Study on Grinding Additives in Cassiterite–Polymetallic Sulfide Ore Grinding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
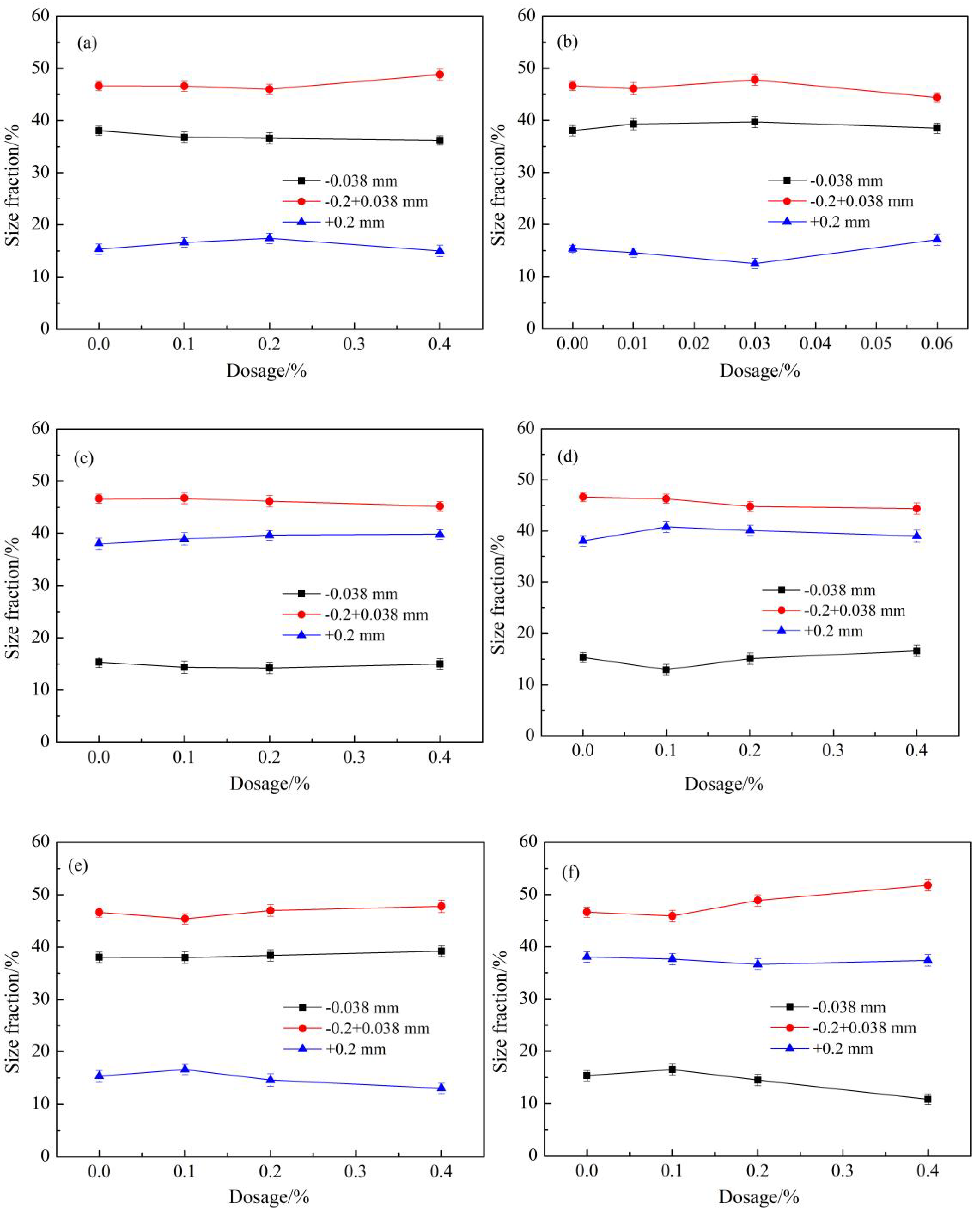
3.1. Effects of Type of Grinding Additives
3.2. Effects of Polyacrylamide Molecular Weight
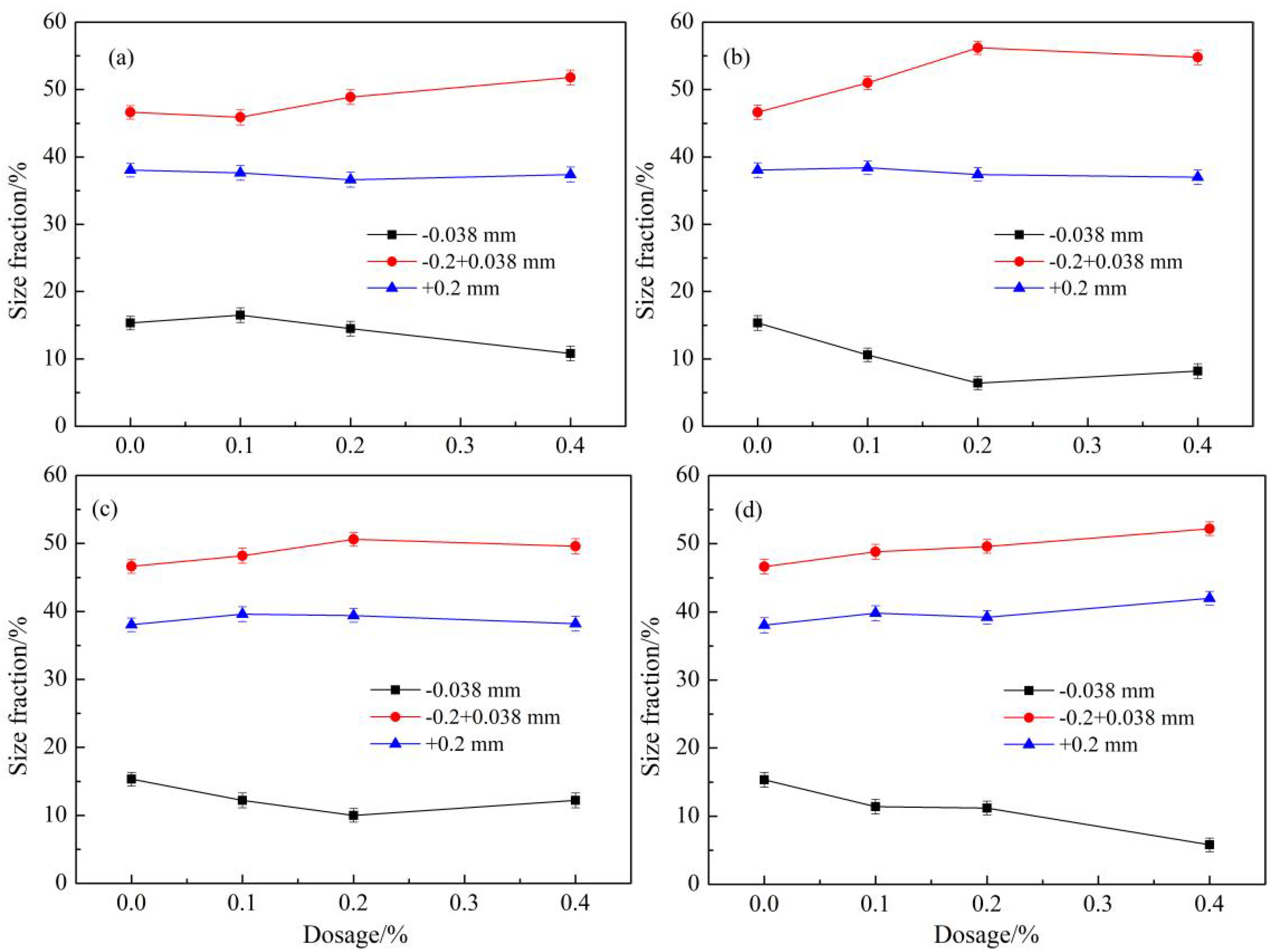
3.3. Effects of Grinding Concentration on the Effectiveness of PAM500
3.4. Effects of Grinding Time on the Effectiveness of PAM500
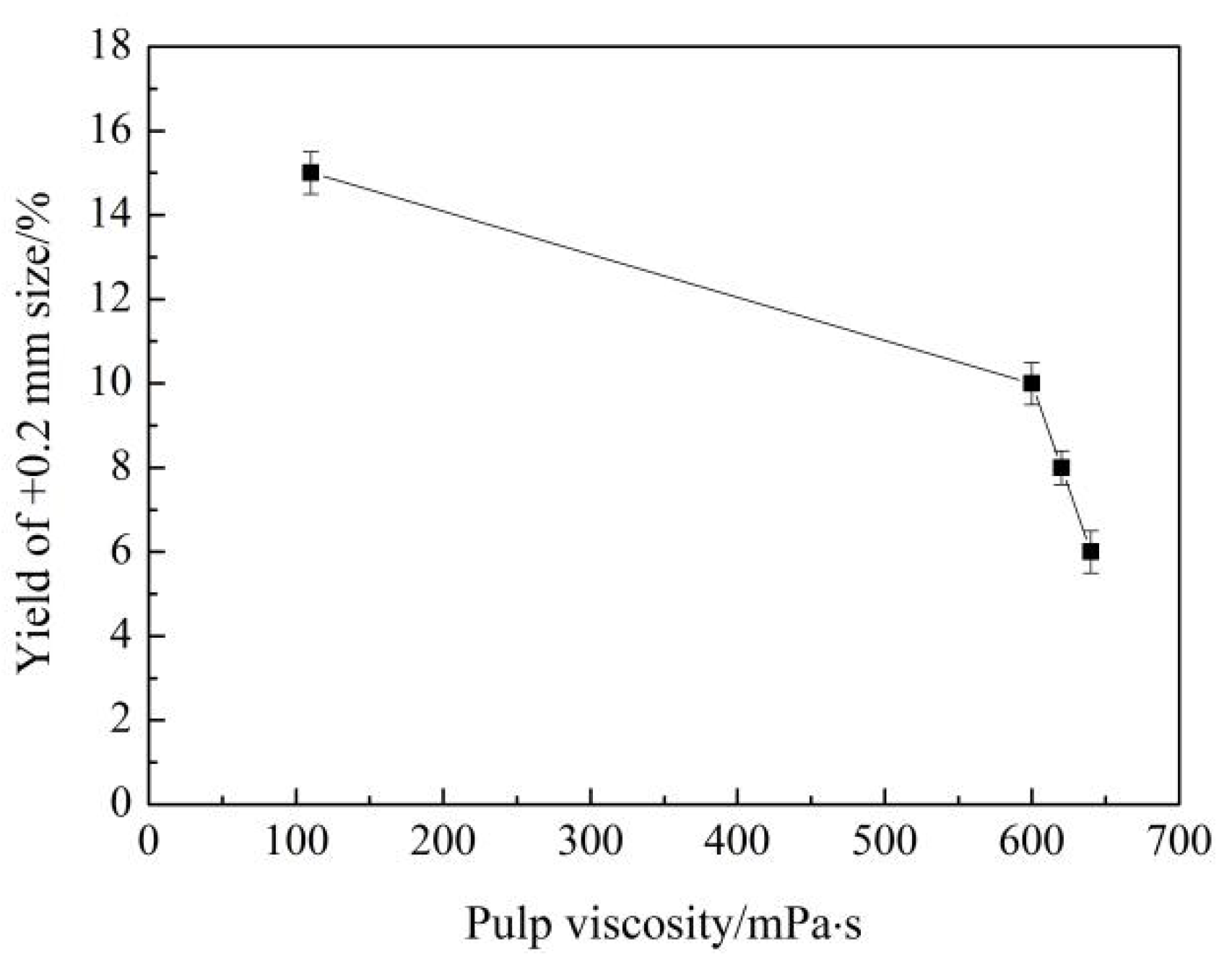
3.5. Mechanism Analysis of Grinding Aids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fuerstenau, D.W.; Phatak, P.B.; Kapur, P.C.; Abouzeid, A.Z.M. Simulation of the grinding of coarse/fine (heterogeneous) systems in a ball mill. Int. J. Miner. Process. 2011, 99, 32–36. [Google Scholar] [CrossRef]
- Klimpel, R.R.; Austin, L.G. Chemical additives for wet grinding of minerals. Powder Technol. 1982, 31, 239–253. [Google Scholar] [CrossRef]
- El-shall, H.; Somasundaran, P. Physico-chemical aspects of grinding: A review of use of additives. Powder Technol. 1984, 38, 275–293. [Google Scholar] [CrossRef]
- Velamakanni, B.V.; Fuerstenau, D.W. The effect of the adsorption of polymeric additives on the wet grinding of minerals 1, mechanisms of suspension stabilization. Powder Technol. 1993, 75, 1–9. [Google Scholar] [CrossRef]
- Velamakanni, B.V.; Fuerstenau, D.W. The effect of the adsorption of polymeric additives on the wet grinding of minerals 2, dispersion and fine grinding of concentrated suspensions. Powder Technol. 1993, 75, 11–19. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Venkataraman, K.S.; Velamakanni, B.V. Effect of chemical additives on the dynamics of grinding media in wet ball mill grinding. Int. J. Miner. Processing 1985, 5, 251–267. [Google Scholar] [CrossRef]
- Sohoni, S.; Sridhar, R.; Mandal, G. The effect of grinding aids on the fine grinding of limestone, quartz and Portland cement clinker. Powder Technol. 1991, 67, 277–286. [Google Scholar] [CrossRef]
- Toprak, N.A.; Altun, O.; Aydogan, N.; Benzer, H. The influences and selection of grinding chemicals in cement grinding circuits. Constr. Build. Mater. 2014, 68, 199–205. [Google Scholar] [CrossRef]
- Sverak, T.S.; Baker, C.G.J.; Kozdas, O. Efficiency of grinding stabilizers in cement clinker processing. Miner. Eng. 2013, 43–44, 52–57. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kimata, M.; Shimane, M.; Shoji, T.; Tsuruta, M. The effect of liquid additives on dry ultrafine grinding of quartz. Powder Technol. 2001, 114, 145–151. [Google Scholar] [CrossRef]
- Nair, P.B.R.; Paramasivam, R. Effect of grinding aids on the time-flow characteristics of the ground product from a batch ball mill. Powder Technol. 1999, 101, 31–42. [Google Scholar] [CrossRef]
- Katsioti, M.; Tsakiridis, P.E.; Giannatos, P.; Tsibouki, Z.; Marinos, J. Characterization of various cement grinding aids and their impact on grindability and cement performance. Constr. Build. Mater. 2009, 23, 1954–1959. [Google Scholar] [CrossRef]
- Choi, H.; Lee, W.; Kim, S. Effect of grinding aids on the kinetics of fine grinding energy consumed of calcite powders by a stirred ball mill. Adv. Powder Technol. 2009, 20, 350–354. [Google Scholar] [CrossRef]
- Choi, H.; Lee, W.; Kim, D.U.; Kumar, S.; Kim, S.S.; Chung, H.S.; Kim, J.H.; Ahn, Y.C. Effect of grinding aids on the grinding energy consumed during grinding of calcite in a stirred ball mill. Miner. Eng. 2009, 23, 54–57. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kimata, M.; Yaguchi, M. Effect and behavior of liquid additive molecules in dry ultrafine grinding of limestone. J. Soc. Powder Technol. Jpn. 2005, 24, 178–184. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Wang, Y.; Forssberg, E. Enhancement of energy efficiency for mechanical production of fine and ultra-fine particles in comminution. China Particuology 2007, 5, 193–201. [Google Scholar] [CrossRef]
- Jeknavorian, A.A.; Barry, E.F.; Serafin, F. Determination of grinding aids in portland cement by pyrolysis gas chromatography-mass spectrometry. Cem. Concr. Res. 1998, 28, 1335–1345. [Google Scholar] [CrossRef]
- Gao, X.J.; Yang, Y.Z.; Deng, H.W. Utilization of beet molasses as a grinding aid in blended cements. Constr. Build. Mater. 2011, 25, 3782–3789. [Google Scholar] [CrossRef]
- Paramasivam, R.; Vedaraman, R. Effect of the physical properties of liquid additives on dry grinding. Powder Technol. 1992, 70, 43–50. [Google Scholar] [CrossRef]
- Duan, X.X. Selective Grinding and Its Application; Metallurgical Industry Press: Beijing, China, 1991. [Google Scholar]
- Chen, B.C. Grinding Principle; Metallurgical Industry Press: Beijing, China, 1989. [Google Scholar]
- Li, T.P. Liu Jincai Control of grinding process parameters-Also on grinding aids. Min. Mach. 2005, 33, 14–17. [Google Scholar]
- Frances, C.; Laguerie, C.; Mazzaotta, B. On the analysis of fine net grinding in a batch ball mill. Chem. Eng. J. 1996, 63, 141–147. [Google Scholar]

| Ingredient | Percent (%) | Ingredient | Percent (%) | Ingredient | Percent (%) | Ingredient | Percent (%) |
|---|---|---|---|---|---|---|---|
| SiO2 | 46 | Zn | 1.2 | Pb | 0.1 | Na2O | 0.01 |
| CaCO3 | 33 | MgO | 1.1 | As | 0.1 | Cd | 0.01 |
| Al2O3 | 6.5 | Mn | 0.3 | Sb | 0.1 | Sr | 0.01 |
| Fe2O3 | 4.8 | Sn | 0.2 | Ni | 0.02 | Zr | 0.01 |
| SO3 | 4 | P2O5 | 0.2 | Cu | 0.02 | Y | <0.01 |
| K2O | 1.4 | Ti | 0.2 | Rb | 0.02 |
| Grinding Concentration (%) | Dosage of PAM500 (%) | Percent of 0.2 mm Size Fraction (%) | Percent of 0.2 to 0.038 mm Size Fraction (%) | Percent of 0.038 mm Size Fraction (%) |
|---|---|---|---|---|
| 60 | 0 | 14.80 | 47.60 | 37.60 |
| 0.1 | 14.60 | 47.90 | 37.50 | |
| 0.2 | 12.90 | 50.80 | 36.30 | |
| 0.4 | 9.00 | 54.20 | 36.80 | |
| 65 | 0 | 15.33 | 46.63 | 38.04 |
| 0.1 | 10.60 | 51.00 | 38.40 | |
| 0.2 | 6.40 | 56.20 | 37.40 | |
| 0.4 | 8.20 | 54.80 | 37.00 | |
| 70 | 0 | 10.30 | 48.80 | 40.90 |
| 0.1 | 8.50 | 51.10 | 40.40 | |
| 0.2 | 10.10 | 50.30 | 39.60 | |
| 0.4 | 13.10 | 48.90 | 38.00 | |
| 75 | 0 | 11.70 | 47.30 | 41.00 |
| 0.1 | 14.10 | 46.90 | 39.00 | |
| 0.2 | 16.50 | 46.80 | 36.70 | |
| 0.4 | 19.20 | 44.00 | 36.80 |
| Grinding Time (min) | Dosage of PAM500 (%) | Percent of 0.2 mm Size Fraction (%) | Percent of 0.2 to 0.038 mm Size Fraction (%) | Percent of 0.038 mm Size Fraction (%) |
|---|---|---|---|---|
| 6 | 0 | 15.33 | 46.63 | 38.04 |
| 0.1 | 10.60 | 51.00 | 38.40 | |
| 0.2 | 6.40 | 56.20 | 37.40 | |
| 0.4 | 8.20 | 54.80 | 37.00 | |
| 8 | 0 | 5.00 | 46.20 | 48.80 |
| 0.1 | 4.00 | 47.00 | 49.00 | |
| 0.2 | 4.20 | 45.10 | 50.70 | |
| 0.4 | 4.40 | 45.80 | 49.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Ma, S.; Zhou, W.; Zhu, P. Study on Grinding Additives in Cassiterite–Polymetallic Sulfide Ore Grinding. Minerals 2022, 12, 472. https://doi.org/10.3390/min12040472
Yang J, Ma S, Zhou W, Zhu P. Study on Grinding Additives in Cassiterite–Polymetallic Sulfide Ore Grinding. Minerals. 2022; 12(4):472. https://doi.org/10.3390/min12040472
Chicago/Turabian StyleYang, Jinlin, Shaojian Ma, Wentao Zhou, and Pengyan Zhu. 2022. "Study on Grinding Additives in Cassiterite–Polymetallic Sulfide Ore Grinding" Minerals 12, no. 4: 472. https://doi.org/10.3390/min12040472





