An Improved Understanding of Chalcopyrite Leaching Mechanisms: The Influence of Anisotropic Crystal Planes
Abstract
:1. Introduction
2. Materials and Methodology
2.1. CuFeS2 Samples
2.2. Leaching Experiments
2.3. Bulk and Surface Analyses
2.4. DFT Calculation
3. Results and Discussion
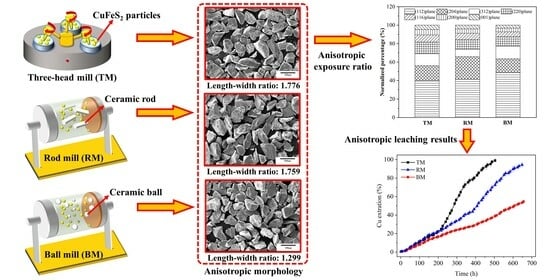
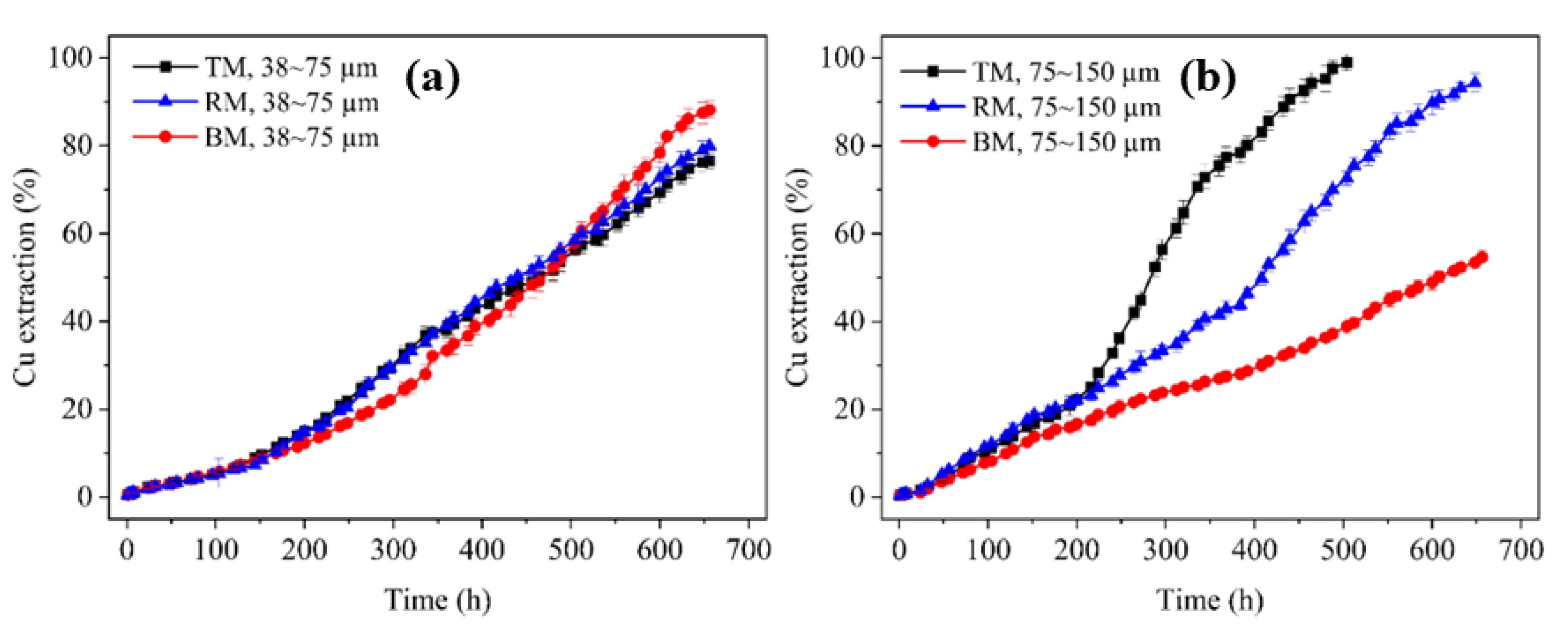
3.1. Anisotropic Leaching Results
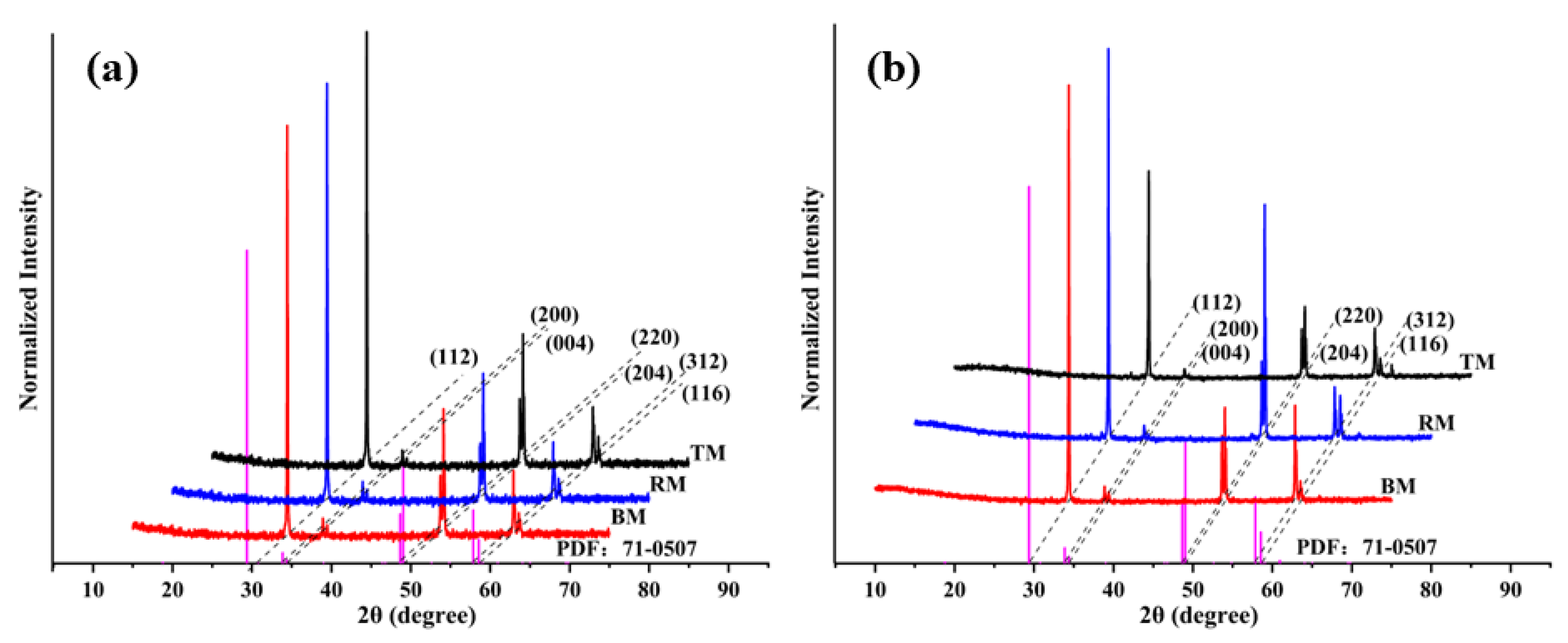
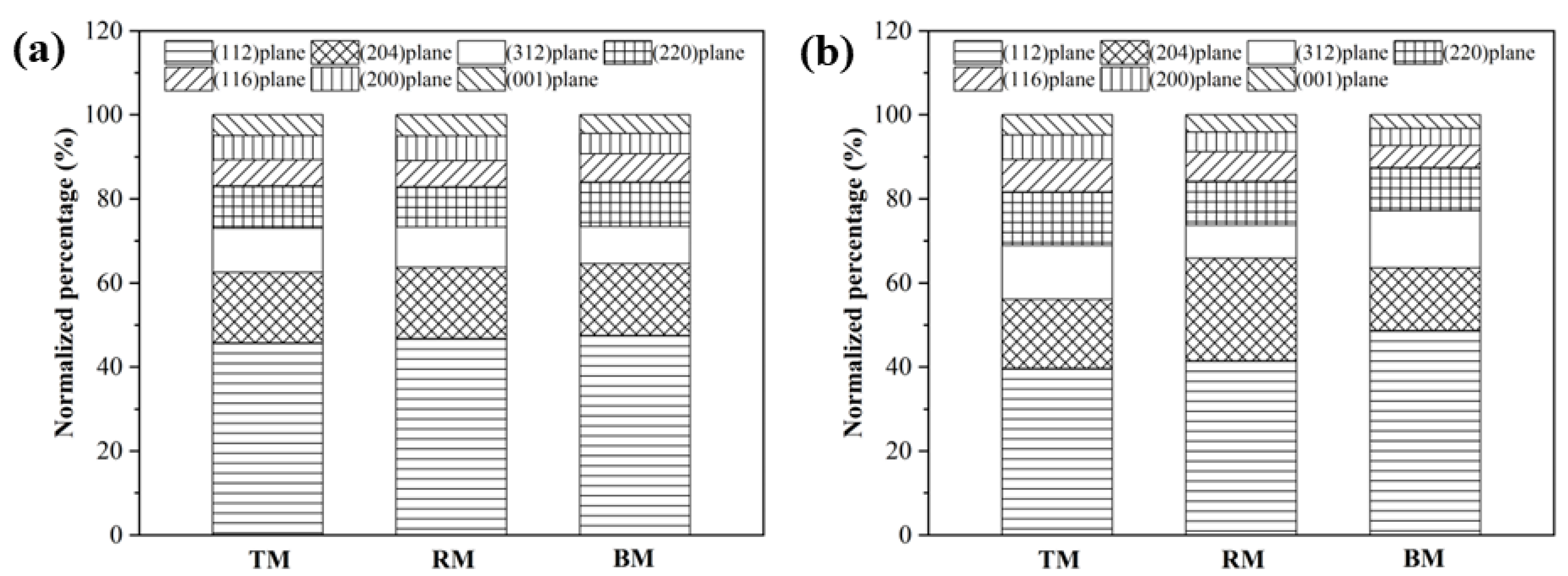
3.2. Anisotropy of CuFeS2 Raw Samples
3.3. XPS Analysis
3.4. The Properties of Anisotropic Crystal Planes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jena, S.S.; Tripathy, S.K.; Mandre, N.R.; Venugopal, R.; Farrokhpay, S. Sustainable use of copper resources: Beneficiation of low-Grade copper ores. Minerals 2022, 12, 545. [Google Scholar]
- Dutrizac, J.E. The kinetics of dissolution of chalcopyrite in ferric ion media. Metall. Trans. B 1978, 9, 431–439. [Google Scholar]
- Córdoba, E.M.; Muoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar]
- Li, W.Q.; Li, Y.B.; Wang, Z.H.; Yang, X.; Chen, W. Selective flotation of chalcopyrite from pyrite via seawater oxidation pretreatment. Int. J. Min. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Li, Y.B.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar]
- Moskalyk, R.R.; Alfantazi, A.M. Review of copper pyrometallurgical practice: Today and tomorrow. Miner. Eng. 2003, 16, 893–919. [Google Scholar]
- Dimitrijević, M.; Kostov, A.; Tasić, V.; Milosević, N. Influence of pyrometallurgical copper production on the environment. J. Hazard. Mater. 2009, 164, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Savić, M.; Mihajlović, I.; Živković, Ž. An anfis–based air quality model for prediction of SO2 concentration in urban area. Serbian J. Manag. 2013, 8, 25–38. [Google Scholar]
- Khoshkhoo, M.; Dopson, M.; Shchukarev, A.; Sandström, Å. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 2014, 149, 220–227. [Google Scholar]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 2014, 146, 96–110. [Google Scholar]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the galvanox™ process. Hydrometallurgy 2012, 113–114, 122–130. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Pan, H.D.; Yang, H.Y.; Tong, L.L.; Zhong, C.B.; Zhao, Y.S. Control method of chalcopyrite passivation in bioleaching. Trans. Nonferrous Met. Soc. China 2012, 22, 2255–2260. [Google Scholar]
- Tshilombo, A. Mechanism and Kinetics of Chalcopyrite Passivation and Depassivation during Ferric and Microbial Leaching. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2004. [Google Scholar]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.R.; van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Harmer, S.L.; Pratt, A.R.; Nesbitt, W.H.; Fleet, M.E. Sulfur species at chalcopyrite (CuFeS2) fracture surfaces. Am. Mineral. 2004, 89, 1026–1032. [Google Scholar] [CrossRef]
- Klauber, C.; Parker, A.; van Bronswijk, W.; Watling, H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Passivation of chalcopyrite during its chemical leaching with ferric ion at 68 °C. Miner. Eng. 2009, 22, 229–235. [Google Scholar] [CrossRef]
- Viramontes-Gamboa, G.; Peña-Gomar, M.M.; Dixon, D.G. Electrochemical hysteresis and bistability in chalcopyrite passivation. Hydrometallurgy 2010, 105, 140–147. [Google Scholar] [CrossRef]
- Li, J.; Kawashima, N.; Kaplun, K.; Absolon, V.J.; Gerson, A.R. Chalcopyrite leaching: The rate controlling factors. Geochim. Cosmochim. Acta 2010, 74, 2881–2893. [Google Scholar] [CrossRef]
- Parker, G.K.; Woods, R.; Hope, G.A. Raman investigation of chalcopyrite oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 160–168. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Ehrig, K. Ore minerals down to the nanoscale: Cu-(Fe)-sulphides from the iron oxide copper gold deposit at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 81, 1218–1235. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Engström, F.; Sandström, Å. New insights into the influence of redox potential on chalcopyrite leaching behaviour. Miner. Eng. 2017, 100, 9–16. [Google Scholar] [CrossRef]
- Wei, Z.L.; Li, Y.B.; Huang, L.Y. New insight into the anisotropic property and wettability of molybdenite: A DFT study. Miner. Eng. 2021, 170, 107058. [Google Scholar] [CrossRef]
- Wei, Z.L.; Li, Y.B.; Gao, H.M.; Zhu, Y.G.; Qian, G.J.; Yao, J. New insights into the surface relaxation and oxidation of chalcopyrite exposed to O2 and H2O: A first-principles DFT study. Appl. Surf. Sci. 2019, 492, 89–98. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.S.T.; Eng, P.J.; Osseo-Asare, K. Applications of in situ synchrotron XRD in hydrometallurgy: Literature review and investigation of chalcopyrite dissolution. Hydrometallurgy 2013, 131, 54–66. [Google Scholar] [CrossRef]
- Xie, H.Y.; Su, X.L.; Zheng, G.; Yan, Y.G.; Liu, W.; Tang, H.; Kanatzidis, M.G.; Uher, C.; Tang, X.F. Nonmagnetic in substituted CuFe1–xInxS2 solid solution thermoelectric. J. Phys. Chem. C 2016, 120, 27895–27902. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.G.; Lv, X.; Tian, Y.; Yang, L.; Zou, G.Q.; Hou, H.S.; Zhao, H.B.; Ji, X.B. Exploration and size engineering from natural chalcopyrite to high-performance electrode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 6154–6165. [Google Scholar] [CrossRef]
- de Oliveira, C.; de Lima, G.F.; de Abreu, H.A.; Duarte, H.A. Reconstruction of the chalcopyrite surfaces—A DFT study. J. Phys. Chem. C 2012, 116, 6357–6366. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. Xrd-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Hu, Y.H.; Gao, Z.Y.; Sun, W.; Liu, X.W. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 439–448. [Google Scholar] [CrossRef]
- de Lima, G.F.; de Oliveira, C.; de Abreu, H.A.; Duarte, H.A. Sulfuric and hydrochloric acid adsorption on the reconstructed sulfur terminated (001) chalcopyrite surface. Int. J. Quantum Chem. 2012, 112, 3216–3222. [Google Scholar] [CrossRef]
- Sokic, M.D.; Markovic, B.; Zivkovic, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Li, C.W.; Sun, W.; Hu, Y.H. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Moimane, T.; Plackowski, C.; Peng, Y.J. The critical degree of mineral surface oxidation in copper sulphide flotation. Miner. Eng. 2020, 145, 106075. [Google Scholar] [CrossRef]
- Li, Y.B.; Chandra, A.P.; Gerson, A.R. Scanning photoelectron microscopy studies of freshly fractured chalcopyrite exposed to O2 and H2O. Geochim. Cosmochim. Acta 2014, 133, 372–386. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, W.; Ji, Z.; Zhou, C.G.; Yuan, S.H. Mechanisms of hydroxyl radicals production from pyrite oxidation by hydrogen peroxide: Surface versus aqueous reactions. Geochim. Cosmochim. Acta 2018, 238, 394–410. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.H.; Lu, F.; Gao, Y.; Liu, R.Q.; Sun, W. Xanthate interaction and flotation separation of H2O2-treated chalcopyrite and pyrite. Trans. Nonferrous Met. Soc. China 2019, 29, 2604–2614. [Google Scholar] [CrossRef]
- Li, Y.B.; Wei, Z.L.; Xiao, Q.; Gao, H.M.; Song, S.X. A fundamental DFT study of chalcopyrite surface evolution due to impurity divalent ions during leaching process. Miner. Eng. 2018, 121, 205–211. [Google Scholar] [CrossRef]
- Silva, J.C.M.; Abreu, H.A.D.; Duarte, H.A. Electronic and structural properties of bulk arsenopyrite and its cleavage surfaces—A DFT study. Rsc Adv. 2014, 5, 2013–2023. [Google Scholar] [CrossRef]
- Hung, A.; Muscat, J.; Yarovsky, I.; Russo, S.P. Density-functional theory studies of pyrite FeS2 (100) and (110) surfaces. Surf. Sci. 2002, 513, 511–524. [Google Scholar] [CrossRef]
- Hung, A.; Muscat, J.; Yarovsky, I.; Russo, S.P. Density-functional theory studies of pyrite FeS2 (111) and (210) surfaces. Surf. Sci. 2002, 520, 111–119. [Google Scholar] [CrossRef]
- de Lima, G.F.; de Oliveira, C.; de Abreu, H.A.; Duarte, H.A. Water adsorption on the reconstructed (001) chalcopyrite surfaces. J. Phys. Chem. C 2011, 115, 10709–10717. [Google Scholar] [CrossRef]
- Xiong, X.L.; Hua, X.M.; Zheng, Y.F.; Lu, X.G.; Li, S.G.; Cheng, H.W.; Xu, Q. Oxidation mechanism of chalcopyrite revealed by X-ray photoelectron spectroscopy and first principles studies. Appl. Surf. Sci. 2018, 427, 233–241. [Google Scholar] [CrossRef]



| Grinding Methods | Average Length (μm) | Average Width (μm) | Length–Width Ratio |
|---|---|---|---|
| TM | 127.762 | 71.953 | 1.776 |
| RM | 124.724 | 70.917 | 1.759 |
| BM | 95.234 | 73.276 | 1.299 |
| Element | Bonding Energy (eV) | CuFeS2 | ||
|---|---|---|---|---|
| BM | RM | TM | ||
| S 2p | 161.4 | 9.26 | 4.93 | 8.08 |
| O 1s | 532.1 | 43.87 | 53.37 | 70.40 |
| Fe 2p | 710.8 | 8.56 | 9.48 | 4.88 |
| Cu 2p | 932.6 | 38.31 | 32.22 | 16.64 |
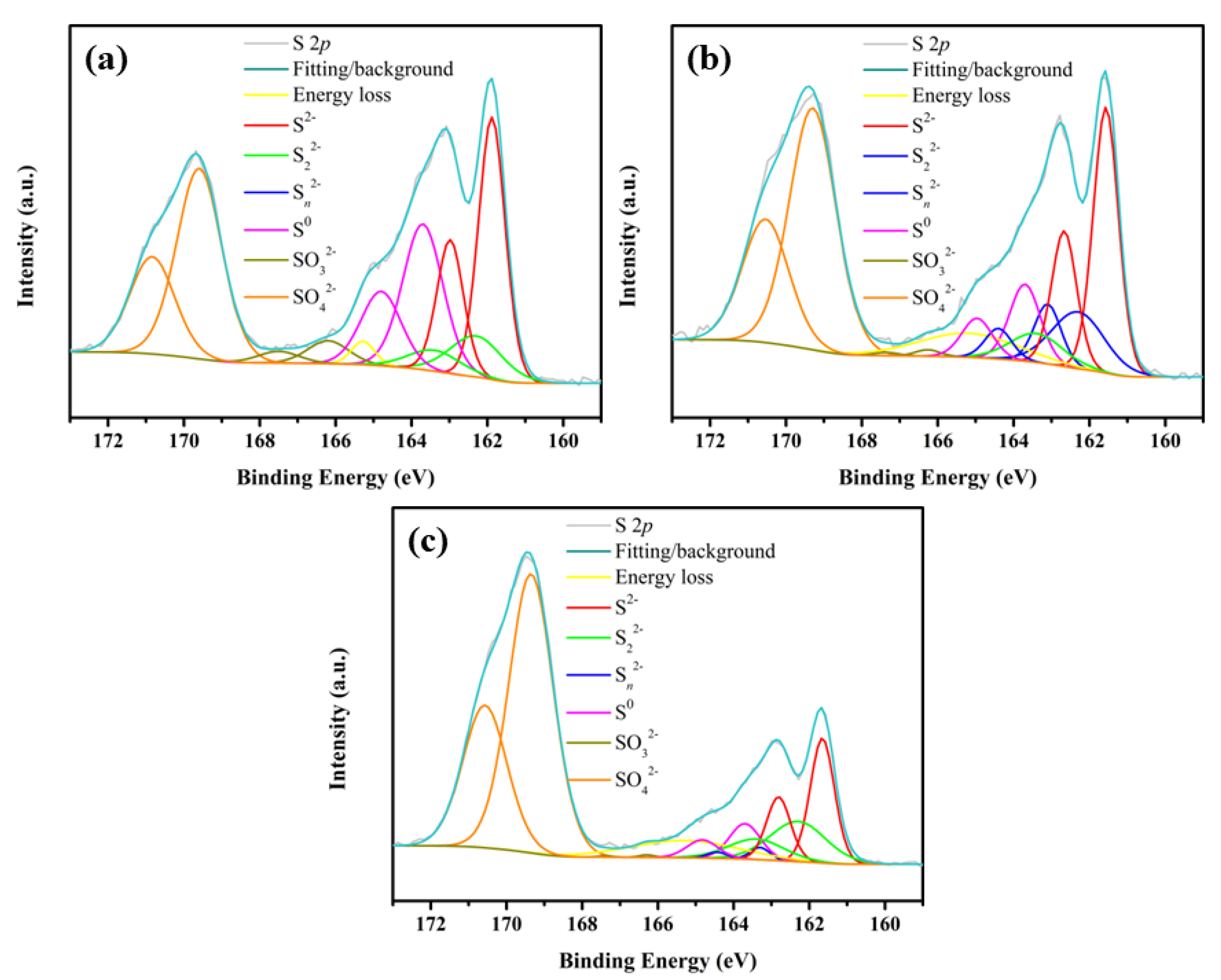
| S Species | Bonding Energy (eV) | Full Width at Half-Maximum (eV) | CuFeS2 | ||
|---|---|---|---|---|---|
| BM | RM | TM | |||
| S2− | 161.4 | 0.7–0.8 | 27.98 | 23.01 | 14.87 |
| S22− | 162.2 | 0.7–0.9 | 8.60 | 10.32 | 11.07 |
| Sn2− | 163.2 | 1.1–1.3 | 0 | 5.50 | 1.19 |
| S0 | 163.7 | 1.0–1.2 | 23.57 | 8.12 | 5.44 |
| SO32− | 166.3 | 1.1–1.3 | 3.81 | 0.54 | 0.20 |
| SO42− | 168.8 | 1.5–1.6 | 34.59 | 39.02 | 61.14 |
| Energy loss | 165.1 | 1.4–1.7 | 1.46 | 5.37 | 6.10 |
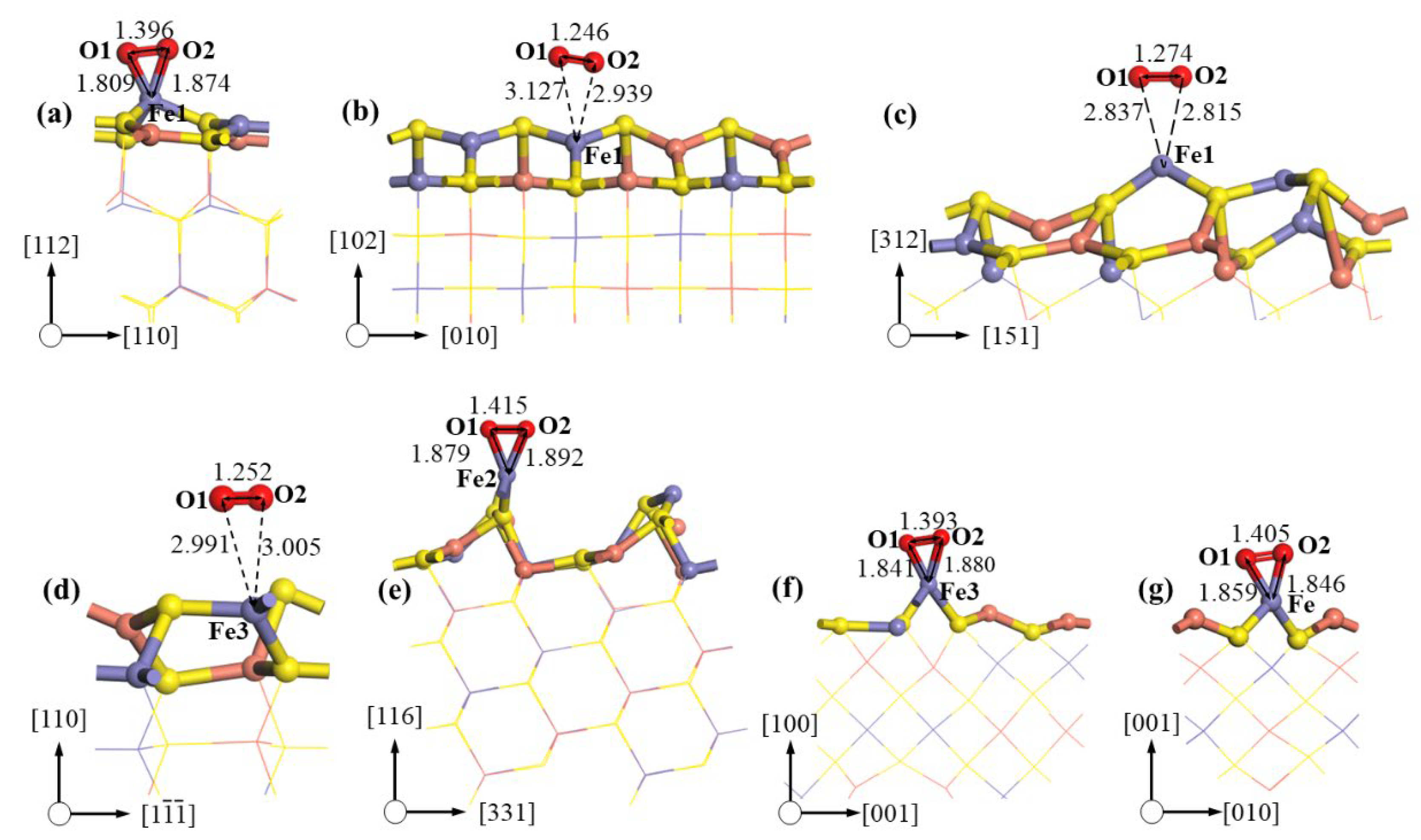
| Planes | Area (nm2) | Surface Energy, Es (J·m−2) | Adsorption Site | Adsorption Energy per Unit Area (kJ·mol−1·nm−2) |
|---|---|---|---|---|
| 112-S | 0.473 | 0.923 a | S1 | −437.384 |
| 112-M | 0.473 | 1.018 a | Fe1 | −1316.631 |
| 102 | 0.774 | 1.128 a | Fe1 | −1372.095 |
| S3 | −470.755 | |||
| 312-M | 1.874 | 1.207 a | Fe1 | −1571.115 |
| 312-S | 1.874 | 1.221 | S2 | −477.523 |
| 110 | 0.408 | 1.332 | Fe3 | −1633.777 |
| S1 | −461.095 | |||
| 116-S | 0.909 | 1.351 | S2 | −527.396 |
| 116-M | 0.909 | 1.494 | Fe2 | −1654.440 |
| 100-S | 0.545 | 1.450 | S3 | −588.869 |
| 100-M | 0.545 | 1.498 | Fe3 | −1858.123 |
| 001-S | 0.274 | 1.495 a | S2 | −690.193 |
| 001-M | 0.274 | 1.566 a | Fe | −2027.415 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Yang, X.; Li, W.; Ma, Q.; Wu, X.; Li, Y. An Improved Understanding of Chalcopyrite Leaching Mechanisms: The Influence of Anisotropic Crystal Planes. Minerals 2023, 13, 1461. https://doi.org/10.3390/min13111461
Wei Z, Yang X, Li W, Ma Q, Wu X, Li Y. An Improved Understanding of Chalcopyrite Leaching Mechanisms: The Influence of Anisotropic Crystal Planes. Minerals. 2023; 13(11):1461. https://doi.org/10.3390/min13111461
Chicago/Turabian StyleWei, Zhenlun, Xu Yang, Wanqing Li, Qiang Ma, Xiaoyong Wu, and Yubiao Li. 2023. "An Improved Understanding of Chalcopyrite Leaching Mechanisms: The Influence of Anisotropic Crystal Planes" Minerals 13, no. 11: 1461. https://doi.org/10.3390/min13111461