Enrichment Characteristics and Mechanisms of Critical Metals in Marine Fe-Mn Crusts and Nodules: A Review
Abstract
:1. Introduction
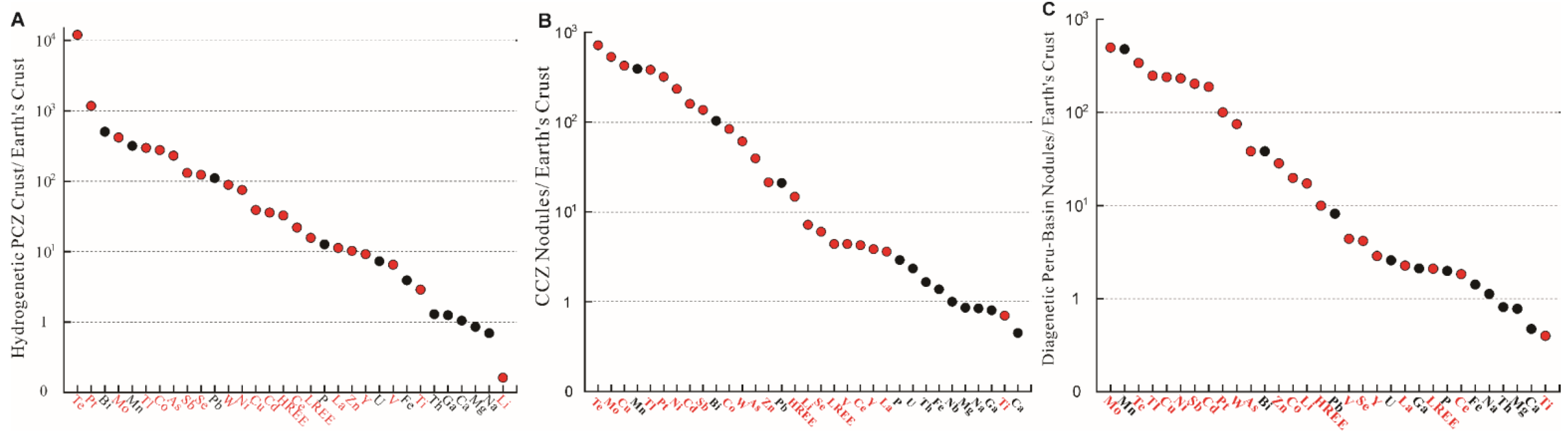
2. Classification of Critical Metals
3. Influencing Factors of Critical Metal Uptake
3.1. Electro-Charge Preference
3.2. Growth Rate
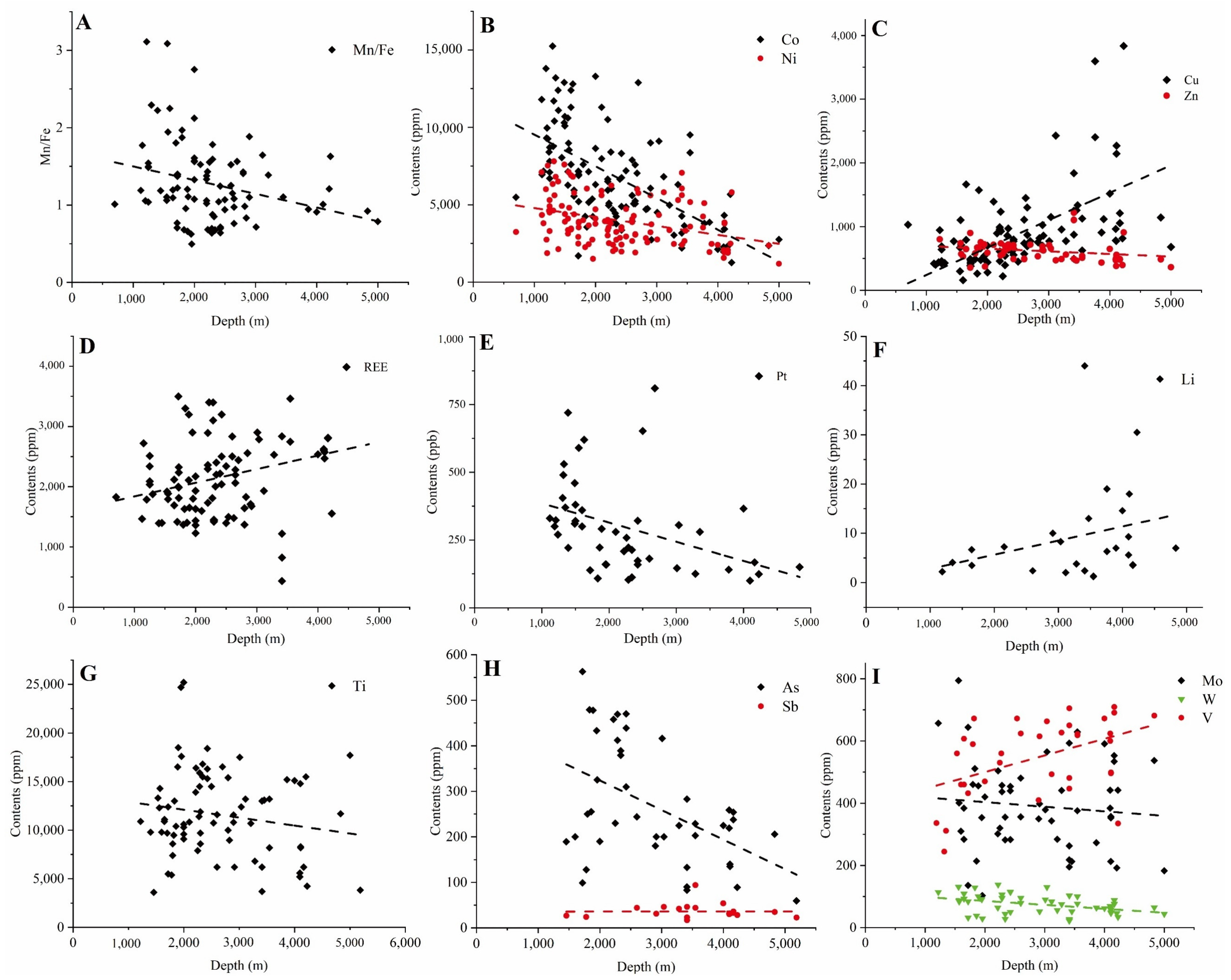
3.3. Water Depth
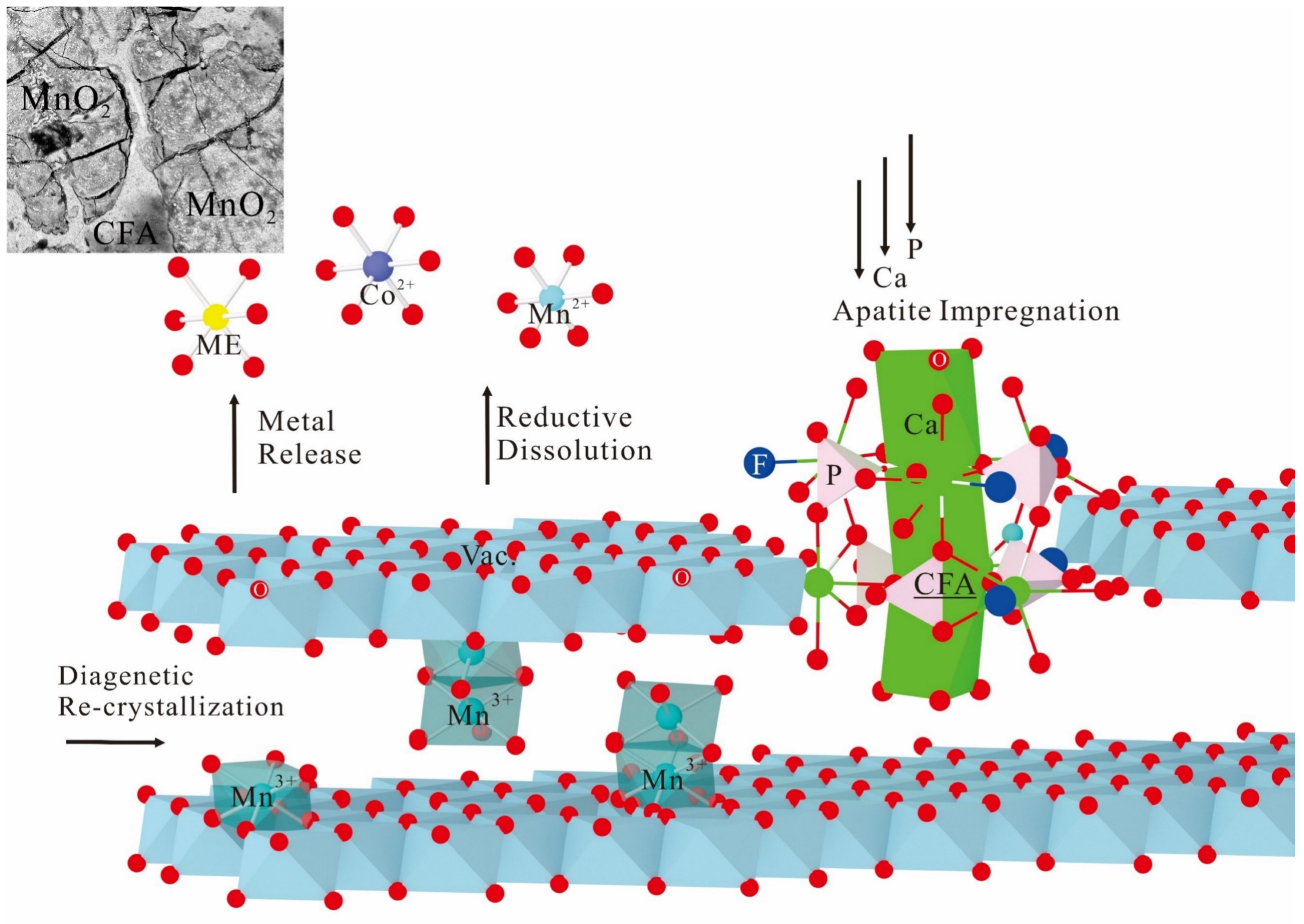
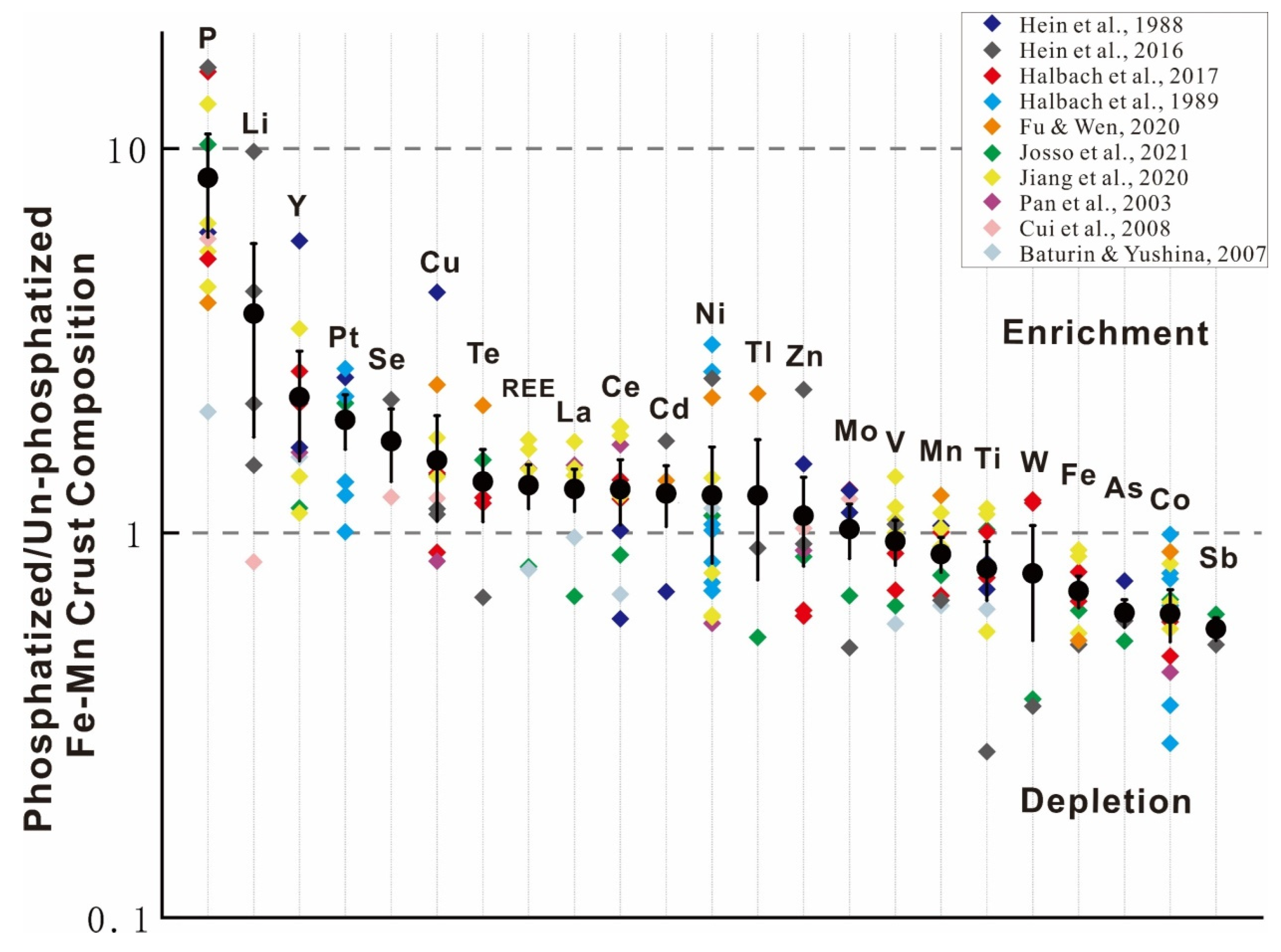
3.4. Post-Depositional Phosphatization
3.5. Structural Uptake
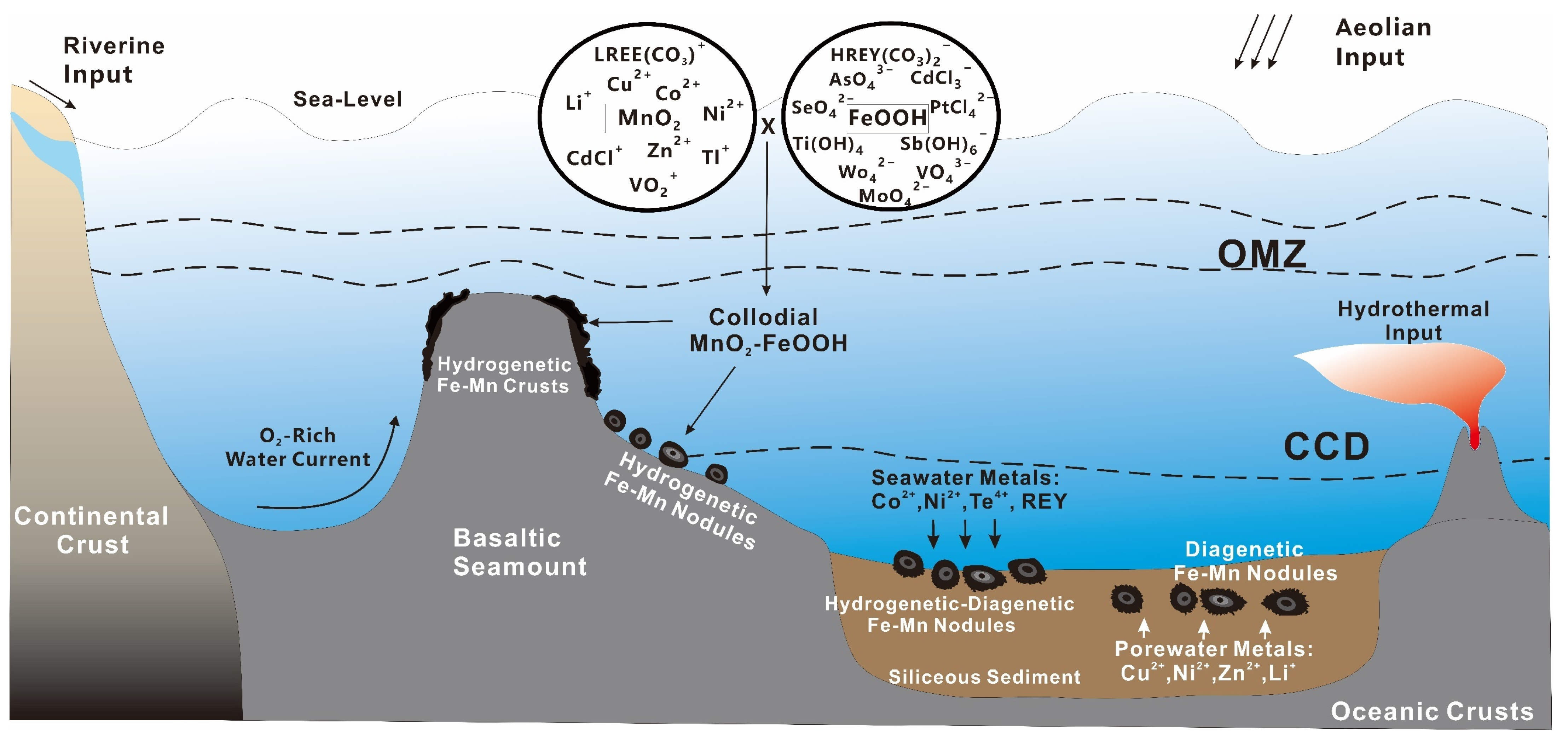
4. Categorization of Critical Metal Enrichment Pathways and Coordination
5. Elaboration of Critical Metal Enrichment Characteristics and Mechanisms
5.1. Major Ore-Forming Metals: Cobalt, Nickel, Copper and Zinc
5.1.1. Cobalt and Nickel
5.1.2. Copper and Zinc
5.2. Rare Earth Elements and Yttrium (REY)
5.3. Platinum Group Elements
5.4. Dispersed Elements
5.4.1. Tellurium
5.4.2. Thallium
5.4.3. Selenium
5.4.4. Cadmium
5.5. Other Critical Elements
5.5.1. Lithium
5.5.2. Molybdenum, Tungsten and Vanadium
5.5.3. Titanium
5.5.4. Antimony and Arsenic
6. Conclusions and Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusty, P.A.J.; Murton, B.J. Deep-Ocean Mineral Deposits: Metal Resources and Windows into Earth Processes. Elements 2018, 14, 301–306. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–291. [Google Scholar]
- Hein, J.R.; Koschinsky, A.; Kuhn, T. Deep-ocean polymetallic nodules as a resource for critical materials. Nat. Rev. Earth Environ. 2020, 1, 158–169. [Google Scholar] [CrossRef]
- Halbach, P.; Jahn, A.; Cherkashov, G. Marine Co-Rich Ferromanganese Crust Deposits: Description and Formation, Occurrences and Distribution, Estimated World-Wide Resources; Springer: Cham, Switzerland, 2017; pp. 65–141. [Google Scholar]
- Kuhn, T.; Wegorzewski, A.; Ruehlemann, C.; Vink, A. Composition, Formation, and Occurrence of Polymetallic Nodules; Springer: Cham, Switzerland, 2017; pp. 23–63. [Google Scholar]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Halbach, P. Processes controlling the heavy-metal distribution in pacific ferromanganese nodules and crusts. Geol. Rundsch. 1986, 75, 235–247. [Google Scholar] [CrossRef]
- Marino, E.; Gonzalez, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrion, M.; Bellido, E.; Somoza, L. High-Resolution Analysis of Critical Minerals and Elements in Fe-Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.; Kang, J.-K.; Roberts, L. Cobalt-Rich Ferromanganese Crusts in the Pacific; CRC Press: Boca Raton, FL, USA, 2000; Volume 17, pp. 239–279. [Google Scholar]
- Sutherland, K.M.; Wankel, S.D.; Hein, J.R.; Hansel, C.M. Spectroscopic Insights Into Ferromanganese Crust Formation and Diagenesis. Geochem. Geophys. Geosystems 2020, 21, e2020GC009074. [Google Scholar] [CrossRef]
- Li, Y.H.; Schoonmaker, J.E. Chemical Composition and Mineralogy of Marine Sediments. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–32. [Google Scholar]
- Manceau, A.; Lanson, M.; Takahashi, Y. Mineralogy and crystal chemistry of Mn, Fe, Co, Ni, and Cu in a deep-sea Pacific polymetallic nodule. Am. Mineral. 2014, 99, 2068–2083. [Google Scholar] [CrossRef]
- Kwon, K.D.; Refson, K.; Sposito, G. Understanding the trends in transition metal sorption by vacancy sites in birnessite. Geochim. Et Cosmochim. Acta 2013, 101, 222–232. [Google Scholar] [CrossRef]
- Bodei, S.; Manceau, A.; Geoffroy, N.; Baronnet, A.; Buatier, M. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments. Geochim. Et Cosmochim. Acta 2007, 71, 5698–5716. [Google Scholar] [CrossRef]
- Manceau, A.; Drits, V.A.; Silvester, E.; Bartoli, C.; Lanson, B. Structural mechanism of Co2+ oxidation by the phyllomanganate buserite. Am. Mineral. 1997, 82, 1150–1175. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H.F.; Xu, W.Q.; Sun, X.M. The structure and crystal chemistry of vernadite in ferromanganese crusts. Acta Crystallogr. Sect. B-Struct. Sci. Cryst. Eng. Mater. 2019, 75, 591–598. [Google Scholar] [CrossRef]
- U.S.G.S. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023.
- Wang, D. Study on critical mineral resources: Significance of research, determination of types, attributes of resources, progress of prospecting, problems of utilization, and direction of exploitation. Acta Geol. Sin. 2019, 93, 1189–1209. [Google Scholar]
- U.S. Department of Commerce. A Federal Strategy to Ensure Secure and Reliable Supplies of Critical Minerals; U.S. Department of Commerce: Washington, DC, USA, 2019.
- Takahashi, Y.; Manceau, A.; Geoffroy, N.; Marcus, M.A.; Usui, A. Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganese oxides. Geochim. Et Cosmochim. Acta 2007, 71, 984–1008. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Crystal-chemistry of Ni in marine ferromanganese crusts and nodules. Am. Mineral. 2007, 92, 1087–1092. [Google Scholar] [CrossRef]
- Usui, A. Nickel and copper accumulation as essential elements in 10-Å manganite of deep-sea manganese nodules. Nature 1979, 279, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Elderfield, H.; Hawkesworth, C.J.; Greaves, M.J.; Calvert, S.E. Rare-earth element geochemistry of oceanic ferromanganese nodules and associated sediments. Geochim. Et Cosmochim. Acta 1981, 45, 513–528. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. REE(III) adsorption onto Mn dioxide (δ-MnO2) and Fe oxyhydroxide: Ce(III) oxidation by δ-MnO2. Geochim. Et Cosmochim. Acta 2001, 65, 695–703. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A.; Dulski, P.; Hein, J.R. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Et Cosmochim. Acta 1996, 60, 1709–1725. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halliday, A.N. Global occurrence of tellurium-rich ferromanganese crusts and a model for the enrichment of tellurium. Geochim. Et Cosmochim. Acta 2003, 67, 1117–1127. [Google Scholar] [CrossRef]
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Strategic and rare elements in Cretaceous-Cenozoic cobalt-rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (northeastern tropical Atlantic). Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]
- Hein, J.R.; Konstantinova, N.; Mikesell, M.; Mizell, K.; Fitzsimmons, J.N.; Lam, P.J.; Jensen, L.T.; Xiang, Y.; Gartman, A.; Cherkashov, G.; et al. Arctic Deep Water Ferromanganese-Oxide Deposits Reflect the Unique Characteristics of the Arctic Ocean. Geochem. Geophys. Geosystems 2017, 18, 3771–3800. [Google Scholar] [CrossRef]
- Muiños, S.B.; Hein, J.R.; Frank, M.; Monteiro, J.H.; Gaspar, L.; Conrad, T.; Pereira, H.G.; Abrantes, F. Deep-sea Fe-Mn Crusts from the Northeast Atlantic Ocean: Composition and Resource Considerations. Mar. Georesources Geotechnol. 2013, 31, 40–70. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R.; Kraemer, D.; Foster, A.L.; Kuhn, T.; Halbach, P. Platinum enrichment and phase associations in marine ferromanganese crusts and nodules based on a multi-method approach. Chem. Geol. 2020, 539, 119426. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Et Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Oishi, Y.; Sakaguchi, A.; Sugiyama, T.; Usui, A.; Takahashi, Y. Chemical processes for the extreme enrichment of tellurium into marine ferromanganese oxides. Geochim. Et Cosmochim. Acta 2014, 131, 150–163. [Google Scholar] [CrossRef]
- Kuhn, T.; Bostick, B.C.; Koschinsky, A.; Halbach, P.; Fendorf, S. Enrichment of Mo in hydrothermal Mn precipitates: Possible Mo sources, formation process and phase associations. Chem. Geol. 2003, 199, 29–43. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Grangeon, S.; Webb, S.M.; Heller, C.; Kuhn, T. Mineralogical transformations in polymetallic nodules and the change of Ni, Cu and Co crystal -chemistry upon burial in sediments. Geochim. Et Cosmochim. Acta 2020, 282, 19–37. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Marcus, M.A.; Uruga, T.; Tanida, H.; Terada, Y.; Usui, A. Tungsten species in natural ferromanganese oxides related to its different behavior from molybdenum in oxic ocean. Geochim. Et Cosmochim. Acta 2013, 106, 364–378. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, X.; Yao, D.; Zhai, S.; Guo, W. Geochemistry of lithium in marine ferromanganese oxide deposits. Deep. -Sea Res. Part I-Oceanogr. Res. Pap. 2007, 54, 85–98. [Google Scholar] [CrossRef]
- Toro, N.; Robles, P.; Jeldres, R.I. Seabed mineral resources, an alternative for the future of renewable energy: A critical review. Ore Geol. Rev. 2020, 126, 103699. [Google Scholar] [CrossRef]
- Lusty, P.A.J.; Hein, J.R.; Josso, P. Formation and occurrence of Ferromanganese Crusts: Earth’s Storehouse for Critical Metals. Elements 2018, 14, 313–318. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.A.; Staudigel, H. Seamount mineral deposits: A source of rare metals for high-technology industries. Oceanography 2010, 23, 184–189. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Verlaan, P.A.; Cronan, D.S.; Morgan, C.L. A comparative analysis of compositional variations in and between marine ferromanganese nodules and crusts in the South Pacific and their environmental controls. Prog. Oceanogr. 2004, 63, 125–158. [Google Scholar] [CrossRef]
- Verlaan, P.A.; Cronan, D.S. Origin and variability of resource-grade marine ferromanganese nodules and crusts in the Pacific Ocean: A review of biogeochemical and physical controls. Geochemistry 2022, 82, 125741. [Google Scholar] [CrossRef]
- Usui, A.; Terashima, S. Deposition of hydrogenetic and hydrothermal manganese minerals in the Ogasawara (Bonin) Arc Area, Northwest Pacific. Mar. Georesources Geotechnol. 1997, 15, 127–154. [Google Scholar] [CrossRef]
- Surya Prakash, L.; Ray, D.; Paropkari, A.L.; Mudholkar, A.V.; Satyanarayanan, M.; Sreenivas, B.; Chandrasekharam, D.; Kota, D.; Kamesh Raju, K.A.; Kaisary, S.; et al. Distribution of REEs and yttrium among major geochemical phases of marine Fe–Mn-oxides: Comparative study between hydrogenous and hydrothermal deposits. Chem. Geol. 2012, 312–313, 127–137. [Google Scholar] [CrossRef]
- Kuhn, T.; Bau, M.; Blum, N.; Halbach, P. Origin of negative Ce anomalies in mixed hydrothermal-hydrogenetic Fe-Mn crusts from the Central Indian Ridge. Earth Planet. Sci. Lett. 1998, 163, 207–220. [Google Scholar] [CrossRef]
- Mikhailik, P.E.; Mikhailik, E.V.; Zarubina, N.V.; Blokhin, M.G. Distribution of rare-earth elements and yttrium in hydrothermal sedimentary ferromanganese crusts of the Sea of Japan. Russ. Geol. Geophys. 2017, 58, 1530–1542. [Google Scholar] [CrossRef]
- Pelleter, E.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Labanieh, S.; Josso, P.; Bollinger, C.; Langlade, J. Ni-Cu-Co-rich hydrothermal manganese mineralization in the Wallis and Futuna back-arc environment (SW Pacific). Ore Geol. Rev. 2017, 87, 126–146. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The Composition Of The Continental-Crust. Geochim. Et Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Fortier, S.M.; Nassar, N.T.; Lederer, G.W.; Brainard, J.; Gambogi, J.; McCullough, E.A. Draft Critical Mineral List—Summary of Methodology and Background Information—U.S. Geological Survey Technical input Document in Response to Secretarial Order No. 3359; Open-File Report 2018-1021; U.S. Geological Survey: Reston, VA, USA, 2018; p. 26. [Google Scholar]
- Gulley, A.L.; Nassar, N.T.; Xun, S. China, the United States, and competition for resources that enable emerging technologies. Proc. Natl. Acad. Sci. USA 2018, 115, 4111–4115. [Google Scholar] [CrossRef]
- Industrie, D.E.E.; Isi, F. Critical Raw Materials for the EU—Report of the Ad-hoc Working Group on Defining Critical Raw Materials; European Commission: Ispra, Italy, 2010. [Google Scholar]
- Sovacool, B.K.; Ali, S.H.; Bazilian, M.; Radley, B.; Nemery, B.; Okatz, J.; Mulvaney, D. Sustainable minerals and metals for a low-carbon future. Science 2020, 367, 30. [Google Scholar] [CrossRef]
- Nassar, N.T.; Graedel, T.E.; Harper, E.M. By-product metals are technologically essential but have problematic supply. Sci. Adv. 2015, 1, e1400180. [Google Scholar] [CrossRef]
- Tu, G.C.; Gao, Z.M. Ore-forming mechanism of the Dispersed Elements. Bull. Chin. Acad. Sci. 2003, 5, 358–361. [Google Scholar] [CrossRef]
- Tu, G.C.; Gao, Z.M.; Hu, R.Z.; Zhang, Q.; Li, C.Y.; Zhao, Z.H.; Zhang, B.G. Geochemistry and Mineralization of the Dispersed Elements; Science Press: Beijing, China, 2003. [Google Scholar]
- Hodkinson, R.A.; Cronan, D.S. Regional and depth variability in the composition of cobalt-rich ferromanganese crusts from the SOPAC area and adjacent parts of the central equatorial Pacific. Mar. Geol. 1991, 98, 437–447. [Google Scholar] [CrossRef]
- Mohwinkel, D.; Kleint, C.; Koschinsky, A. Phase associations and potential selective extraction methods for selected high-tech metals from ferromanganese nodules and crusts with siderophores. Appl. Geochem. 2014, 43, 13–21. [Google Scholar] [CrossRef]
- Byrne, R.H. Inorganic speciation of dissolved elements in seawater: The influence of pH on concentration ratios. Geochem. Trans. 2002, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Maeno, M.Y.; Ohashi, H.; Yonezu, K.; Miyazaki, A.; Okaue, Y.; Watanabe, K.; Ishida, T.; Tokunaga, M.; Yokoyama, T. Sorption behavior of the Pt(II) complex anion on manganese dioxide (δ-MnO2): A model reaction to elucidate the mechanism by which Pt is concentrated into a marine ferromanganese crust. Miner. Depos. 2016, 51, 211–218. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Feng, X.H.; Lee, S.S.; Reinhart, B.; Elzinga, E.J. Sorption and oxidation of Co(II) at the surface of birnessite: Impacts of aqueous Mn(II). Chem. Geol. 2023, 618, 121281. [Google Scholar] [CrossRef]
- Zhu, H.W.; Xiao, X.Y.; Guo, Z.H.; Peng, C.; Wang, X.H.; Yang, A.D. Characteristics and behaviour of vanadium(V) adsorption on goethite and birnessite. Environ. Earth Sci. 2020, 79, 240. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Takahashi, Y.; Terada, Y.; Sakata, M. Antimony(V) Incorporation into Synthetic Ferrihydrite, Goethite, and Natural Iron Oxyhydroxides. Environ. Sci. Technol. 2010, 44, 3712–3718. [Google Scholar] [CrossRef]
- Takematsu, N.; Sato, Y.; Okabe, S. Factors controlling the chemical-composition of marine manganese nodules and crusts—A review and synthesis. Mar. Chem. 1989, 26, 41–56. [Google Scholar] [CrossRef]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Tokumaru, A.; Sakaguchi, A.; Yamaoka, K.; Kato, S.; et al. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Pacific, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef]
- Halbach, P.; Marbler, H. Marine ferromanganese crusts: Contents, distribution and enrichment of strategic minor and trace elements. In BGR-Report; BGR: Berlin, Germany, 2009; pp. 1–73. [Google Scholar]
- Halbach, P.; Segl, M.; Puteanus, D.; Mangini, A. Co-fluxes and growth-rates in ferromanganese deposits from central pacific seamount areas. Nature 1983, 304, 716–719. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimizu, H.; Usui, A.; Kagi, H.; Nomura, M. Direct observation of tetravalent cerium in ferromanganese nodules and crusts by X-ray-absorption near-edge structure (XANES). Geochim. Et Cosmochim. Acta 2000, 64, 2929–2935. [Google Scholar] [CrossRef]
- Manheim, F.T.; Lanebostwick, C.M. Cobalt in ferromanganese crusts as a monitor of hydrothermal discharge on the Pacific sea-floor. Nature 1988, 335, 59–62. [Google Scholar] [CrossRef]
- Puteanus, D.; Halbach, P. Correlation of co concentration and growth-rate—A method for age-determination of ferromanganese crusts. Chem. Geol. 1988, 69, 73–85. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Wen, H.J. Variabilities and enrichment mechanisms of the dispersed elements in marine Fe–Mn deposits from the Pacific Ocean. Ore Geol. Rev. 2020, 121, 103470. [Google Scholar] [CrossRef]
- Conrad, T.; Hein, J.R.; Paytan, A.; Clague, D.A. Formation of Fe-Mn crusts within a continental margin environment. Ore Geol. Rev. 2017, 87, 25–40. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.M.; Ren, Y.Z.; Jiang, X.D. Mineralogy, geochemistry and genesis of the polymetallic crusts and nodules from the South China Sea. Ore Geol. Rev. 2017, 89, 206–227. [Google Scholar] [CrossRef]
- Ivanova, Y.M.; Mikhailik, P.E.; Mikhailik, E.V.; Zarubina, N.V.; Blokhin, M.G. Chemical Composition and Genesis of Ferromanganese Crusts from the Sonne Ridge (Kuril Basin, Sea of Okhotsk). Russ. Geol. Geophys. 2019, 60, 1026–1042. [Google Scholar] [CrossRef]
- Douville, E.; Bienvenu, P.; Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Appriou, P.; Gamo, T. Yttrium and rare earth elements in fluids from various deep-sea hydrothermal systems. Geochim. Et Cosmochim. Acta 1999, 63, 627–643. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Song, W.; Sun, Z.; Huang, W.; Hu, G.; Yuan, X.; Kao, S.-J. High-Resolution Analysis of Fe-Mn Oxyhydroxide in Ferromanganese Nodules from the Northwestern Pacific Ocean and Insights on Element Mobility. Minerals 2023, 13, 415. [Google Scholar] [CrossRef]
- Ren, J.B.; He, G.W.; Deng, X.G.; Deng, X.Z.; Yang, Y.; Yao, H.Q.; Yang, S.X. Metallogenesis of Co-rich ferromanganese nodules in the northwestern Pacific: Selective enrichment of metallic elements from seawater. Ore Geol. Rev. 2022, 143, 104778. [Google Scholar] [CrossRef]
- Mikhailik, P.E.; Khanchuk, A.I.; Mikhailik, E.V.; Rashidov, V.A.; Savelyev, D.P.; Zarubina, N.V. Ferromanganese Crusts of the North Pacific Ocean. Russ. J. Pac. Geol. 2023, 17, 101–133. [Google Scholar] [CrossRef]
- Yi, L.; Li, Y.; Mikhailik, P.; Qi, Y.; Deng, C. Magnetic and geochemical scanning reveals growth history of marine ferromanganese crust on Detroit Seamount, northwest Pacific since the early Miocene. Quat. Int. 2023, 671, 52–61. [Google Scholar] [CrossRef]
- Halbach, P.; Puteanus, D. The influence of the carbonate dissolution rate on the growth and composition of Co-rich ferromanganese crusts from Central Pacific seamount areas. Earth Planet. Sci. Lett. 1984, 68, 73–87. [Google Scholar] [CrossRef]
- Vonstackelberg, U.; Beiersdorf, H. The formation of manganese nodules between the Clarion and Clipperton fracture-zones southeast of Hawaii. Mar. Geol. 1991, 98, 411–423. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, D.; Zhai, S. Factors controlling the concentration of the transition metals Cu, Co and Ni in the ferromanganese deposits: An overview. Mar. Geol. Quat. Geol. 2004, 24, 41–48. [Google Scholar]
- Hein, J.R.; Schwab, W.C.; Davis, A.S. Cobalt-rich and platinum-rich ferromanganese crusts and associated substrate rocks from the Marshall-islands. Mar. Geol. 1988, 78, 255–283. [Google Scholar] [CrossRef]
- Halbach, P.; Sattler, C.D.; Teichmann, F.; Wahsner, M. Cobalt-rich and platinum-bearing manganese crust deposits on seamounts: Nature, formation, and metal potential. Mar. Min. 1989, 8, 23–39. [Google Scholar]
- Nozaki, Y. Elemental Distribution: Overview. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 255–260. [Google Scholar]
- Hein, J.R.; Conrad, T.A.; Frank, M.; Christl, M.; Sager, W.W. Copper-nickel-rich, amalgamated ferromanganese crust-nodule deposits from Shatsky Rise, NW Pacific. Geochem. Geophys. Geosystems 2012, 13, Q10022. [Google Scholar] [CrossRef]
- De Carlo, E.H.; McMurtry, G.M. Rare-earth element geochemistry of ferromanganese crusts from the Hawaiian Archipelago, central Pacific. Chem. Geol. 1992, 95, 235–250. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.; Mizell, K.; Banakar, V.K.; Frey, F.A.; Sager, W.W. Controls on ferromanganese crust composition and reconnaissance resource potential, Ninetyeast Ridge, Indian Ocean. Deep.-Sea Res. Part I-Oceanogr. Res. Pap. 2016, 110, 1–19. [Google Scholar] [CrossRef]
- Jiang, X.J.; Lin, X.H.; Yao, D.; Guo, W.D. Enrichment mechanisms of rare earth elements in marine hydrogenetic ferromanganese crusts. Sci. China-Earth Sci. 2011, 54, 197–203. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Sun, X.-M.; Chou, Y.-M.; Hein, J.R.; He, G.-W.; Fu, Y.; Li, D.-f.; Liao, J.-L.; Ren, J.-B. Geochemistry and origins of carbonate fluorapatite in seamount Fe-Mn crusts from the Pacific Ocean. Mar. Geol. 2020, 423, 106135. [Google Scholar] [CrossRef]
- Halbach, P.; Kriete, C.; Prause, B.; Puteanus, D. Mechanisms to explain the platinum concentration in ferromanganese seamount crusts. Chem. Geol. 1989, 76, 95–106. [Google Scholar] [CrossRef]
- Bruland, K.W. Oceanographic distributions of cadmium, zinc, nickel, and copper in the North Pacific. Earth Planet. Sci. Lett. 1980, 47, 176–198. [Google Scholar] [CrossRef]
- Varghese, S.; Ramesh, R.P.; Pillai, R.; Joseph, S.R.; Gopakumar, B.; Sathikumar, R.; Joshi, R.K.; Guha, P.D.; Manoj, R.V.; Nagasundaram, M. Accumulation and enrichment of platinum group elements in hydrogenous Fe-Mn crust and nodules from the Andaman Sea, India. Curr. Sci. 2021, 120, 1740–1748. [Google Scholar] [CrossRef]
- Savelyev, D.P.; Khanchuk, A.I.; Savelyeva, O.L.; Moskaleva, S.V.; Mikhailik, P.E. First Find of Platinum in Cosmogenic Spherules of Ferromanganese Crusts (Fedorov Guyot, Magellan Seamounts, Pacific Ocean). Dokl. Earth Sci. 2020, 491, 199–203. [Google Scholar] [CrossRef]
- Koschinsky, A.; Stascheit, A.; Bau, M.; Halbach, P. Effects of phosphatization on the geochemical and mineralogical composition of marine ferromanganese crusts. Geochim. Et Cosmochim. Acta 1997, 61, 4079–4094. [Google Scholar] [CrossRef]
- Hein, J.R.; Yeh, H.W.; Gunn, S.H.; Sliter, W.V.; Benninger, L.M.; Wang, C.H. Two Major Cenozoic Episodes of Phosphogenesis Recorded in Equatorial Pacific Seamount Deposits. Paleoceanography 1993, 8, 293–311. [Google Scholar] [CrossRef]
- Cui, Y.C.; Shi, X.F.; Liu, J.H.; Ren, X.W. Effects of Phosphatization on the Elemental Association of Cobalt-Rich Crusts. Geol. Sci. Techology Inf. 2008, 27, 61–67. [Google Scholar]
- Ren, X.W.; Liu, J.H.; Chui, Y.C.; Shi, X.F.; Yin, J.W. Effects of phosphatization on enrichment of cobalt in the Co-rich Fe-Mn crusts from seamount MP2 of the Line islands in the Central Pacific. Adv. Mar. Sci. 2011, 29, 324–329. [Google Scholar]
- Pan, J.; De Carlo, E.H.; Liu, S. Effect of phosphatization on element concentration of cobalt-rich ferromanganese crusts. In Proceedings of the ISOPE Ocean Mining Symposium, Seoul, Republic of Korea, 15–19 September 2003; pp. 20–26. [Google Scholar]
- Josso, P.; Lusty, P.; Chenery, S.; Murton, B. Controls on metal enrichment in ferromanganese crusts: Temporal changes in oceanic metal flux or phosphatisation? Geochim. Et Cosmochim. Acta 2021, 308, 60–74. [Google Scholar] [CrossRef]
- Oldham, V.E.; Jones, M.R.; Tebo, B.M.; Luther, G.W. Oxidative and reductive processes contributing to manganese cycling at oxic-anoxic interfaces. Mar. Chem. 2017, 195, 122–128. [Google Scholar] [CrossRef]
- Baturin, G.N.; Yushina, I.G. Rare earth elements in phosphate-ferromanganese crusts on Pacific seamounts. Lithol. Miner. Resour. 2007, 42, 101–117. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, K.D. Tunnel cation and water structures of todorokite: Insights into metal partitioning and isotopic fractionation. Geochim. Et Cosmochim. Acta 2022, 333, 153–165. [Google Scholar] [CrossRef]
- van Genuchten, C.M.; Pena, J. Sorption selectivity of birnessite particle edges: A d-PDF analysis of Cd(II) and Pb(II) sorption by δ-MnO2 and ferrihydrite. Environ. Sci.-Process. Impacts 2016, 18, 1030–1041. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T.; Dohrmann, R.; Wirth, R.; Grangeon, S. Mineralogical characterization of individual growth structures of Mn-nodules with different Ni+Cu content from the central Pacific Ocean. Am. Mineral. 2015, 100, 2497–2508. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman spectroscopy study of manganese oxides: Tunnel structures. Am. Mineral. 2020, 105, 1175–1190. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman spectroscopy study of manganese oxides: Layer structures. Am. Mineral. 2021, 106, 351–366. [Google Scholar] [CrossRef]
- Hens, T.; Brugger, J.; Etschmann, B.; Paterson, D.; Brand, H.E.A.; Whitworth, A.; Frierdich, A.J. Nickel exchange between aqueous Ni(II) and deep-sea ferromanganese nodules and crusts. Chem. Geol. 2019, 528, 119276. [Google Scholar] [CrossRef]
- Grangeon, S.; Lanson, B.; Lanson, M. Solid-state transformation of nanocrystalline phyllomanganate into tectomanganate: Influence of initial layer and interlayer structure. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 828–838. [Google Scholar] [CrossRef]
- Zhu, M.; Ginder-Vogel, M.; Sparks, D.L. Cation Effects on the Layer Structure of Biogenic Mn-Oxides. Environ. Sci. Technol. 2010, 44, 4465–4471. [Google Scholar] [CrossRef]
- Yang, P.; Lee, S.; Post, J.E.; Xu, H.; Wang, Q.; Xu, W.; Zhu, M. Trivalent manganese on vacancies triggers rapid transformation of layered to tunneled manganese oxides (TMOs): Implications for occurrence of TMOs in low-temperature environment. Geochim. Et Cosmochim. Acta 2018, 240, 173–190. [Google Scholar] [CrossRef]
- Tournassat, C.; Charlet, L.; Bosbach, D.; Manceau, A. Arsenic(III) Oxidation by Birnessite and Precipitation of Manganese(II) Arsenate. Environ. Sci. Technol. 2002, 36, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Steinmann, S.N. Nature of High- and Low-Affinity Metal Surface Sites on Birnessite Nanosheets. ACS Earth Space Chem. 2021, 5, 66–76. [Google Scholar] [CrossRef]
- Grangeon, S.; Fernandez-Martinez, A.; Claret, F.; Marty, N.; Tournassat, C.; Warmont, F.; Gloter, A. In-situ determination of the kinetics and mechanisms of nickel adsorption by nanocrystalline vernadite. Chem. Geol. 2017, 459, 24–31. [Google Scholar] [CrossRef]
- Sherman, D.M.; Peacock, C.L. Surface complexation of Cu on birnessite (δ-MnO2): Controls on Cu in the deep ocean. Geochim. Et Cosmochim. Acta 2010, 74, 6721–6730. [Google Scholar] [CrossRef]
- Cruz-Hernandez, Y.; Villalobos, M.; Marcus, M.A.; Pi-Puig, T.; Zanella, R.; Martinez-Villegas, N. Tl(I) sorption behavior on birnessite and its implications for mineral structural changes. Geochim. Et Cosmochim. Acta 2019, 248, 356–369. [Google Scholar] [CrossRef]
- Atkins, A.L.; Shaw, S.; Peacock, C.L. Release of Ni from birnessite during transformation of birnessite to todorokite: Implications for Ni cycling in marine sediments. Geochim. Et Cosmochim. Acta 2016, 189, 158–183. [Google Scholar] [CrossRef]
- Simanova, A.A.; Peña, J. Time-Resolved Investigation of Cobalt Oxidation by Mn(III)-Rich δ-MnO2 Using Quick X-ray Absorption Spectroscopy. Environ. Sci. Technol. 2015, 49, 10867–10876. [Google Scholar] [CrossRef]
- Peña, J.; Kwon, K.D.; Refson, K.; Bargar, J.R.; Sposito, G. Mechanisms of nickel sorption by a bacteriogenic birnessite. Geochimica Et Cosmochimica Acta 2010, 74, 3076–3089. [Google Scholar] [CrossRef]
- Simanova, A.A.; Kwon, K.D.; Bone, S.E.; Bargar, J.R.; Refson, K.; Sposito, G.; Peña, J. Probing the sorption reactivity of the edge surfaces in birnessite nanoparticles using nickel(II). Geochim. Et Cosmochim. Acta 2015, 164, 191–204. [Google Scholar] [CrossRef]
- Wick, S.; Pena, J.; Voegelin, A. Thallium Sorption onto Manganese Oxides. Environ. Sci. Technol. 2019, 53, 13168–13178. [Google Scholar] [CrossRef]
- Peña, J.; Bargar, J.R.; Sposito, G. Copper sorption by the edge surfaces of synthetic birnessite nanoparticles. Chem. Geol. 2015, 396, 196–207. [Google Scholar] [CrossRef]
- Sun, Q.; Cui, P.-X.; Zhu, M.; Fan, T.-T.; Ata-Ul-Karim, S.T.; Gu, J.-H.; Wu, S.; Zhou, D.-M.; Wang, Y.-J. Cd(II) retention and remobilization on δ-MnO2 and Mn(III)-rich δ-MnO2 affected by Mn(II). Environ. Int. 2019, 130, 104932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peacock, C.; Kwon, K.D.; Gu, X.; Feng, X.; Li, W. Site-specific isotope fractionation during Zn adsorption onto birnessite: Insights from X-ray absorption spectroscopy, density functional theory and surface complexation modeling. Geochim. Et Cosmochim. Acta 2023, 348, 68–84. [Google Scholar] [CrossRef]
- Manceau, A.; Simionovici, A.; Findling, N.; Glatzel, P.; Detlefs, B.; Wegorzewski, A.V.; Mizell, K.; Hein, J.R.; Koschinsky, A. Crystal Chemistry of Thallium in Marine Ferromanganese Deposits. ACS Earth Space Chem. 2022, 6, 1269–1285. [Google Scholar] [CrossRef]
- Huang, W.; Hu, B.; Song, W.; Zhao, J.; Lu, J.; Meng, X.; Jiang, Y.; Cui, R.; Ding, X. Enrichment and constraints of critical metals in ferromanganese crusts from 13°20′ N seamount of the southern Kyushu-Palau Ridge. Mar. Geol. Quat. Geol. 2022, 42, 137–148. [Google Scholar]
- Ren, Y.; Sun, X.; Guan, Y.; Xiao, Z.; Liu, Y.; Liao, J.; Guo, Z. Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe-Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study. Minerals 2019, 9, 8. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.H.; Zhang, B.Y.; Guo, S.H. Scavenging of Cd through Fe/Mn oxides within natural surface coatings. J. Environ. Sci. 2006, 18, 1199–1203. [Google Scholar] [CrossRef]
- Sun, Q.; Cui, P.-X.; Fan, T.-T.; Wu, S.; Zhu, M.; Alves, M.E.; Zhou, D.-M.; Wang, Y.-J. Effects of Fe(II) on Cd(II) immobilization by Mn(III)-rich δ-MnO2. Chem. Eng. J. 2018, 353, 167–175. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Kubo, S.; Tanaka, M.; Senda, R.; Iizuka, T.; Tanimizu, M.; Takahashi, Y. Stable isotope fractionation of tungsten during adsorption on Fe and Mn (oxyhydr) oxides. Geochim. Et Cosmochim. Acta 2017, 204, 52–67. [Google Scholar] [CrossRef]
- Arai, Y. X-ray Absorption Spectroscopic Investigation of Molybdenum Multinuclear Sorption Mechanism at the Goethite−Water Interface. Environ. Sci. Technol. 2010, 44, 8491–8496. [Google Scholar] [CrossRef]
- Qin, H.; Uesugi, S.; Yang, S.; Tanaka, M.; Kashiwabara, T.; Itai, T.; Usui, A.; Takahashi, Y. Enrichment mechanisms of antimony and arsenic in marine ferromanganese oxides: Insights from the structural similarity. Geochim. Et Cosmochim. Acta 2019, 257, 110–130. [Google Scholar] [CrossRef]
- Yang, K.L.; Zhou, J.S.; Lou, Z.M.; Zhou, X.R.; Liu, Y.L.; Li, Y.Z.; Baig, S.A.; Xu, X.H. Removal of Sb(V) from aqueous solutions using Fe-Mn binary oxides: The influence of iron oxides forms and the role of manganese oxides. Chem. Eng. J. 2018, 354, 577–588. [Google Scholar] [CrossRef]
- Lan, S.; Ying, H.; Wang, X.; Liu, F.; Tan, W.; Huang, Q.; Zhang, J.; Feng, X. Efficient catalytic As(III) oxidation on the surface of ferrihydrite in the presence of aqueous Mn(II). Water Res. 2018, 128, 92–101. [Google Scholar] [CrossRef]
- Bai, Y.H.; Jefferson, W.A.; Liang, J.S.; Yang, T.T.; Qu, J.H. Antimony oxidation and adsorption by in-situ formed biogenic Mn oxide and Fe-Mn oxides. J. Environ. Sci. 2017, 54, 126–134. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Bostick, B.; Suarez, D.L. Arsenic(III) oxidation and arsenic(V) adsorption reactions on synthetic birnessite. Environ. Sci. Technol. 2002, 36, 976–981. [Google Scholar] [CrossRef]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Li, G.T. Removal mechanism of As(III) by a novel Fe-Mn binary oxide adsorbent: Oxidation and sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to Goethite: Influence on Sb(III) Oxidation and Mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef]
- Lafferty, B.J.; Ginder-Vogel, M.; Zhu, M.; Livi, K.J.T.; Sparks, D.L. Arsenite Oxidation by a Poorly Crystalline Manganese-Oxide. 2. Results from X-ray Absorption Spectroscopy and X-ray Diffraction. Environ. Sci. Technol. 2010, 44, 8467–8472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, F.D.; Liu, H.J.; Qu, J.H.; Liu, R.P. Respective Role of Fe and Mn Oxide Contents for Arsenic Sorption in Iron and Manganese Binary Oxide: An X-ray Absorption Spectroscopy Investigation. Environ. Sci. Technol. 2014, 48, 10316–10322. [Google Scholar] [CrossRef]
- Tokoro, C.; Yatsugi, Y.; Koga, H.; Owada, S. Sorption Mechanisms of Arsenate during Coprecipitation with Ferrihydrite in Aqueous Solution. Environ. Sci. Technol. 2010, 44, 638–643. [Google Scholar] [CrossRef]
- Takematsu, N.; Sato, Y.; Okabe, S.; Usui, A. Uptake of selenium and other oxyanionic elements in marine ferromanganese concretions of different origins. Mar. Chem. 1990, 31, 271–283. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Chao, T.T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese-dioxide. Geochim. Et Cosmochim. Acta 1990, 54, 739–751. [Google Scholar] [CrossRef]
- Wu, Z.; Lanson, B.; Feng, X.; Yin, H.; Tan, W.; He, F.; Liu, F. Transformation of the phyllomanganate vernadite to tectomanganates with small tunnel sizes: Favorable geochemical conditions and fate of associated Co. Geochim. Et Cosmochim. Acta 2021, 295, 224–236. [Google Scholar] [CrossRef]
- Wang, Z.; Kwon, K.D.; Peacock, C.; Mo, X.X.; Gou, W.X.; Feng, X.H.; Li, W. Zn stable isotope fractionation during adsorption onto todorokite: A molecular perspective from X-ray absorption spectroscopy and density functional theory. Geochim. Et Cosmochim. Acta 2022, 327, 116–136. [Google Scholar] [CrossRef]
- Marchig, V.; Vonstackelberg, U.; Hufnagel, H.; Durn, G. Compositional changes of surface sediments and variability of manganese nodules in the Peru Basin. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 3523–3547. [Google Scholar] [CrossRef]
- Manceau, A.; Steinmann, S.N. Density Functional Theory Modeling of the Oxidation Mechanism of Co(II) by Birnessite. ACS Earth Space Chem. 2022, 6, 2063–2075. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Geoffroy, N. Natural speciation of Ni, Zn, Ba, and As in ferromanganese coatings on quartz using X-ray fluorescence, absorption, and diffraction. Geochim. Et Cosmochim. Acta 2007, 71, 95–128. [Google Scholar] [CrossRef]
- Manceau, A.; Schlegel, M.L.; Musso, M.; Sole, V.A.; Gauthier, C.; Petit, P.E.; Trolard, F. Crystal chemistry of trace elements in natural and synthetic goethite. Geochim. Et Cosmochim. Acta 2000, 64, 3643–3661. [Google Scholar] [CrossRef]
- Li, D.; Fu, Y.; Sun, X.; Wei, Z. Critical metal enrichment mechanism of deep-sea hydrogenetic nodules: Insights from mineralogy and element mobility. Ore Geol. Rev. 2020, 118, 103371. [Google Scholar] [CrossRef]
- Peacock, C.L. Physiochemical controls on the crystal-chemistry of Ni in birnessite: Genetic implications for ferromanganese precipitates. Geochim. Et Cosmochim. Acta 2009, 73, 3568–3578. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Sorption of Ni by birnessite: Equilibrium controls on Ni in seawater. Chem. Geol. 2007, 238, 94–106. [Google Scholar] [CrossRef]
- Sorensen, J.V.; Gueguen, B.; Stewart, B.D.; Pena, J.; Rouxel, O.; Toner, B.M. Large nickel isotope fractionation caused by surface complexation reactions with hexagonal birnessite. Chem. Geol. 2020, 537, 119481. [Google Scholar] [CrossRef]
- Little, S.H.; Sherman, D.M.; Vance, D.; Hein, J.R. Molecular controls on Cu and Zn isotopic fractionation in Fe-Mn crusts. Earth Planet. Sci. Lett. 2014, 396, 213–222. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Copper(II) sorption onto goethite, hematite and lepidocrocite: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy1 1Associate editor: D. L. Sparks. Geochim. Et Cosmochim. Acta 2004, 68, 2623–2637. [Google Scholar] [CrossRef]
- Shi, D.D.; Wang, S.Z.; Li, Y.; Di, Y.W.; Wang, T. The adsorption mechanism of Zn2+ by triclinic birnessite and the mechanism. Environ. Technol. 2022, 43, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Wang, Q.; Sun, J.Y.; Borkiewicz, O.J.; Huang, R.X.; Saad, E.M.; Fields, B.; Chen, S.; Zhu, M.Q.; Tang, Y.Z. Effect of Zn coprecipitation on the structure of layered Mn oxides. Chem. Geol. 2018, 493, 234–245. [Google Scholar] [CrossRef]
- Yin, H.; Wang, X.; Qin, Z.; Ginder-Vogel, M.; Zhang, S.; Jiang, S.; Liu, F.; Li, S.; Zhang, J.; Wang, Y. Coordination geometry of Zn2+ on hexagonal turbostratic birnessites with different Mn average oxidation states and its stability under acid dissolution. J. Environ. Sci. 2018, 65, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.H.; Sholkovitz, E.R. Chapter 158 Marine chemistry and geochemistry of the lanthanides. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Hoboken, NJ, USA, 1996; Volume 23, pp. 497–593. [Google Scholar]
- Aplin, A.C. Rare-earth element geochemistry of central pacific ferromanganese encrustations. Earth Planet. Sci. Lett. 1984, 71, 13–22. [Google Scholar] [CrossRef]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Et Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
- Byrne, R.H.; Kim, K.-H. Rare earth element scavenging in seawater. Geochim. Et Cosmochim. Acta 1990, 54, 2645–2656. [Google Scholar] [CrossRef]
- Koeppenkastrop, D.; De Carlo, E.H. Sorption of rare-earth elements from seawater onto synthetic mineral particles: An experimental approach. Chem. Geol. 1992, 95, 251–263. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A. Oxidative scavenging of cerium on hydrous Fe oxide: Evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts. Geochem. J. 2009, 43, 37–47. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Guo, Q.; Cao, J.; Wang, H.; Liu, C. Rare earth element geochemistry characteristics of seawater and porewater from deep sea in western Pacific. Sci. Rep. 2017, 7, 16539. [Google Scholar] [CrossRef]
- Khanchuk, A.I.; Mikhailik, P.E.; Mikhailik, E.V.; Zarubina, N.V.; Blokhin, M.G. Peculiarities of the distribution of rare-earth elements and yttrium in mineral phases of the ferromanganese crusts from the Detroit guyot (Pacific Ocean). Dokl. Earth Sci. 2015, 465, 1243–1247. [Google Scholar] [CrossRef]
- De Carlo, E.H.; Wen, X.-Y. The Influence of Redox Reactions on the Uptake of Dissolved Ce by Suspended Fe and Mn Oxide Particles. Aquat. Geochem. 1997, 3, 357–389. [Google Scholar] [CrossRef]
- Nakada, R.; Takahashi, Y.; Tanimizu, M. Cerium stable isotope ratios in ferromanganese deposits and their potential as a paleo-redox proxy. Geochim. Et Cosmochim. Acta 2016, 181, 89–100. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. Rare earth element partitioning between Fe oxyhydroxide precipitates and aqueous NaCl solutions doped with NaHCO3: Determinations of rare earth element complexation constants with carbonate ions. Geochem. J. 2000, 34, 439–454. [Google Scholar] [CrossRef]
- Quinn, K.A.; Byrne, R.H.; Schijf, J. Comparative Scavenging of Yttrium and the Rare Earth Elements in Seawater: Competitive Influences of Solution and Surface Chemistry. Aquat. Geochem. 2004, 10, 59–80. [Google Scholar] [CrossRef]
- Goldberg, E.D. Heavy metal analyses in the marine environment—Approaches to quality control. Mar. Chem. 1987, 22, 117–124. [Google Scholar] [CrossRef]
- Sharma, M. Platinum Group Elements and Their Isotopes in the Ocean. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2019; pp. 174–180. [Google Scholar]
- Banakar, V.K.; Hein, J.R.; Rajan, R.P.; Chodankar, A.R. Platinum group elements and gold in ferromanganese crusts from Afanasiy-Nikitin seamount, equatorial Indian Ocean: Sources and fractionation. J. Earth Syst. Sci. 2007, 116, 3–13. [Google Scholar] [CrossRef]
- Baturin, G.N.; Konopleva, E.V.; Dubinchuk, V.T.; Mel’nikov, M.E. Platinum and gold in the ferromanganese crusts of the Pacific Ocean. Oceanology 2005, 45, 269–276. [Google Scholar]
- Asavin, A.M.; Anikeev, L.I.; Kazakov, V.A.; Andreev, S.I.; Sapozhnikov, D.A.; Roshchina, I.A.; Kogarko, L.N. Trace element and PGE distribution in layered ferromanganese crusts. Geochem. Int. 2008, 46, 1179–1205. [Google Scholar] [CrossRef]
- Berezhnaya, E.D.; Dubinin, A.V.; Rimskaya-Korsakova, M.N.; Safin, T.H. Accumulation of Platinum Group Elements in Hydrogenous Fe-Mn Crust and Nodules from the Southern Atlantic Ocean. Minerals 2018, 8, 275. [Google Scholar] [CrossRef]
- Qiu, Z.; Dong, Y.; Ma, W.; Zhang, W.; Yang, K.; Zhao, H. Geochemical characteristics of platinum-group elements in polymetallic nodules from the Northwest Pacific Ocean. Acta Oceanol. Sin. 2020, 39, 34–42. [Google Scholar] [CrossRef]
- Berezhnaya, E.D.; Dubinin, A.V.; Mikhailik, E.V. Platinum Group Elements in Ferromanganese Crusts of the Atlantic Ocean: Forms and Sources of Matter. Oceanology 2021, 61, 390–403. [Google Scholar] [CrossRef]
- Halbach, P.; Puteanus, D.; Manheim, F.T. Platinum concentrations in ferromanganese seamount crusts from the central pacific. Naturwissenschaften 1984, 71, 577–579. [Google Scholar] [CrossRef]
- Cobelo-García, A.; López-Sánchez, D.E.; Almécija, C.; Santos-Echeandía, J. Behavior of platinum during estuarine mixing (Pontevedra Ria, NW Iberian Peninsula). Mar. Chem. 2013, 150, 11–18. [Google Scholar] [CrossRef]
- Li, Z.; Sun, X.; Li, D.; Liang, Y.; Li, S.; Peng, J. Platinum enrichment in marine ferromanganese oxides: Constraints from surface adsorption behavior on synthetic feroxyhyte (δ-FeOOH). Chem. Geol. 2023, 615, 121204. [Google Scholar] [CrossRef]
- Boyle, E.A.; Sclater, F.; Edmond, J.M. On the marine geochemistry of cadmium. Nature 1976, 263, 42–44. [Google Scholar] [CrossRef]
- Lee, D.S.; Edmond, J.M. Tellurium species in seawater. Nature 1985, 313, 782–785. [Google Scholar] [CrossRef]
- Biver, M.; Quentel, F.; Filella, M. Direct determination of tellurium and its redox speciation at the low nanogram level in natural waters by catalytic cathodic stripping voltammetry. Talanta 2015, 144, 1007–1013. [Google Scholar] [CrossRef]
- Harada, T.; Takahashi, Y. Origin of the difference in the distribution behavior of tellurium and selenium in a soil–water system. Geochim. Et Cosmochim. Acta 2008, 72, 1281–1294. [Google Scholar] [CrossRef]
- Fukami, Y.; Kashiwabara, T.; Amakawa, H.; Shibuya, T.; Usui, A.; Suzuki, K. Tellurium stable isotope composition in the surface layer of ferromanganese crusts from two seamounts in the Northwest Pacific Ocean. Geochim. Et Cosmochim. Acta 2022, 318, 279–291. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T.; Azarnova, L.A.; Mel’nikov, M.E. Species of molybdenum, thallium, and tellurium in ferromanganese crusts of oceanic Seamounts. Oceanology 2007, 47, 415–422. [Google Scholar] [CrossRef]
- Peacock, C.L.; Moon, E.M. Oxidative scavenging of thallium by birnessite: Explanation for thallium enrichment and stable isotope fractionation in marine ferromanganese precipitates. Geochim. Et Cosmochim. Acta 2012, 84, 297–313. [Google Scholar] [CrossRef]
- Cutter, G.A.; Cutter, L.S. Sources and cycling of selenium in the western and equatorial Atlantic Ocean. Deep.-Sea Res. Part Ii-Top. Stud. Oceanogr. 2001, 48, 2917–2931. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Webster, J.G.; Miskelly, G.M. Goethite adsorption of Cu(II), Pb(II), Cd(II), and Zn(II) in the presence of sulfate: Properties of the ternary complex. Geochim. Et Cosmochim. Acta 2009, 73, 1548–1562. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Villalobos, M.; Tan, W.; Liu, F. Sorption behavior of heavy metals on birnessite: Relationship with its Mn average oxidation state and implications for types of sorption sites. Chem. Geol. 2012, 292–293, 25–34. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Liao, B.H.; Lin, L.N.; Qiu, W.W.; Song, Z.G. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Galer, S.J.G.; Abouchami, W. Mass-dependent cadmium isotopic variations in nature with emphasis on the marine environment. Earth Planet. Sci. Lett. 2009, 277, 262–272. [Google Scholar] [CrossRef]
- Randall, S.R.; Sherman, D.M.; Ragnarsdottir, K.V. An extended X-ray absorption fine structure spectroscopy investigation of cadmium sorption on cryptomelane (KMn8O16). Chem. Geol. 1998, 151, 95–106. [Google Scholar] [CrossRef]
- Stoffynegli, P.; Mackenzie, F.T. Mass balance of dissolved lithium in the oceans. Geochim. Et Cosmochim. Acta 1984, 48, 859–872. [Google Scholar] [CrossRef]
- Rajani, P.R.; Manoj, R.V.; Joshi, R.K.; Jishnu, B.K.; Nagasundaram, M. Base metals- and Lithium- rich ferromanganese oxide deposits from the South Andaman Sea, Northeastern Indian Ocean: Mode of occurrence and genesis. J. Asian Earth Sci. 2022, 234, 105272. [Google Scholar] [CrossRef]
- Chan, L.-H.; Hein, J.R. Lithium contents and isotopic compositions of ferromanganese deposits from the global ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1147–1162. [Google Scholar] [CrossRef]
- Feng, Q.; Yanagisawa, K.; Yamasaki, N. Synthesis of Birnessite-Type Lithium Manganese Oxide. J. Ceram. Soc. Jpn. 1996, 104, 897–899. [Google Scholar] [CrossRef]
- Sohrin, Y.; Isshiki, K.; Kuwamoto, T.; Nakayama, E. Tungsten in north pacific waters. Mar. Chem. 1987, 22, 95–103. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Tanimizu, M.; Usui, A. Molecular-scale mechanisms of distribution and isotopic fractionation of molybdenum between seawater and ferromanganese oxides. Geochim. Et Cosmochim. Acta 2011, 75, 5762–5784. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Vanadium(V) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim. Et Cosmochim. Acta 2004, 68, 1723–1733. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sherman, D.M. Molecular speciation of Mo (VI) on goethite and its implications for molybdenum and its isotopic cycle in ocean. Geochim. Et Cosmochim. Acta 2021, 313, 116–132. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Tiberg, C. Molybdenum binding to soil constituents in acid soils: An XAS and modelling study. Chem. Geol. 2015, 417, 279–288. [Google Scholar] [CrossRef]
- Naeem, A.; Westerhoff, P.; Mustafa, S. Vanadium removal by metal (hydr)oxide adsorbents. Water Res. 2007, 41, 1596–1602. [Google Scholar] [CrossRef]
- Ren, Y.Z.; Guan, Y.; Sun, X.M.; Xu, L.; Xiao, Z.L.; Deng, Y.Q.; He, W.T. Nano-mineralogy and growth environment of Fe-Mn polymetallic crusts and nodules from the South China Sea. Front. Mar. Sci. 2023, 10, 1141926. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, D.; Lin, X.; Zhai, S. Geochemistry of Cu, Co, Ni, Ti, and Mg in diagenetic ferromanganese nodules. Mar. Geol. Quat. Geol. 2007, 27, 47–54. [Google Scholar]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.; Alves, M.E.; Ata-Ul-Karim, S.T.; Zhou, D.-M.; He, J.-Z.; Cui, P.-X.; Wang, Y.-J. The oxidation and sorption mechanism of Sb on δ-MnO2. Chem. Eng. J. 2018, 342, 429–437. [Google Scholar] [CrossRef]
- Marcus, M.A.; Manceau, A.; Kersten, M. Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule. Geochim. Et Cosmochim. Acta 2004, 68, 3125–3136. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Mitsunobu, S.; Das, A.; Itai, T.; Tanimizu, M.; Takahashi, Y. Oxidation states of antimony and arsenic in marine ferromanganese oxides related to their fractionation in oxic marine environment. Chem. Lett. 2008, 37, 756–757. [Google Scholar] [CrossRef]
- Manceau, A. The mechanism of anion adsorption on iron-oxides—Evidence for the bonding of arsenate tetrahedra on free Fe(O,OH)6 edges. Geochim. Et Cosmochim. Acta 1995, 59, 3647–3653. [Google Scholar] [CrossRef]
- Villalobos, M.; Escobar-Quiroz, I.N.; Salazar-Camacho, C. The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III). Geochim. Et Cosmochim. Acta 2014, 125, 564–581. [Google Scholar] [CrossRef]
- Wang, S.L.; Mulligan, C.N. Speciation and surface structure of inorganic arsenic in solid phases: A review. Environ. Int. 2008, 34, 867–879. [Google Scholar] [CrossRef]


| Element | PCZ | CCZ | PB | |||
|---|---|---|---|---|---|---|
| Mean | N | Mean | N | Mean | N | |
| Mn (wt.%) | 22.8 | 362 | 28.1 | 54 | 34.2 | - |
| Fe | 16.9 | 362 | 5.92 | 54 | 6.12 | - |
| Co (ppm) | 6662 | 362 | 2011 | 54 | 475 | - |
| Ni | 4209 | 362 | 13,159 | 54 | 13,008 | - |
| Cu | 976 | 362 | 10,631 | 54 | 5988 | - |
| Zn | 668 | 325 | 1385 | 54 | 1845 | - |
| Ce | 1322 | 83 | 255 | 54 | 110 | - |
| La | 272 | 83 | 108 | 54 | 68 | - |
| Y | 221 | 294 | 92 | 54 | 69 | - |
| LREE | 2044.3 | 83 | 570.53 | 54 | 272.97 | - |
| HREE | 425.23 | 83 | 191.57 | 54 | 129.54 | - |
| Li | 2.92 | 33 | 129 | 54 | 311 | - |
| Te | 60 | 43 | 3.6 | 54 | 1.7 | - |
| Cd | 3.59 | 285 | 16 | 12 | 18.8 | - |
| Se | 14.8 | 1 | 0.72 | 12 | 0.5 | - |
| Tl | 155 | 34 | 199 | 12 | 129 | - |
| As | 393 | 328 | 67 | 12 | 65 | - |
| Sb | 39.3 | 43 | 41 | 12 | 61 | - |
| V | 641 | 328 | 429 | 54 | 431 | - |
| W | 89 | 36 | 61 | 54 | 75 | - |
| Mo | 461 | 328 | 587 | 54 | 547 | - |
| Ti | 11,600 | 345 | 2800 | 54 | 1600 | - |
| Pt (ppb) | 470 | 60 | 128 | 12 | 40 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Fu, Y. Enrichment Characteristics and Mechanisms of Critical Metals in Marine Fe-Mn Crusts and Nodules: A Review. Minerals 2023, 13, 1532. https://doi.org/10.3390/min13121532
Huang S, Fu Y. Enrichment Characteristics and Mechanisms of Critical Metals in Marine Fe-Mn Crusts and Nodules: A Review. Minerals. 2023; 13(12):1532. https://doi.org/10.3390/min13121532
Chicago/Turabian StyleHuang, Sucheng, and Yazhou Fu. 2023. "Enrichment Characteristics and Mechanisms of Critical Metals in Marine Fe-Mn Crusts and Nodules: A Review" Minerals 13, no. 12: 1532. https://doi.org/10.3390/min13121532





