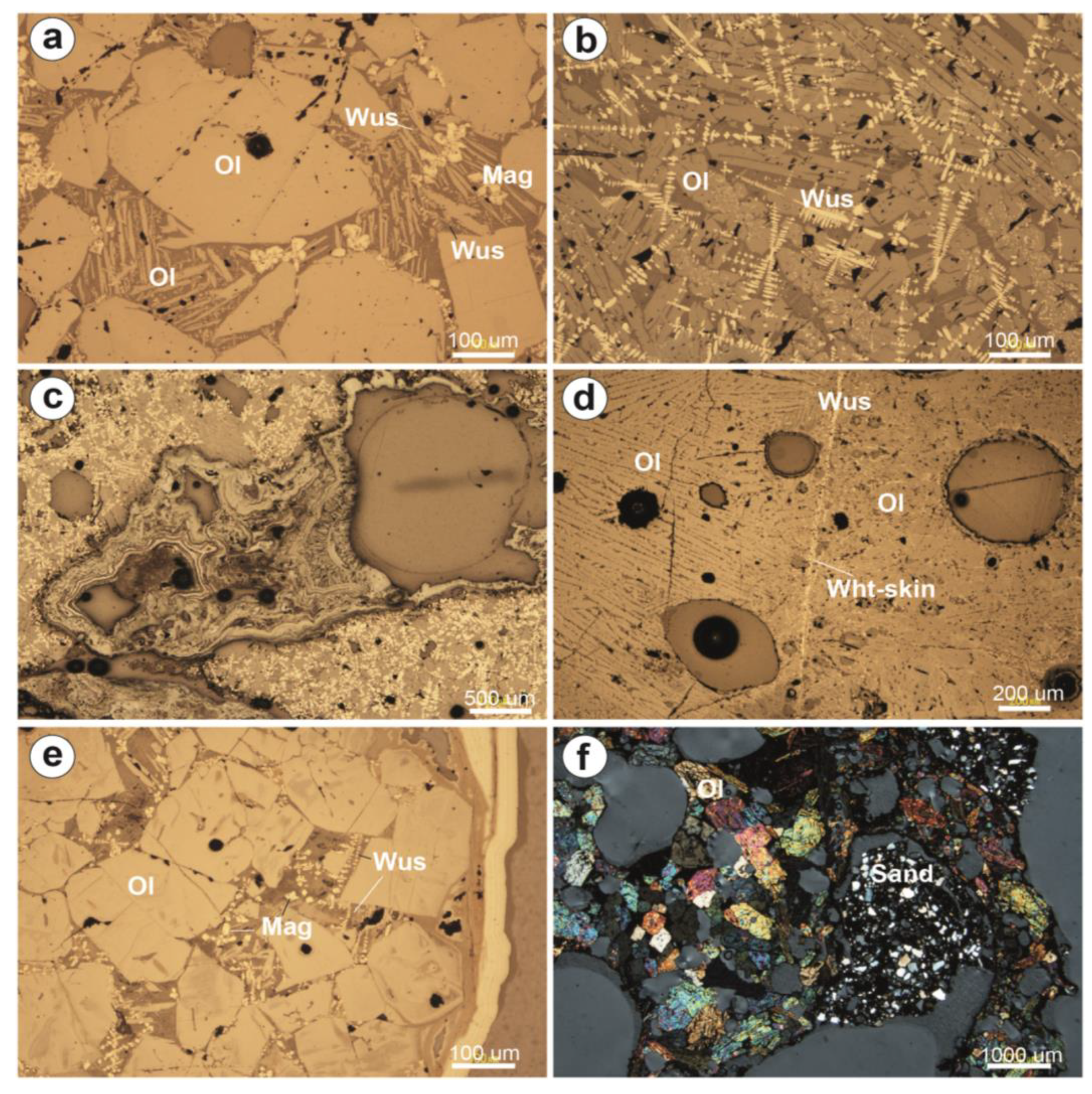
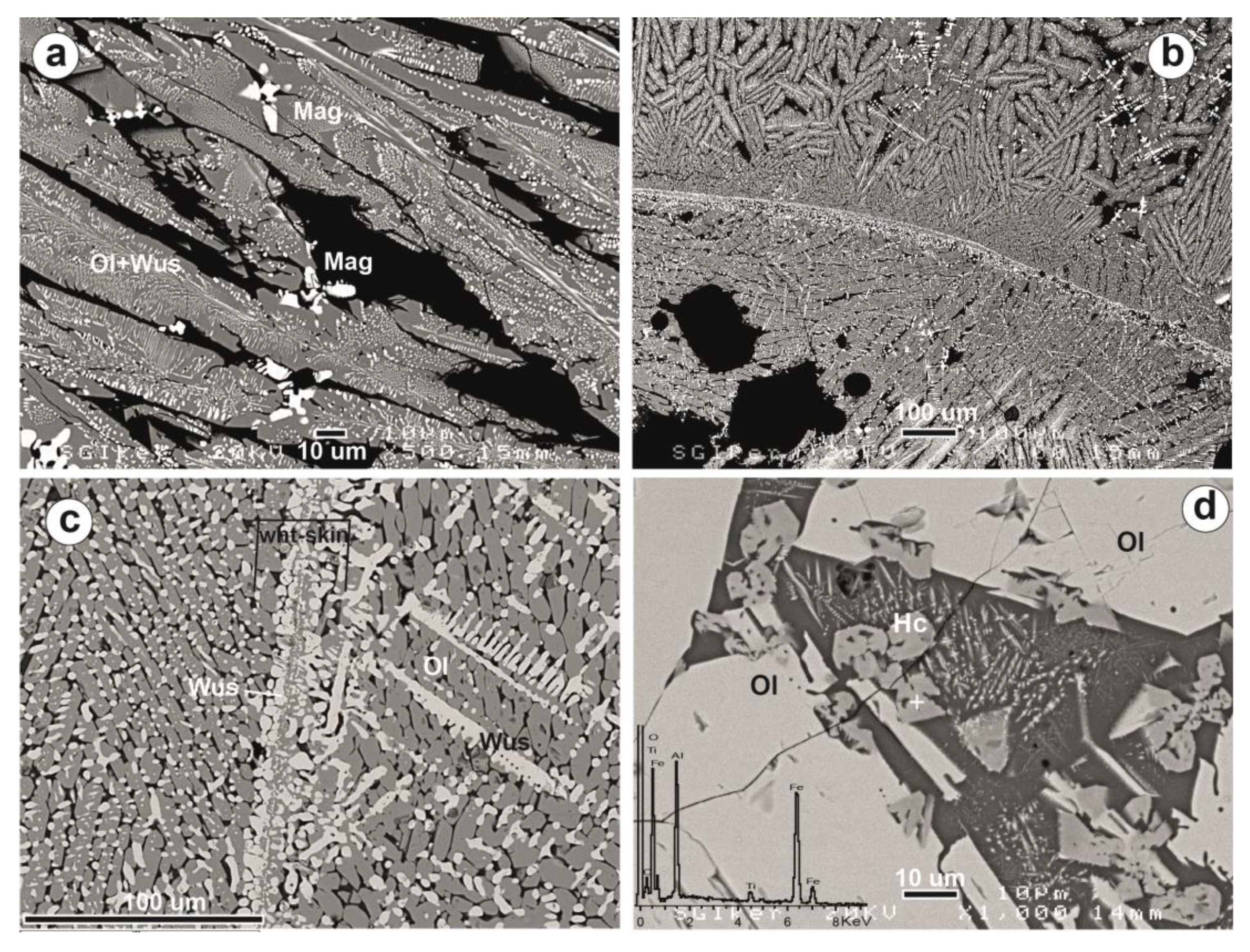

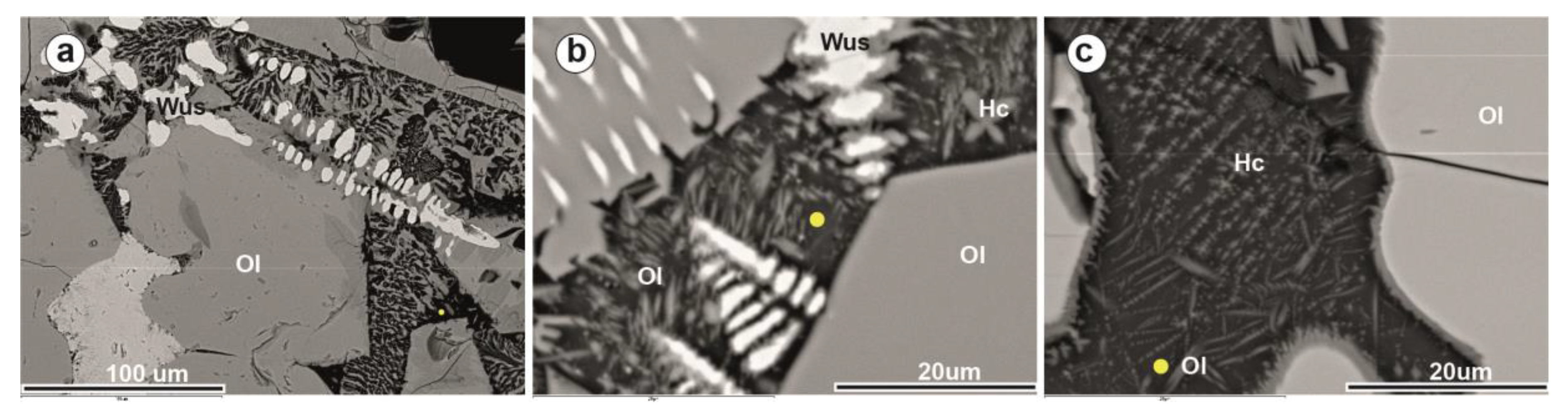
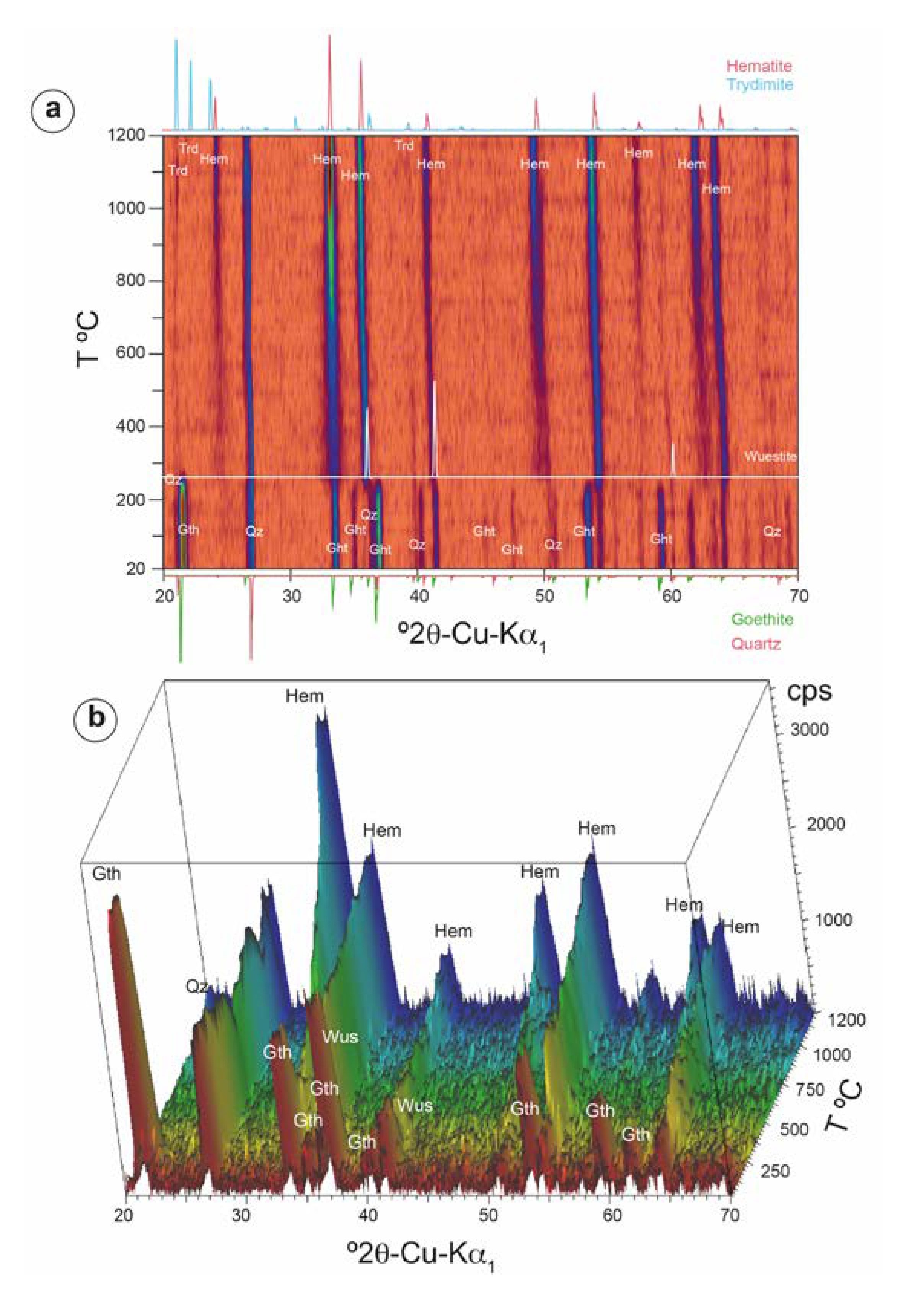
| Site | Period | Age | Sample | Slag Type | Slag Features | |
|---|---|---|---|---|---|---|
| Group 1 | OIOLA IV | Middle Ages | 10th to 13th centuries AD | OI-26-2 | Tap-slags | Strong magnetism and porosity |
| OI-32 | ||||||
| OI-33-44E | ||||||
| OI-33-45E | ||||||
| Group 2 | OIOLA IV | Middle Ages | 10th to 13th centuries AD | OI-18 | Plano-convex | Moderate magnetism and porosity |
| OI-22-13E | Tap-slags | |||||
| OI-22-18E | ||||||
| OI-22-51E | ||||||
| Group 3 | OIOLA IV | Middle Ages | 10th to 13th centuries AD | OI-19 | Tap-slags | Low/absence of magnetism and low porosity |
| OI-20 | ||||||
| OI-21 | ||||||
| OI-22-16E | ||||||
| OI-22-23E | ||||||
| OI-23-22E | ||||||
| OI-25-43E | ||||||
| OI-25-50E | ||||||
| OI-26-1 | ||||||
| OI-27 | ||||||
| OI-28 | ||||||
| OI-31 | ||||||
| OI-27-57E | Plano-convex | |||||
| Group 4 | OIOLA II | Late Roman Period | 4th century AD | OI-II-02 | Tap-slags | Strong magnetismand low porosity |
| OI-II-03 | ||||||
| OI-II-04 | ||||||
| OI-II-05 | ||||||
| OI-II-06 | ||||||
| OI-II-07 | ||||||
| OI-II-08 | ||||||
| OI-II-09 | ||||||
| OI-II-10 | ||||||
| Group 5 | OIOLA II | Late Roman Period | 4th century AD | OI-II-12 | Cinder slags |
| Sample | Period | Mineralogy | Slag Group | |||
|---|---|---|---|---|---|---|
| Quartz | Magnetite | Olivine | Wuestite | |||
| OI-26-2 | Middle Ages 10th to 13th centuries AD | 15 | 20 | 40 | 26 | Group 1 |
| OI-32 | 38 | 15 | 32 | 15 | ||
| OI-33-44E | 30 | 17 | 27 | 26 | ||
| OI-33-45E | 75 | 18 | 6 | 1 | ||
| OI-18 | 16 | 60 | 24 | Group 2 | ||
| OI-22-13E | 5 | 11 | 57 | 27 | ||
| OI-22-18E | 18 | 7 | 59 | 15 | ||
| OI-22-51E | 20 | 9 | 56 | 15 | ||
| OI-19 | 43 | 45 | 11 | Group 3 | ||
| OI-20 | 3 | 70 | 27 | |||
| OI-21 | 76 | 26 | ||||
| OI-22-16E | 87 | 13 | ||||
| OI-22-23E | 7 | 93 | ||||
| OI-23-22E | 17 | 73 | 10 | |||
| OI-25-43E | 7 | 3 | 77 | 13 | ||
| OI-25-50E | 83 | 14 | ||||
| OI-26-1 | 51 | 49 | ||||
| OI-27 | 20 | 74 | 6 | |||
| OI-27-57E | 81 | 19 | ||||
| OI-28 | 19 | 38 | 43 | |||
| OI-31 | 2 | 84 | 14 | |||
| OI-II-02 | Late Roman Period 4th century AD | 20 | 10 | 54 | 15 | Group 4 |
| OI-II-03 | 6 | 26 | 68 | |||
| OI-II-04 | 19 | 21 | 60 | |||
| OI-II-05 | 17 | 67 | 16 | |||
| OI-II-06 | 21 | 13 | 52 | 14 | ||
| OI-II-07 | 4 | 15 | 68 | 13 | ||
| OI-II-08 | 5 | 28 | 67 | |||
| OI-II-09 | 24 | 77 | ||||
| OI-II-10 | 11 | 70 | 19 | |||
| OI-II-12 | 16 | 23 | 44 | 17 | Group 5 | |
| Group 1 | Group 2 | Group 3 | Group 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Medieval | Medieval | Medieval | Roman | |||||
| average | SD | average | SD | average | SD | average | SD | |
| n= | 18 | 28 | 15 | 12 | ||||
| SiO2 | 32.44 | 0.25 | 32.64 | 0.72 | 32.68 | 0.33 | 34.64 | 0.16 |
| MgO | 0.58 | 0.08 | 0.76 | 0.29 | ||||
| FeO | 64.33 | 0.12 | 64.06 | 2.50 | 63.45 | 1.48 | 63.31 | 0.30 |
| CaO | 1.25 | 0.87 | 0.48 | 0.17 | 0.39 | 0.13 | ||
| MnO | 2.46 | 0.08 | 0.90 | 0.10 | 2.73 | 1.12 | 1.13 | 0.15 |
| Group 1 | Group 2 | Group 3 | Group 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Medieval | Medieval | Medieval | Roman | |||||
| average | SD | average | SD | average | SD | average | SD | |
| n= | 24 | 28 | 56 | 48 | ||||
| SiO2 | 46.74 | 1.15 | 41.72 | 3.83 | 42.41 | 3.16 | 43.41 | 3.54 |
| Al2O3 | 13.32 | 0.70 | 18.30 | 1.41 | 20.65 | 2.16 | 18.33 | 1.59 |
| TiO2 | 1.61 | 0.16 | 0.56 | 0.12 | 0.37 | 0.06 | 0.53 | 0.04 |
| FeO | 17.26 | 0.16 | 22.06 | 7.76 | 20.99 | 6.96 | 20.89 | 2.68 |
| CaO | 15.21 | 0.43 | 11.04 | 2.54 | 7.83 | 2.76 | 8.94 | 2.38 |
| Na2O | 0.63 | 0.07 | 0.59 | 0.06 | 1.09 | 0.10 | 0.68 | 0.12 |
| K2O | 0.86 | 0.03 | 5.20 | 0.50 | 6.69 | 0.62 | 5.05 | 1.93 |
| P2O5 | 2.33 | 0.26 | 0.63 | 0.09 | 0.64 | 0.10 | 0.63 | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portillo-Blanco, H.; Zuluaga, M.C.; Ortega, L.A.; Alonso-Olazabal, A.; Cepeda-Ocampo, J.J.; Martínez Salcedo, A. Mineralogical Characterization of Slags from the Oiola Site (Biscay, Spain) to Assess the Development in Bloomery Iron Smelting Technology from the Roman Period to the Middle Ages. Minerals 2020, 10, 321. https://doi.org/10.3390/min10040321
Portillo-Blanco H, Zuluaga MC, Ortega LA, Alonso-Olazabal A, Cepeda-Ocampo JJ, Martínez Salcedo A. Mineralogical Characterization of Slags from the Oiola Site (Biscay, Spain) to Assess the Development in Bloomery Iron Smelting Technology from the Roman Period to the Middle Ages. Minerals. 2020; 10(4):321. https://doi.org/10.3390/min10040321
Chicago/Turabian StylePortillo-Blanco, Haizea, Maria Cruz Zuluaga, Luis Angel Ortega, Ainhoa Alonso-Olazabal, Juan José Cepeda-Ocampo, and Ana Martínez Salcedo. 2020. "Mineralogical Characterization of Slags from the Oiola Site (Biscay, Spain) to Assess the Development in Bloomery Iron Smelting Technology from the Roman Period to the Middle Ages" Minerals 10, no. 4: 321. https://doi.org/10.3390/min10040321
APA StylePortillo-Blanco, H., Zuluaga, M. C., Ortega, L. A., Alonso-Olazabal, A., Cepeda-Ocampo, J. J., & Martínez Salcedo, A. (2020). Mineralogical Characterization of Slags from the Oiola Site (Biscay, Spain) to Assess the Development in Bloomery Iron Smelting Technology from the Roman Period to the Middle Ages. Minerals, 10(4), 321. https://doi.org/10.3390/min10040321





