Progress in the Application of Biomimetic Mineralization for Tooth Repair
Abstract
:1. Introduction
2. Structure and Properties of Teeth
2.1. Enamel
2.1.1. Hierarchical Structure of Enamel
2.1.2. Mechanical Properties of Enamel

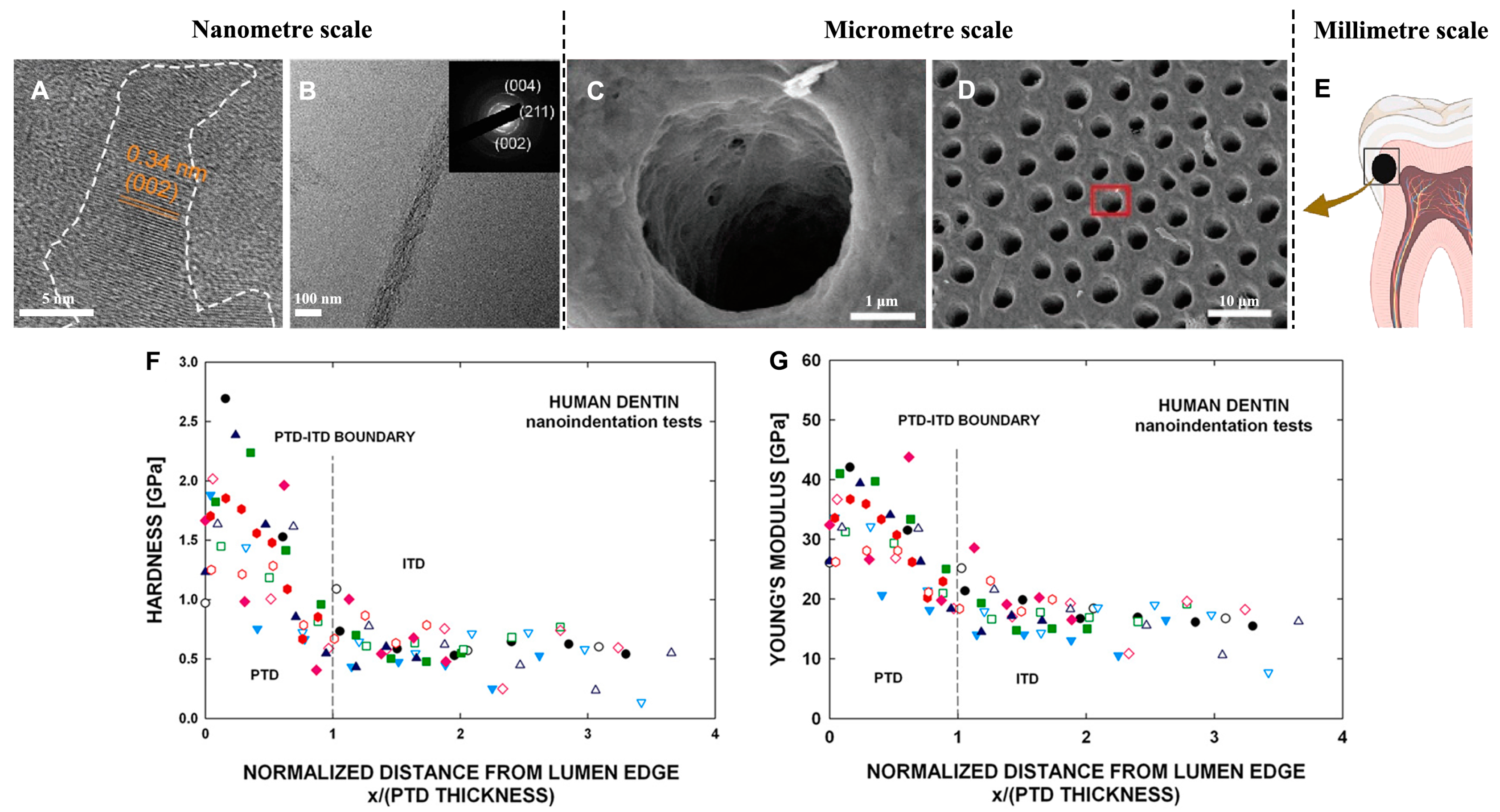
2.2. Dentin
2.2.1. The Structure of Dentin
2.2.2. Mechanical Properties of Dentin
3. Tooth Restoration
3.1. Dental Caries
3.2. Clinical Therapeutic Strategies and Their Limitations
4. Biomimetic Mineralization for the Remineralization of Enamel and Dentin
4.1. Enamel Remineralization
4.1.1. Amelogenin and Amelogenin-Inspired Peptides
4.1.2. Calcium Phosphate Particle-Based Systems
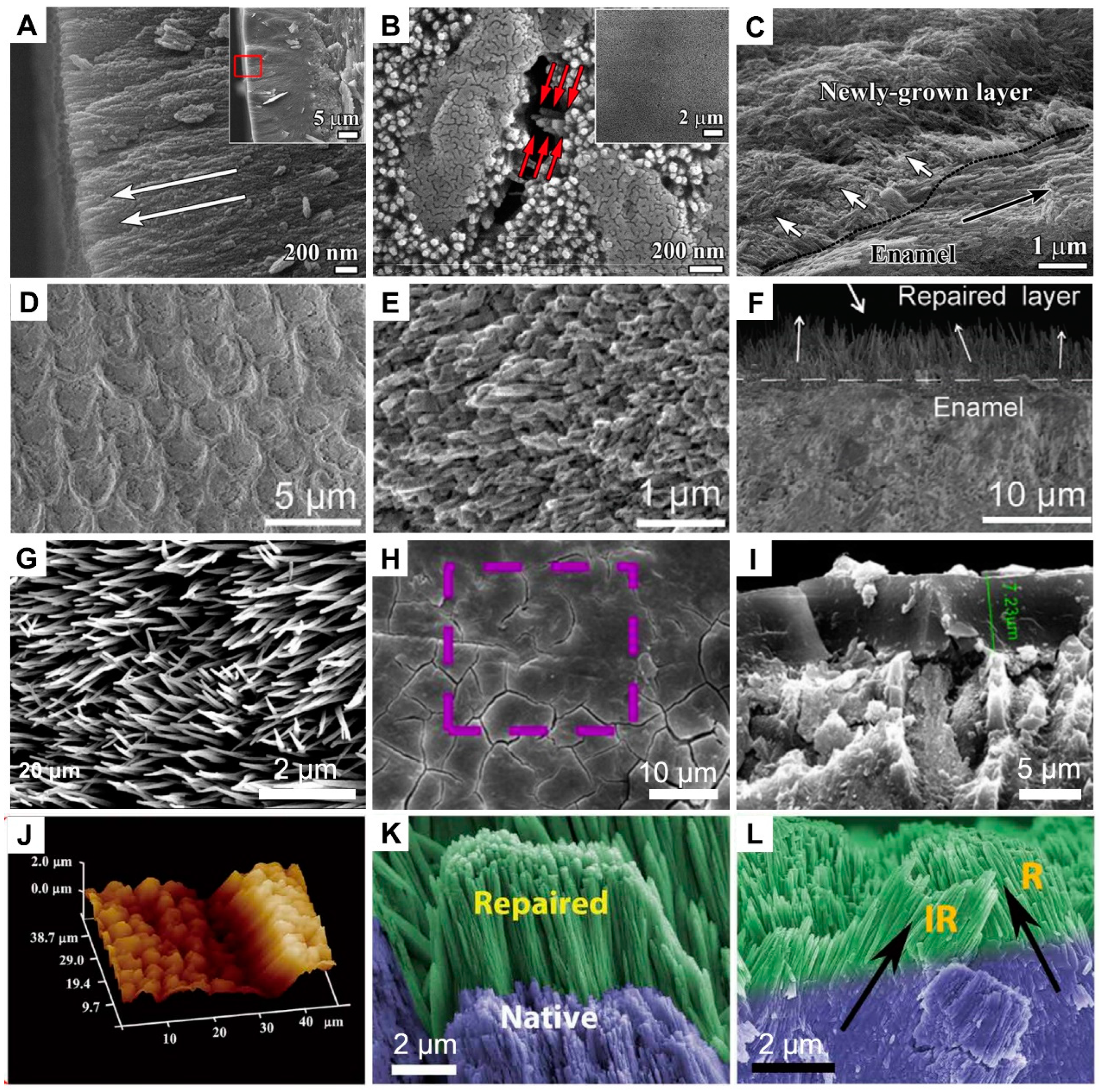
4.2. Dentin Remineralization
4.2.1. Proteins or Peptides for Dentin Remineralization
4.2.2. NCP Surrogate
4.2.3. Based on the Promotion of Collagen Remineralization for the Repair of Dentin

4.2.4. ACP Nanoparticles for Dentin Remineralization
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kruzic, J.J.; Hoffman, M.; Arsecularatne, J.A. Fatigue and wear of human tooth enamel: A review. J. Mech. Behav. Biomed. Mater. 2023, 138, 21. [Google Scholar] [CrossRef]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.H.; Kang, S.H.; Yoon, C.H.; Lee, H.J.; Yun, P.Y.; Youn, T.J.; Chae, I.H. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: A population-based study from Korea. Eur. Heart J. 2019, 40, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Ungar, P.S. Mammalian dental function and wear: A review. Biosurface Biotribol. 2015, 1, 25–41. [Google Scholar] [CrossRef]
- Zolotarev, V.M.; Grisimov, V.N. Architectonics and Optical Properties of Dentin and Dental Enamel. Opt. Spectrosc. 2001, 90, 753–759. [Google Scholar] [CrossRef]
- Zhao, X.; O’Brien, S.; Shaw, J.; Abbott, P.; Munroe, P.; Habibi, D.; Xie, Z. The origin of remarkable resilience of human tooth enamel. Appl. Phys. Lett. 2013, 103, 241901. [Google Scholar] [CrossRef]
- Gavrilovski, D.S.; Blagojevic, N.S.; Gavrilovski, M.P. Modelling glass-ceramic enamel properties. J. Serb. Chem. Soc. 2002, 67, 135–142. [Google Scholar] [CrossRef]
- Lubarsky, G.V.; Lemoine, P.; Meenan, B.J.; Deb, S.; Mutreja, I.; Carolan, P.; Petkov, N. Enamel proteins mitigate mechanical and structural degradations in mature human enamel during acid attack. Mater. Res. Express 2014, 1, 025404. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Cui, F.-Z.; Ge, J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef]
- White, S.N.; Luo, W.; Paine, M.L.; Fong, H.; Sarikaya, M.; Snead, M.L. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J. Dent. Res. 2001, 80, 321–326. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M.V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J. Mech. Behav. Biomed. Mater. 2008, 1, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, I.H.; Han, S.Y. The Reason why a Sheath Exists in Enamel. Int. J. Precis. Eng. Manuf. 2015, 16, 807–811. [Google Scholar] [CrossRef]
- Cao, C.; Mei, M.; Li, Q.-l.; Lo, E.; Chu, C. Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review. Materials 2015, 8, 2873–2886. [Google Scholar] [CrossRef]
- Popowics, T.E.; Rensberger, J.M.; Herring, S.W. Enamel microstructure and microstrain in the fracture of human and pig molar cusps. Arch. Oral Biol. 2004, 49, 595–605. [Google Scholar] [CrossRef]
- Chai, H.; Lee, J.J.W.; Constantino, P.J.; Lucas, P.W.; Lawn, B.R. Remarkable resilience of teeth. Proc. Natl. Acad. Sci. USA 2009, 106, 7289–7293. [Google Scholar] [CrossRef]
- Lawn, B.R.; Lee, J.J.W.; Chai, H. Teeth: Among Nature’s Most Durable Biocomposites. Annu. Rev. Mater. Res. 2010, 40, 55–75. [Google Scholar] [CrossRef]
- Cuy, J.L.; Man, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Imbeni, V.; Kruzic, J.J.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O. The dentin–enamel junction and the fracture of human teeth. Nat. Mater. 2005, 4, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Baldassarri, M.; Margolis, H.C.; Beniash, E. Compositional Determinants of Mechanical Properties of Enamel. J. Dent. Res. 2008, 87, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.H.; Swain, M.V.; Swadener, G.; Munroe, P.; Hoffman, M. Effect of microstructure upon elastic behaviour of human tooth enamel. J. Biomech. 2009, 42, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cui, F.Z.; Wang, X.M.; Feng, H.L. Property variations in the prism and the organic sheath within enamel by nanoindentation. Biomaterials 2005, 26, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef]
- Xu, C.; Reed, R.; Gorski, J.P.; Wang, Y.; Walker, M.P. The distribution of carbonate in enamel and its correlation with structure and mechanical properties. J. Mater. Sci. 2012, 47, 8035–8043. [Google Scholar] [CrossRef]
- Alkattan, R.; Lippert, F.; Tang, Q.; Eckert, G.J.; Ando, M. The influence of hardness and chemical composition on enamel demineralization and subsequent remineralization. J. Dent. 2018, 75, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Carrilho, M.R.; Breschi, L.; Tay, F.R.; Pashley, D.H. Dentin basic structure and composition—An overview. Endod. Top. 2009, 20, 3–29. [Google Scholar] [CrossRef]
- Carda, C.; Peydró, A. Ultrastructural patterns of human dentinal tubules, odontoblasts processes and nerve fibres. Tissue Cell 2006, 38, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; D’Souza, R.; Feng, J.Q. Dentin matrix protein 1 (DMP1): New and important roles for biomineralization and phosphate homeostasis. J. Dent. Res. 2007, 86, 1134–1141. [Google Scholar] [CrossRef]
- Butler, W.T. Dentin matrix proteins and dentinogenesis. Connect Tissue Res. 1995, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Oida, S.; Yamakoshi, Y. Dentin Sialophosphoprotein-derived proteins in the dental pulp. J. Dent. Res. 2015, 94, 1120–1127. [Google Scholar] [CrossRef]
- Suzuki, S.; Sreenath, T.; Haruyama, N.; Honeycutt, C.; Terse, A.; Cho, A.; Kohler, T.; Mueller, R.; Goldberg, M.; Kulkarni, A.B. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009, 28, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.S.; Fang, P.-A.; Zhang, X.; Jayaraman, T.; Sfeir, C.; Beniash, E. Primary Structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules 2011, 12, 2933–2945. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A.J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Wang, Y.; Azais, T.; Robin, M.; Vallee, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.-M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, R.; Shen, L.; Yang, Y.; Wang, G.; Yang, B. The multi-scale meso-mechanics model of viscoelastic dentin. J. Mech. Behav. Biomed. Mater. 2022, 136, 105525. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.P. The tooth: An analogue for biomimetic materials design and processing. Dent. Mater. 2020, 36, 25–42. [Google Scholar] [CrossRef]
- Carreon, A.H.; Funkenbusch, P.D. Nanoscale properties and deformation of human enamel and dentin. J. Mech. Behav. Biomed. Mater. 2019, 97, 74–84. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Du, W.; Zhou, X.-D.; Yu, H.-Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ziskind, D.; Hasday, M.; Cohen, S.R.; Wagner, H.D. Young’s modulus of peritubular and intertubular human dentin by nano-indentation tests. J. Struct. Biol. 2011, 174, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Jung, G.S.; Danti, S.; Buehler, M.J. Mechanics of mineralized collagen fibrils upon transient loads. ACS Nano 2020, 14, 8307–8316. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudsy, L.; Hu, Y.-W.; Xu, H.; Yang, P.-F. Mineralized collagen fibrils: An essential component in determining the mechanical behavior of cortical bone. ACS Biomater. Sci. Eng. 2023, 9, 2203–2219. [Google Scholar] [CrossRef]
- Wu, H.; Shao, C.; Shi, J.; Hu, Z.; Zhou, Y.; Chen, Z.; Tang, R.; Xie, Z.; Jin, W. Hyaluronic acid-mediated collagen intrafibrillar mineralization and enhancement of dentin remineralization. Carbohydr. Polym. 2023, 319, 121174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Duan, Y.; Shan, S.; Sun, K.; Wang, G.; Shao, C.; Tang, Z.; Xu, Z.; Zhou, Y.; Chen, Z.; et al. Deep and compact dentinal tubule occlusion via biomimetic mineralization and mineral overgrowth. Nanoscale 2022, 14, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.H. Teeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Ulrich, L. Dental glass ionomer cements as permanent filling materials?—Properties, limitations and future trends. Materials 2009, 3, 76–96. [Google Scholar]
- Olegário, I.; Ladewig, N.; Hesse, D.; Bonifácio, C.; Braga, M.; Imparato, J.; Mendes, F.; Raggio, D. Is it worth using low-cost glass ionomer cements for occlusal ART restorations in primary molars? 2-year survival and cost analysis of a Randomized clinical trial. J. Dent. 2020, 101, 103446. [Google Scholar] [CrossRef]
- Rueggeberg, F.A. From vulcanite to vinyl, a history of resins in restorative dentistry. J. Prosthet. Dent. 2002, 87, 364–379. [Google Scholar] [CrossRef]
- Brunthaler, A.; König, F.; Lucas, T.; Schedle, W.S. Longevity of direct resin composite restorations in posterior teeth: A review. Clin. Oral Investig. 2003, 7, 63–70. [Google Scholar] [CrossRef]
- Kanzow, P.; Wiegand, A. Retrospective analysis on the repair vs. replacement of composite restorations. Dent. Mater. 2020, 36, 108–118. [Google Scholar] [CrossRef]
- Sarrett, D. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent. Mater. 2005, 21, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, S.; Lim, L.H.; Lee, Z.T.; Lim, K.H.; Sia, S.Y. Efficacy of three enamel protecting agents on shear bond strength of orthodontic brackets bonded to demineralised enamel with conventional adhesive. J. Clin. Diagn. Res. 2021, 15, ZC18–ZC21. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic aspects of restorative dentistry biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Li, Z.; Ding, L.; Wang, X.; Niu, Y.; Qin, X.; Zhou, X.; Zhang, L. Anti-biofilm and remineralization effects of chitosan hydrogel containing amelogenin-derived peptide on initial caries lesions. Regen. Biomater. 2018, 5, 69–76. [Google Scholar] [CrossRef]
- Ruan, Q.C.; Moradian-Oldak, J. An amelogenin–chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.Y.; Jin, B.A.; Mu, Z.; Lu, H.; Zhao, Y.Q.; Wu, Z.F.; Yan, L.M.; Zhang, Z.S.; Zhou, Y.C.; Pan, H.H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, X.Y. Principles of mimicking and engineering the self-organized structure of hard tissues. J. Biol. Chem. 2004, 279, 41286–41293. [Google Scholar] [CrossRef]
- Jiang, H.D.; Liu, X.Y.; Zhang, G.; Li, Y. Kinetics and template nucleation of self-assembled hydroxyapatite nanocrystallites by chondroitin sulfate. J. Biol. Chem. 2005, 280, 42061–42066. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; Simmer, J.P.; Lau, E.C.; Sarte, P.E.; Fincham, A.G. Detection of monodisperse aggregates of a recombinant amelogenin by dynamic light scattering. Biopolymers 2010, 34, 1339–1347. [Google Scholar] [CrossRef]
- Engelberth, S.A.; Bacino, M.S.; Sandhu, S.; Li, W.; Bonde, J.; Habelitz, S. Progression of self-assembly of amelogenin protein supramolecular structures in simulated enamel fluid. Biomacromolecules 2018, 19, 3917–3924. [Google Scholar] [CrossRef]
- Du, C.; Falini, G.; Fermani, S.; Abbott, C.; Moradian-Oldak, J. Supramolecular Assembly of Amelogenin Nanospheres into Birefringent Microribbons. Science 2005, 307, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Fincham, A.G.; Moradian-Oldak, J.; Diekwisch, T.G.H.; Lyaruu, D.M.; Wright, J.T.; Bringas, P.; Slavkin, H.C. Evidence for amelogenin “Nanospheres” as functional components of secretory-stage enamel matrix. J. Struct. Biol. 1995, 115, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.; Ruan, Q.; Mukherjee, K.; Nutt, S.; Moradian-Oldak, J. The presence of MMP-20 reinforces biomimetic enamel regrowth. J. Dent. Res. 2018, 97, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.J.; Han, S.L.; Wang, K.; Zheng, S.N.; Zheng, W.Y.; Peng, X.; Niu, Y.M.; Li, W.; Zhang, L.L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 7, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Deng, J.J.; Deng, X.L.; Fang, C.Q.; Zhang, X.; Yang, P. Controlling enamel remineralization by Amyloid-Like amelogenin mimics. Adv. Mater. 2020, 32, e2002080. [Google Scholar] [CrossRef]
- Yang, S.; He, H.; Wang, L.; Jia, X.; Feng, H. Oriented crystallization of hydroxyapatite by the biomimetic amelogenin nanospheres from self-assemblies of amphiphilic dendrons. Chem. Commun. 2011, 47, 10100–10102. [Google Scholar] [CrossRef]
- Yang, J.; Cao, S.; Li, J.; Xin, J.; Chen, X.; Wu, W.; Xu, F.; Li, J. Staged self-assembly of PAMAM dendrimers into macroscopic aggregates with a microribbon structure similar to that of amelogenin. Soft Matter 2013, 9, 7553–7559. [Google Scholar] [CrossRef]
- Chen, L.; Liang, K.N.; Li, J.S.; Wu, D.; Zhou, X.D.; Li, J.Y. Regeneration of biomimetic hydroxyapatite on etched human enamel by anionic PAMAM template in vitro. Arch. Oral Biol. 2013, 58, 975–980. [Google Scholar] [CrossRef]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [PubMed]
- Steve, W.; Julia, M.; Yael, P.; Yurong, M.A.; Lia, A. Overview of the amorphous precursor phase strategy in biomineralization. Front. Mater. Sci. China 2009, 3, 104–108. [Google Scholar]
- Lowenstam, H.A.; Weiner, S. Transformation of amorphous calcium phosphate to crystalline dahillite in the radular teeth of chitons. Science 1985, 227, 51–53. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.K.; Gilbert, P. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Zhang, S.H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Sun, X.; Kishen, A.; Deng, X.; Yang, X.; Wang, H.; Cong, C.; Wang, Y.; Wu, M. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J. Mater. Sci. Mater. Med. 2014, 25, 2619–2628. [Google Scholar] [CrossRef]
- Fletcher, J.; Walsh, D.; Fowler, C.E.; Mann, S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. CrystEngComm 2011, 13, 3692–3697. [Google Scholar] [CrossRef]
- Iafisco, M.; Degli Esposti, L.; Ramirez-Rodriguez, G.B.; Carella, F.; Gomez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-Lopez, J.M. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Que, K.H.; Wang, H.R.; An, R.; Chen, Z.; Qiu, Z.J.; Lin, M.L.; Song, J.H.; Yang, J.; Lu, D.Y.; et al. Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides. Dent. Mater. 2017, 33, 1217–1228. [Google Scholar] [CrossRef]
- Weir, M.D.; Chow, L.C.; Xu, H.H.K. Remineralization of Demineralized Enamel via Calcium Phosphate Nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef]
- Li, L.; Mao, C.Y.; Wang, J.M.; Xu, X.R.; Pan, H.H.; Deng, Y.; Gu, X.H.; Tang, R.K. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 2011, 23, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Xiao, Z.H.; Yang, J.; Lu, D.Y.; Kishen, A.; Li, Y.Q.; Chen, Z.; Que, K.H.; Zhang, Q.; Deng, X.L.; et al. Oriented and ordered biomimetic remineralization of the surface of demineralized dental enamel using HAP@ACP nanoparticles guided by glycine. Sci. Rep. 2017, 7, 40701. [Google Scholar] [CrossRef]
- Gericke, A.; Qin, C.; Sun, Y.; Redfern, R.; Redfern, D.; Fujimoto, Y.; Taleb, H.; Butler, W.T.; Boskey, A.L. Different forms of DMP1 play distinct roles in mineralization. J. Dent. Res. 2010, 89, 355–359. [Google Scholar] [CrossRef]
- Liang, K.; Xiao, S.; Shi, W.; Li, J.; Yang, X.; Gao, Y.; Gou, Y.; Hao, L.; He, L.; Cheng, L.; et al. 8DSS-promoted remineralization of demineralized dentin in vitro. J. Mater. Chem. B 2015, 3, 6763–6772. [Google Scholar] [CrossRef]
- Liang, K.; Xiao, S.; Liu, H.; Shi, W.; Li, J.; Gao, Y.; He, L.; Zhou, X.; Li, J. 8DSS peptide induced effective dentinal tubule occlusion in vitro. Dent. Mater. 2018, 34, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Padovano, J.D.; Ravindran, S.; Snee, P.T.; Ramachandran, A.; Bedran-Russo, A.K.; George, A. DMP1-derived peptides promote remineralization of human dentin. J. Dent. Res. 2015, 94, 608–614. [Google Scholar] [CrossRef]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Han, S.; Ding, L.; Qin, X.; Hui, D.; Het, T.; Tian, T.; Lu, Z.; Zhang, L. Promoting effect of a calcium-responsive self-assembly β-sheet peptide on collagen intrafibrillar mineralization. Regen. Biomater. 2022, 9, rbac059. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Qin, X.; Ren, Q.; Hui, D.; Tian, T.; He, T.; Li, W.; Zhang, L.L. Rational design of β-sheet peptides with self-assembly into nanofibres on remineralisation of initial caries lesions. Chin. J. Dent. Res. 2020, 23, 131–141. [Google Scholar] [PubMed]
- Mukherjee, K.; Visakan, G.; Phark, J.-H.; Moradian-Oldak, J. Enhancing collagen mineralization with amelogenin peptide: Toward the restoration of dentin. ACS Biomater. Sci. Eng. 2020, 6, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R. Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Burwell, A.K.; Thula-Mata, T.; Gower, L.B.; Habeliz, S.; Kurylo, M.; Ho, S.P.; Chien, Y.-C.; Cheng, J.; Cheng, N.F.; Gansky, S.A.; et al. Functional remineralization of dentin lesions using polymer-induced liquid-precursor process. PLoS ONE 2012, 7, e38852. [Google Scholar] [CrossRef] [PubMed]
- Bacino, M.; Girn, V.; Nurrohman, H.; Saeki, K.; Marshall, S.J.; Gower, L.; Saeed, E.; Stewart, R.; Le, T.; Marshall, G.W.; et al. Integrating the PILP-mineralization process into a restorative dental treatment. Dent. Mater. 2019, 35, 53–63. [Google Scholar] [CrossRef]
- Qi, Y.; Ye, Z.; Fok, A.; Holmes, B.N.; Espanol, M.; Ginebra, M.-P.; Aparicio, C. Effects of molecular weight and concentration of poly(Acrylic Acid) on biomimetic mineralization of collagen. ACS Biomater. Sci. Eng. 2018, 4, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Bu, H.; Zhang, Y.; Tang, P.; Li, G. Molecular weight and concentration of poly (acrylic acid) dual-responsive homogeneous and intrafibrillar collagen mineralization using an in situ co-organization strategy. Polym. Compos. 2021, 42, 4448–4460. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Z.; Li, W. Poly(acrylic acid)-assisted intrafibrillar mineralization of type I collagen: A review. Macromol. Rapid Commun. 2023, 44, e2200827. [Google Scholar] [CrossRef]
- Butler, W.T. Dentin matrix proteins. Eur. J. Oral Sci. 1998, 106, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Gulseren, G.; Tansik, G.; Garifullin, R.; Tekinay, A.B.; Guler, M.O. Dentin phosphoprotein mimetic peptide nanofibers promote biomineralization. Macromol. Biosci. 2019, 19, e1800080. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Butler, W.T.; Qin, C. Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 2010, 51, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef]
- Mai, S.; Kim, Y.K.; Toledano, M.; Breschi, L.; Ling, J.Q.; Pashley, D.H.; Tay, F.R. Phosphoric acid esters cannot replace polyvinylphosphonic acid as phosphoprotein analogs in biomimetic remineralization of resin-bonded dentin. Dent. Mater. 2009, 25, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.-S.; Kim, J.; Kim, Y.K.; Liu, Y.; Dickens, S.H.; Pashley, D.H.; Ling, J.-Q.; Tay, F.R. A chemical phosphorylation-inspired design for Type I collagen biomimetic remineralization. Dent. Mater. 2010, 26, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Qi, Y.; Niu, L.-N.; Elshafiy, S.; Mao, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. The use of sodium trimetaphosphate as a biomimetic analog of matrix phosphoproteins for remineralization of artificial caries-like dentin. Dent. Mater. 2011, 27, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhao, R.; Jiang, S.; Yao, S.; Wu, Z.; Jin, B.; Yang, Y.; Pan, H.; Tang, R. Citrate improves collagen mineralization via Interface Wetting: A physicochemical understanding of biomineralization control. Adv. Mater. 2018, 30, 1704876. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Q.; Li, Y.; Yang, M.; Dong, M.; He, X.; Zheng, S.; Cao, C.Y.; Zhou, Z.; Zhao, Y.; et al. A dopamine acrylamide molecule for promoting collagen biomimetic mineralization and regulating crystal growth direction. ACS Appl. Mater. Interfaces 2021, 13, 39142–39156. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Jiao, K.; Tonggu, L.; Wang, L.G.; Zhang, S.L.; Yang, Y.D.; Zhang, L.; Bian, J.H.; Hao, D.X.; Wang, C.Y.; et al. Contribution of biomimetic collagen-ligand interaction to intrafibrillar mineralization. Sci. Adv. 2019, 5, eaav9075. [Google Scholar] [CrossRef]
- Amornkitbamrung, U.; In, Y.; Wang, Z.; Song, J.; Oh, S.H.; Hong, M.-H.; Shin, H. Axis-Oriented platelets of crystalline hydroxyapatite in biomimetic intrafibrillar mineralization of polydopamine-functionalized collagen type I. ACS Omega 2022, 7, 4821–4831. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Y.; Ren, Q.; Ding, L.; Han, S.; Hu, D.; Lu, Z.; Wang, L.; Zhang, Y.; Zhang, L. Mineralization promotion and protection effect of carboxymethyl chitosan biomodification in biomimetic mineralization. Int. J. Biol. Macromol. 2023, 234, 123720. [Google Scholar] [CrossRef]
- Huang, X.-Q.; Yang, H.-Y.; Luo, T.; Huang, C.; Tay, F.R.; Niu, L.-N. Hollow mesoporous zirconia delivery system for biomineralization precursors. Acta Biomater. 2018, 67, 366–377. [Google Scholar] [CrossRef]
- Cuylear, D.L.; Elghazali, N.A.; Kapila, S.D.; Desai, T.A. Calcium phosphate delivery systems for regeneration and biomineralization of mineralized tissues of the craniofacial complex. Mol. Pharm. 2023, 20, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Shi, J.; Wu, Q.; Yang, L.; Cao, S. Hollow hydroxyapatite/polyelectrolyte hybrid microparticles with controllable size, wall thickness and drug delivery properties. J. Mater. Chem. B 2015, 3, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-J.; Yang, H.-Y.; Niu, L.-N.; Mao, J.; Huang, C.; Pashley, D.H.; Tay, F.R. Translation of a solution-based biomineralization concept into a carrier-based delivery system via the use of expanded-pore mesoporous silica. Acta Biomater. 2016, 31, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Guo, Y.; Zhang, H.; Qiu, Y.; Li, J.; Ma, D.; Li, Z.; Zhen, P.; Liu, B.; et al. Novel core-shell CHX/ACP nanoparticles effectively improve the mechanical, antibacterial and remineralized properties of the dental resin composite. Dent. Mater. 2021, 37, 636–647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Shan, S.; Chen, Z.; Shao, C. Progress in the Application of Biomimetic Mineralization for Tooth Repair. Minerals 2023, 13, 1433. https://doi.org/10.3390/min13111433
Tang Z, Shan S, Chen Z, Shao C. Progress in the Application of Biomimetic Mineralization for Tooth Repair. Minerals. 2023; 13(11):1433. https://doi.org/10.3390/min13111433
Chicago/Turabian StyleTang, Zhenhang, Songzhe Shan, Zhuo Chen, and Changyu Shao. 2023. "Progress in the Application of Biomimetic Mineralization for Tooth Repair" Minerals 13, no. 11: 1433. https://doi.org/10.3390/min13111433




