Silica Polymorphs Formation in the Jänisjärvi Impact Structure: Tridymite, Cristobalite, Quartz, Trace Stishovite and Coesite
Abstract
:1. Introduction
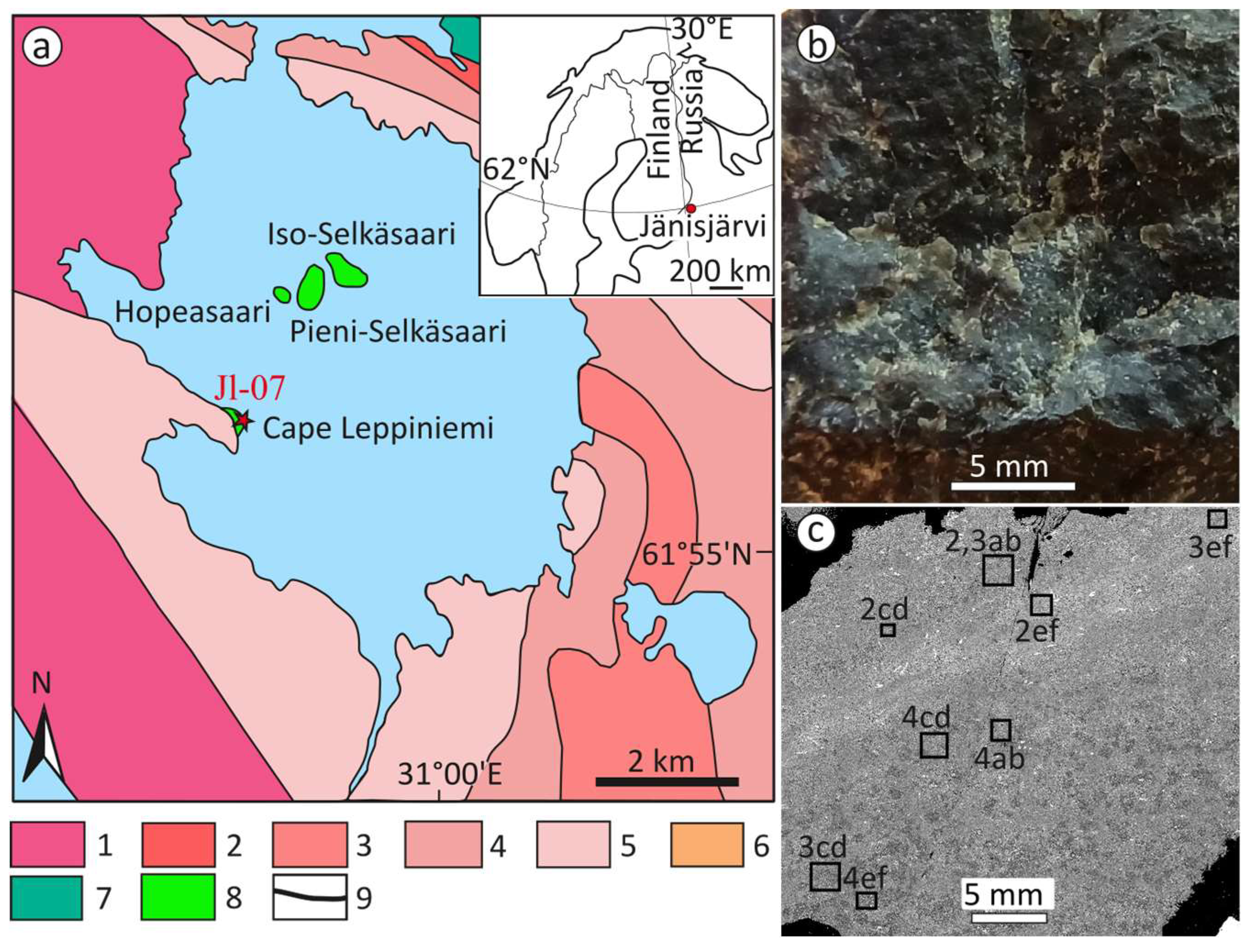
2. Materials and Methods
3. Results
3.1. Mineralogical Composition of Impact Melt Rocks
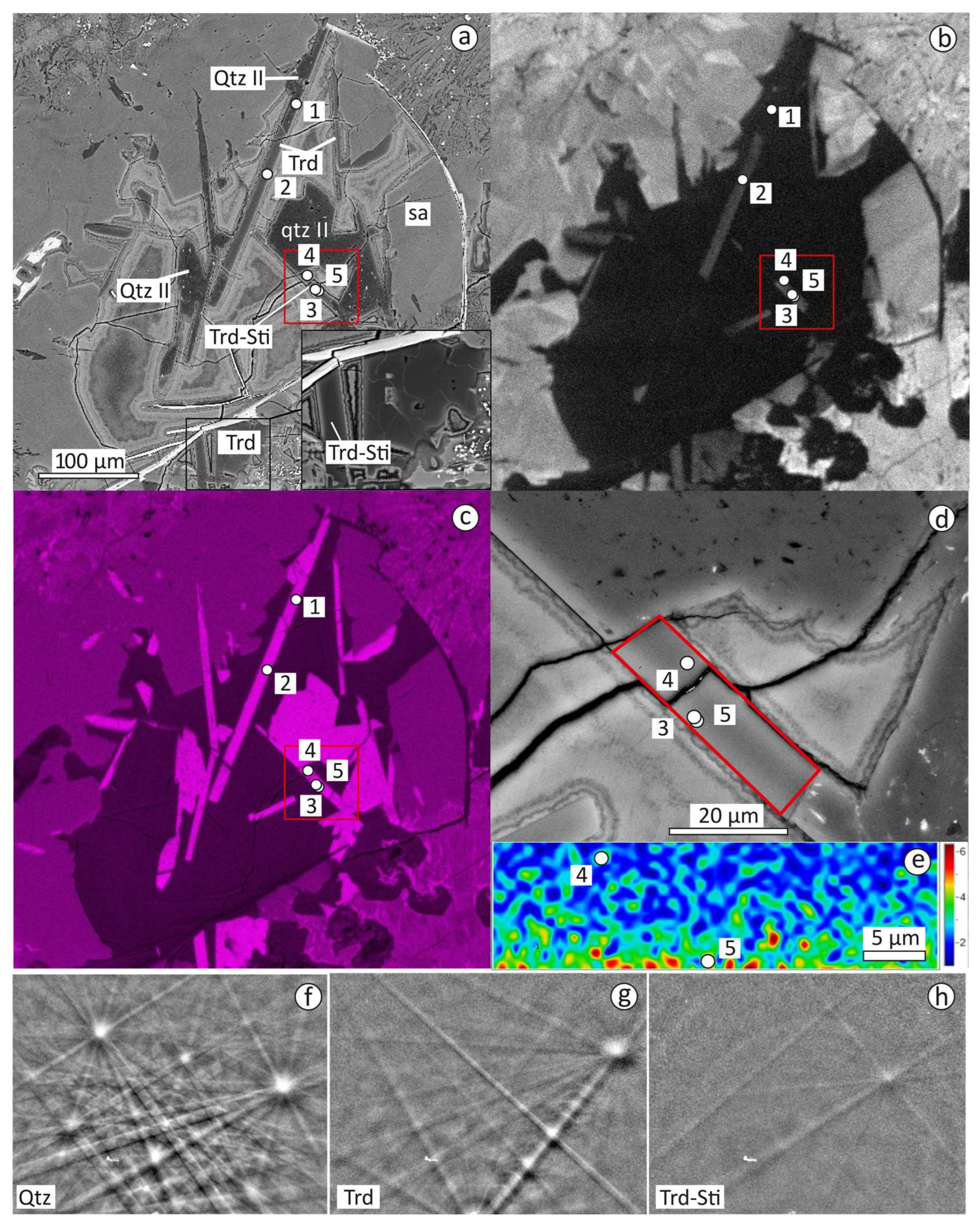
3.2. Textural Relationships of Tridymite, Cristabolite and Quartz
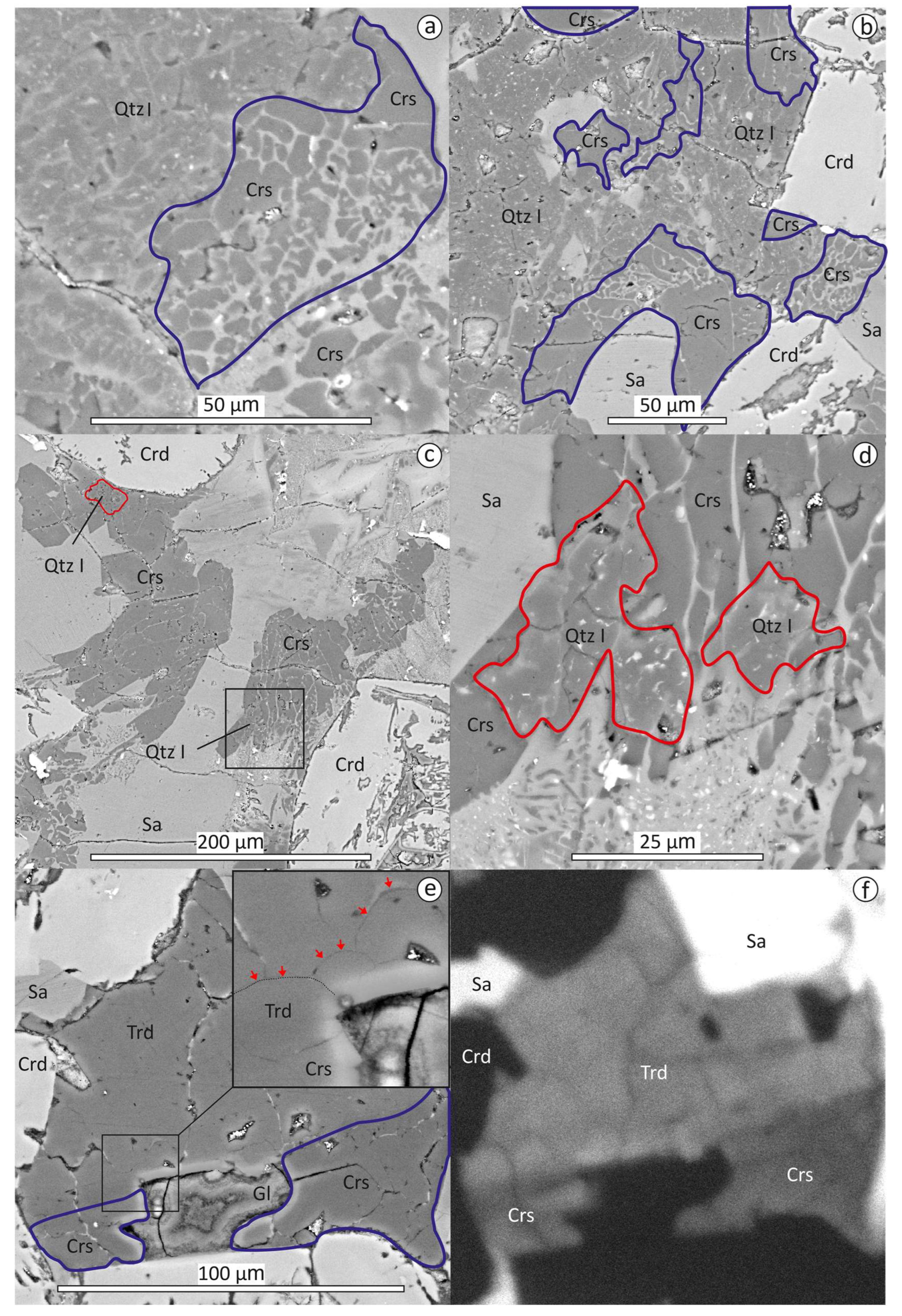
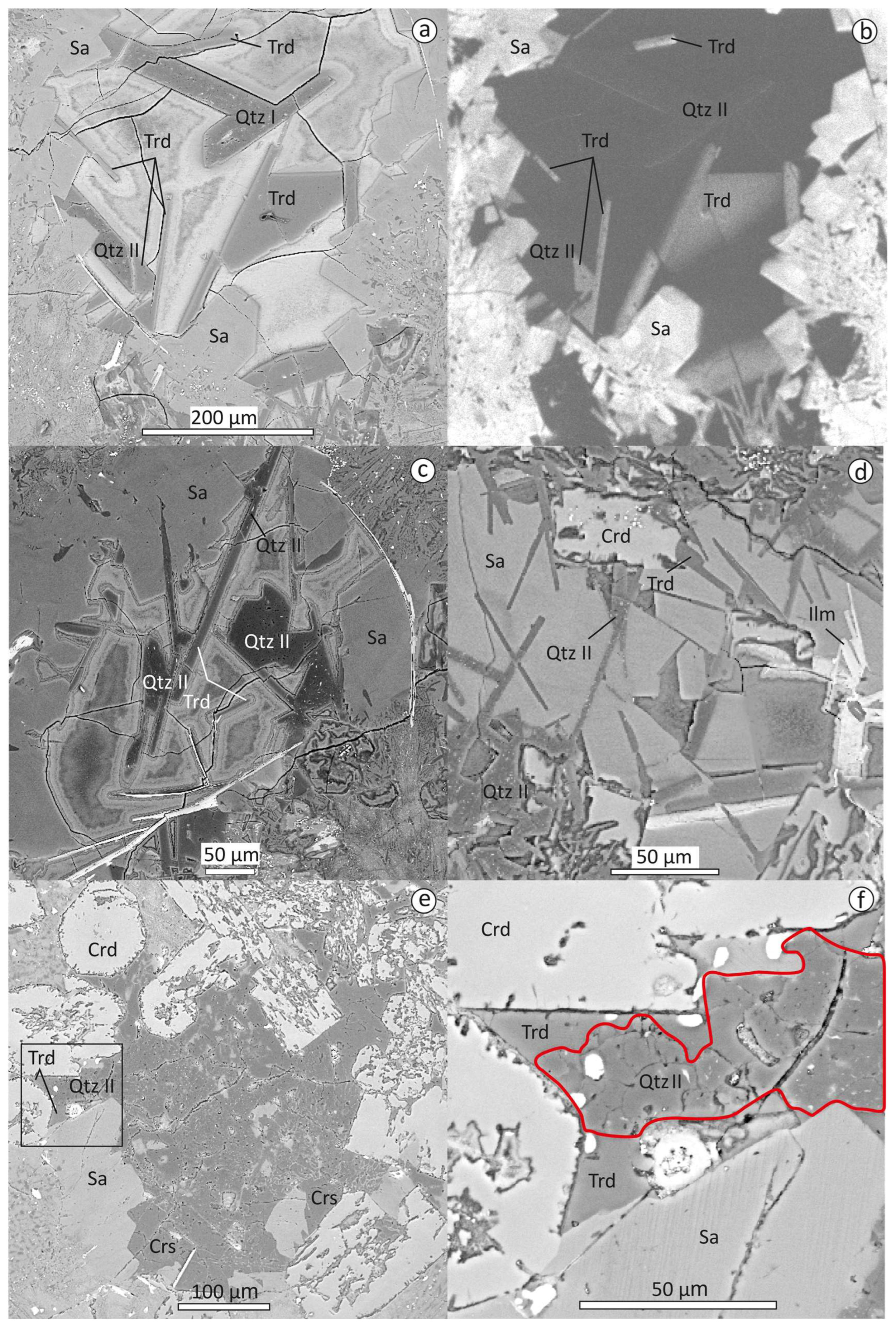
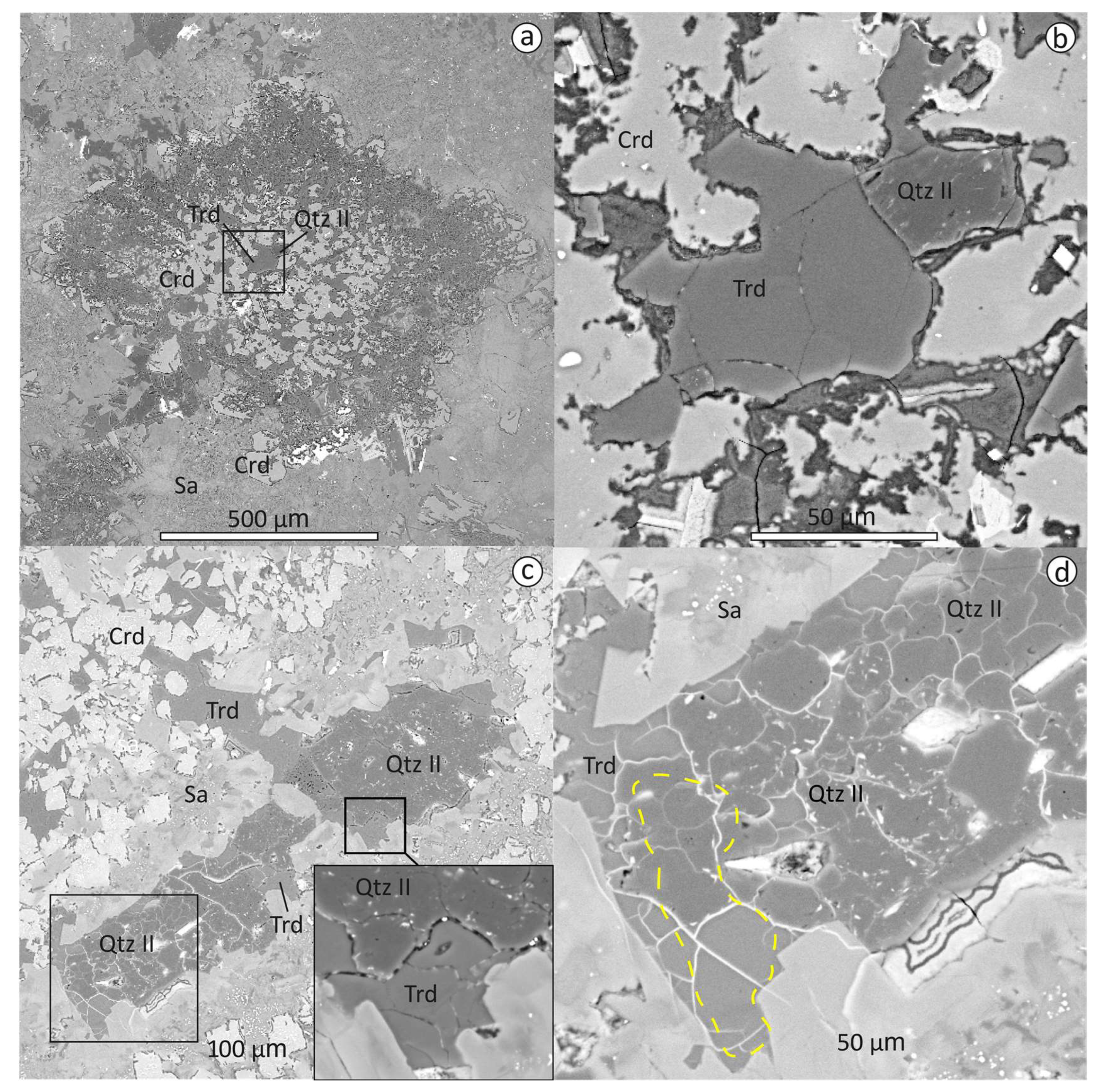
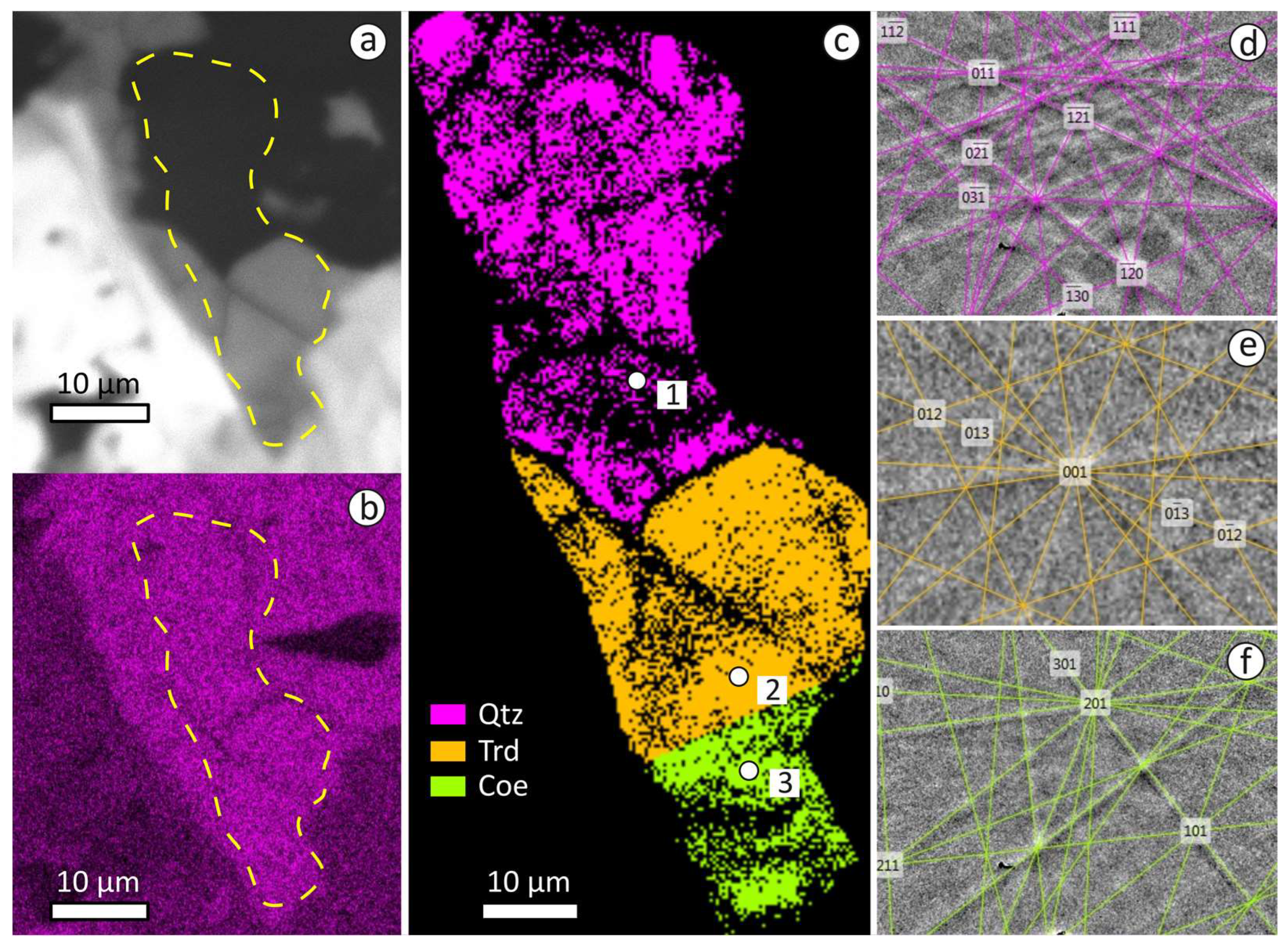
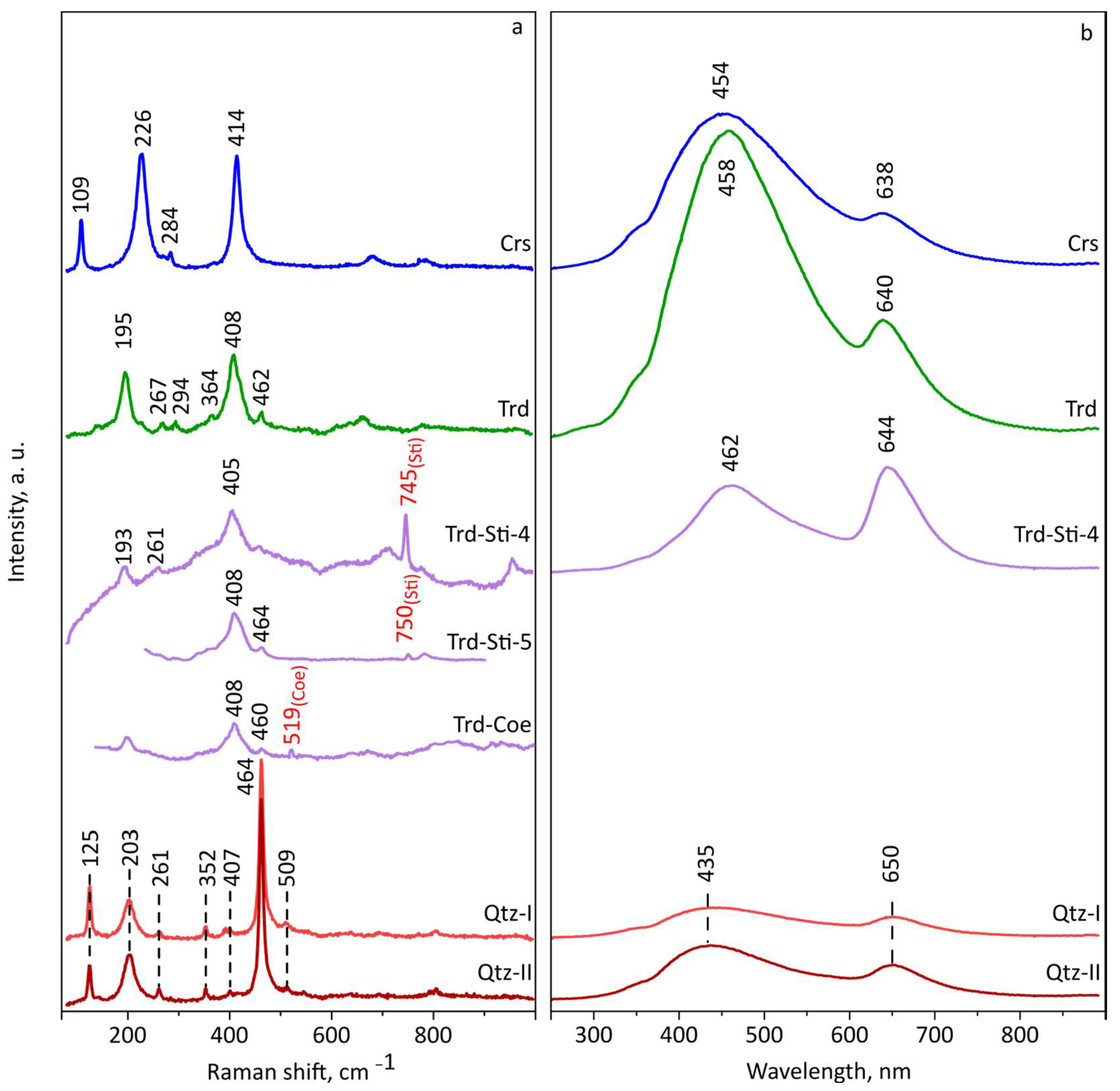
3.3. EPMA Data, Raman and CL Spectra of Tridymite, Cristobalite, Quartz, Traces of Stishovite and Coesite
4. Discussion
4.1. Tridymite, Cristabolite and Quartz Paramorphs Formation
4.2. Traces of Stishovite and Coesite in Tridymite
4.3. Formation of Ballen Textures
4.4. The Formation Sequence of Silica Polymorphs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stöffler, D.; Hamann, C.; Metzler, K. Shock metamorphism of planetary silicate rocks and sediments: Proposal for an updated classification system. Meteorit. Planet. Sci. 2018, 53, 5–49. [Google Scholar] [CrossRef]
- Heaney, P.J.; Prewitt, C.T.; Gibbs, G.V. (Eds.) Silica: Physical Behavior, Geochemistry, and Materials Applications; Walter de Gruyter GmbH & Co KG.: Washington, DC, USA, 2018; Volume 29, 605p. [Google Scholar]
- Damby, D.E.; Llewellin, E.W.; Horwell, C.J.; Williamson, B.J.; Najorka, J.; Cressey, G.; Carpenter, M. The α–β phase transition in volcanic cristobalite. J. Appl. Crystallogr. 2014, 47, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.E.; Naney, M.T.; Westrich, H.R.; Eichelberger, J.C. Crystallization history of Obsidian dome, Inyo domes, California. Bull. Volcanol. 1989, 51, 161–176. [Google Scholar] [CrossRef]
- Martel, C.; Bourdier, J.-L.; Pichavant, M.; Traineau, H. Textures, water content and degassing of silica andesites from recept plinian and dome-forming eruption at Mount Pelee volcano (Martinique, Lesser Antilles arc). J. Volcanol. Geotherm. Res. 2000, 96, 91–206. [Google Scholar] [CrossRef]
- Watkins, J.; Manga, M.; Huber, C.; Martin, M. Diffusion-controlled spheru lite growth in obsidian inferred from H2O concentration profiles. Contrib. Mineral. Petrol. 2009, 157, 163–172. [Google Scholar] [CrossRef]
- Horwell, C.J.; Williamson, B.J.; Llewellin, E.W.; Damby, D.E.; Le Blond, J.S. The nature and formation of cristobalite at the Soufrière Hills volcano, Montserrat: Implications for the petrology and stability of silicic lava domes. Bull. Volcanol. 2013, 75, 696. [Google Scholar] [CrossRef]
- Davydova, V.O.; Shcherbakov, V.D.; Plechov, P.Y.; Koulakov, I.Y. Petrological evidence of rapid evolution of the magma plumbing system of Bezymianny volcano in Kamchatka before the December 20th, 2017 eruption. J. Volcanol. Geotherm. Res. 2022, 421, 107422. [Google Scholar] [CrossRef]
- Heled, Y.; Rowe, M.C.; Chambefort, I.; Wilson, C.J. Significance of tridymite distribution during cooling and vapor-phase alteration of ignimbrites. Am. Min. 2022, 107, 460–475. [Google Scholar] [CrossRef]
- Leroux, H.; Cordier, P. Magmatic cristobalite and quartz in the NWA 856 Martian meteorite. Meteorit. Planet. Sci. 2006, 41, 913–923. [Google Scholar] [CrossRef]
- Seddio, S.M.; Korotev, R.L.; Jolliff, B.L.; Wang, A. Silica polymorphs in lunar granite: Implications for granite petrogenesis on the Moon. Am. Min. 2015, 100, 1533–1543. [Google Scholar] [CrossRef]
- Kayama, M.; Nagaoka, H.; Niihara, T. Lunar and martian silica. Minerals 2018, 8, 267. [Google Scholar] [CrossRef]
- Payré, V.; Siebach, K.L.; Thorpe, M.T.; Antoshechkina, P.; Rampe, E.B. Tridymite in a lacustrine mudstone in Gale Crater, Mars: Evidence for an explosive silicic eruption during the Hesperian. Earth Planet. Sci. Lett. 2022, 594, 117694. [Google Scholar] [CrossRef]
- Shoval, S.; Champagnon, B.; Panczer, G. The quartz-cristobalite transforma tion in heated chert rock composed of micro and crypto-quartz by micro-Raman and FT-IR spectroscopic methods. J. Therm. Anal. 1997, 50, 203–213. [Google Scholar] [CrossRef]
- Smith, D.K. Opal, cristobalite, and tridymite: Non-crystallinity versus crystallinity, nomenclature of the silica minerals and bibliography. Powder Diffr. 1998, 13, 2–19. [Google Scholar] [CrossRef]
- Vishnevsky, S.; Montanari, A. Popigai impact structure (Arctic Siberia, Russia): Geology, petrology, geochemistry, and geochronology of glassbearing impactites. In Large Meteorite Impacts and Planetary Evolution II. Geological Society of America Special; Paper, 339; Dressler, B.O., Sharpton, V.L., Eds.; The Geological Society of America: Boulder, CO, USA, 1999; pp. 19–59. [Google Scholar]
- Whitehead, J.; Grieve, R.A.F.; Spray, J.G. Mineralogy and petrology of melt rocks from the Popigai impact structure, Siberia. Meteorit. Planet. Sci. 2002, 37, 623–647. [Google Scholar] [CrossRef]
- Osinski, G.R. Impact melt rocks from the Ries impact structure, Germany: An origin as impact melt flows? Earth Planet. Sci. Lett. 2004, 226, 529–543. [Google Scholar] [CrossRef]
- Ferrière, L.; Koeberl, C.; Reimold, W.U. Characterisation of ballen quartz and cristobalite in impact breccias: New observations and constraints on ballen formation. Eur. J. Mineral. 2009, 21, 203–217. [Google Scholar] [CrossRef]
- Trepmann, C.A.; Dellefant, F.; Kaliwoda, M.; Hess, K.U.; Schmahl, W.W.; Hölzl, S. Quartz and cristobalite ballen in impact melt rocks from the Ries impact structure, Germany, formed by dehydration of shock-generated amorphous phases. Meteorit. Planet. Sci. 2020, 55, 2360–2374. [Google Scholar] [CrossRef]
- Jackson, J.C.; Horton, J.W., Jr.; Chou, I.M.; Belkin, H.E. Monoclinic tridymite in clast-rich impact melt rock from the Chesapeake Bay impact structure. Am. Min. 2011, 96, 81–88. [Google Scholar] [CrossRef]
- Ivanova, D.A.; Shcherbakov, V.D.; Plechov, P.Y.; Nekrylov, N.A.; Davydova, V.O.; Turova, M.A.; Stepanov, O.V. Cristobalite in extrusive rocks of Bezymianny volcano. New Data Min. 2018, 52, 51–58. [Google Scholar]
- Carstens, H. Thermal history of impact melt rocks in the Fennoscandian shield. Contrib. Mineral. Petrol. 1975, 50, 145–155. [Google Scholar] [CrossRef]
- Bischoff, A.; Stöffler, D. Chemical and structural changes induced by thermal annealing of shocked feldspar inclusions in impact melt rocks from Lappajarvi crater, Finland. J. Geophys. Res. 1984, 89, 645–656. [Google Scholar] [CrossRef]
- Fel’dman, V.I.; Granovsky, L.B.; Sazaonova, L.V.; Nikishina, N.N.; Butenko, T.G.; Naumova, I.G. Some peculiarities of geochemistry of impactites of Janisjarvi, SW Karelia, and Kara, Polar Ural, astroblemes. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 19–23 March 1979. [Google Scholar]
- Zamiatina, D.A.; Zamyatin, D.A.; Mikhalevskii, G.B. Ballen Quartz in Jänisjärvi Impact Melt Rock with High Concentrations of Fe, Mg, and Al: EPMA, EDS, EBSD, CL, and Raman Spectroscopy. Minerals 2022, 12, 886. [Google Scholar] [CrossRef]
- Jourdan, F.; Renne, P.R.; Reimold, W.U. High-precision 40Ar/39Ar age of the Jänisjärvi impact structure (Russia). Earth Planet. Sci. Lett. 2008, 265, 438–449. [Google Scholar] [CrossRef]
- Masaitis, V.L. Impact structures of the northeast of Eurasia: The territories of Russia and neighboring countries. Meteorit. Planet. Sci. 1999, 34, 691–711. [Google Scholar] [CrossRef]
- Sazonova, L.V.; Feldman, V.I.; Shcherbovsky, E.Y. Features of the chemical composition and optical properties of the rock-forming minerals of the tagamites from Jänisjärvi astrobleme. Dokl. Akad. Nauk SSSR 1985, 281, 138–142. (In Russian) [Google Scholar]
- Fel’dman, V.I.; Sazonova, L.V.; Kozlov, E.A. Shock metamorphism of some rock-forming minerals: Experimental results and natural observations. J. Petrol. 2006, 14, 540–566. [Google Scholar] [CrossRef]
- Marakushev, A.A. (Ed.) Impactites; Moscow State University: Moscow, Russia, 1981; p. 240. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; De Gruyter, W., Ed.; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2015; pp. 1–30. [Google Scholar] [CrossRef]
- Maksimova, A.A.; Zamiatina, D.A.; Zamyatin, D.A.; Ryanskaya, A.D.; Chukin, A.V.; Oshtrakh, M.I. Impact melt rock from Jänisjärvi astrobleme: Study of the iron-bearing phases using Raman spectroscopy, X-ray diffraction, and Mössbauer spectroscopy. Meteorit. Planet. Sci. 2022, 57, 912–925. [Google Scholar] [CrossRef]
- Kingma, K.J.; Hemley, R.J. Raman spectroscopic study of microcrystalline silica. Am. Min. 1994, 79, 269–273. [Google Scholar]
- Zamyatin, D.A. Application of Raman Spectroscopy for Studying Shocked Zircon from Terrestrial and Lunar Impactites: A Systematic Review. Minerals 2022, 12, 969. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, S.; Huang, H.; Wu, X.; Liu, H. Structural characteristics and elasticities of coesite and coesite-II at high pressure. New J. Phys. 2020, 22, 093044. [Google Scholar] [CrossRef]
- Papike, J.J.; Cameron, M. Crystal chemistry of silicate minerals of geophysical interest. Rev. Geophys. Space Phys. 1976, 14, 37–80. [Google Scholar] [CrossRef]
- Swamy, V.; Saxena, S.K.; Sundman, B.; Zhang, J. A thermodynamic assessment of silica phase diagram. J. Geophys. Res. Solid Earth 1994, 99, 11787–11794. [Google Scholar] [CrossRef]
- Dapiaggi, M.; Pagliari, L.; Pavese, A.; Sciascia, L.; Merli, M.; Francescon, F. The formation of silica high temperature polymorphs from quartz: Influence of grain size and mineralising agents. J. Eur. Ceram. Soc. 2015, 35, 4547–4555. [Google Scholar] [CrossRef]
- Putnis, A.; Putnis, C.V. The mechanism of reequilibration of solids in the presence of a fluid phase. J. Solid State Chem. 2007, 180, 1783–1786. [Google Scholar] [CrossRef]
- Götze, J.; Plötze, M.; Habermann, D. Origin, spectral characteristics and practical applications of the cathodoluminescence (CL) of quartz—A review. Mineral. Petrol. 2001, 71, 225–250. [Google Scholar] [CrossRef]
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Stevens-Kalceff, M. Cathodoluminescence microcharacterization of point defects in α-quartz. Miner. Mag. 2009, 73, 585–605. [Google Scholar] [CrossRef]
- Sittner, J.; Götze, J. Cathodoluminescence (CL) Characteristics of Quartz from Different Metamorphic Rocks within the Kaoko Belt (Namibia). Minerals 2018, 8, 190. [Google Scholar] [CrossRef]
- Gucsik, A. (Ed.) Cathodoluminescence and Its Application in the Planetary Sciences; Springer: Berlin/Heidelberg, Germany, 2009; p. 160. [Google Scholar] [CrossRef]
- Augustsson, C.; Reker, A. Cathodolumenescence spectra of quartz as provenance indicators revisited. J. Sediment. Res. 2012, 82, 559–570. [Google Scholar] [CrossRef]
- Sippel, R.F. Luminescence petrography of the Apollo 12 rocks and comparative features in terrestrial rocks and meteorites. In Lunar and Planetary Science Conference Proceedings; Pergamon Press: New York, NY, USA, 1971; Volume 2, p. 247. [Google Scholar]
- Kayama, M.; Nishido, H.; Ninagawa, K. Cathodoluminescence characterization of tridymite and cristobalite: Effects of electron irradiation and sample temperature. Am. Mineral. 2009, 94, 1018–1028. [Google Scholar] [CrossRef]
- Schipper, C.I.; Rickard, W.D.; Reddy, S.M.; Saxey, D.W.; Castro, J.M.; Fougerouse, D.; Lilly, K. Volcanic SiO2-cristobalite: A natural product of chemical vapor deposition. Am. Mineral. J. Earth Planet. Mater. 2020, 105, 510–524. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Wise, W.S.; Zussman, J. Rock-forming Minerals: Framework Silicates: Silica Minerals, Feldspathoids and the Zeolites, 4B; The Geological Society: London, UK, 2004; 982p. [Google Scholar]
- Lakshtanov, D.L.; Sinogeikin, S.V.; Bass, J.D. High-temperature phase transitions and elasticity of silica polymorphs. Phys. Chem. Miner. 2007, 34, 11–22. [Google Scholar] [CrossRef]
- Martel, C.; Pichavant, M.; Di Carlo, I.; Champallier, R.; Wille, G.; Castro, J.M.; Kushnir, A.R. Experimental constraints on the crystallization of silica phases in silicic magmas. J. Petrol. 2021, 62, egab004. [Google Scholar] [CrossRef]
- Mason, B. Lunar tridymite and cristobalite. Am. Min. 1972, 57, 1530–1535. [Google Scholar]
- Langenhorst, F.; Deutsch, A. Shock metamorphism of minerals. Elements 2012, 8, 31–36. [Google Scholar] [CrossRef]
- Stöffler, D.; Langenhorst, F. Shock metamorphism of quartz in nature and experiment: I. Basic observation and theory. Meteoritics 1994, 29, 155–181. [Google Scholar] [CrossRef]
- Stähle, V.; Altherr, R.; Koch, M.; Nasdala, L. Shock-induced growth and metastability of stishovite and coesite in lithic clasts from suevite of the Ries impact crater (Germany). Contrib. Mineral. Petrol. 2008, 155, 457–472. [Google Scholar] [CrossRef]

| Mineral | SiO2 | Al2O3 | FeO | MgO | CaO | Na2O | K2O | TiO2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cristobalite | 93.97 | 1.47 | 0.51 | 0.12 | 0.10 | 0.12 | 0.05 | bdl | 96.43 |
| 94.91 | 1.23 | bdl | bdl | bdl | 0.15 | 0.07 | bdl | 96.55 | |
| 95.62 | 1.19 | 0.41 | 0.08 | 0.07 | 0.06 | 0.05 | bdl | 97.54 | |
| 95.36 | 1.07 | 0.13 | bdl | 0.04 | 0.13 | bdl | 0.14 | 96.92 | |
| 94.32 | 0.70 | bdl | bdl | bdl | 0.11 | bdl | 0.12 | 95.44 | |
| 93.41 | 1.38 | bdl | bdl | 0.09 | 0.18 | 0.45 | bdl | 95.72 | |
| 95.58 | 1.09 | bdl | bdl | bdl | 0.11 | 0.04 | bdl | 97.13 | |
| 96.30 | 0.41 | bdl | bdl | bdl | 0.06 | 0.04 | 0.12 | 97.07 | |
| 91.35 | 3.21 | bdl | bdl | 0.19 | 0.52 | 1.08 | 0.13 | 96.55 | |
| 94.26 | 1.42 | bdl | bdl | 0.05 | 0.30 | bdl | bdl | 96.22 | |
| Quartz after cristobalite (Qtz I) | 92.48 | 0.94 | 0.86 | 0.14 | 0.17 | 0.12 | 0.15 | 0.10 | 94.97 |
| 94.99 | 0.60 | bdl | bdl | bdl | 0.14 | 0.10 | bdl | 96.00 | |
| Tridymite | 98.88 | 0.38 | 0.35 | bdl | bdl | 0.13 | 0.05 | 0.1 | 99.91 |
| 95.94 | 1.38 | 0.72 | 0.15 | 0.05 | 0.17 | 0.08 | 0.11 | 98.60 | |
| 99.45 | 0.46 | 0.47 | bdl | bdl | 0.17 | 0.06 | bdl | 100.71 | |
| 98.81 | 0.42 | 0.49 | bdl | bdl | 0.12 | 0.05 | bdl | 100.00 | |
| 99.01 | 0.47 | 0.43 | bdl | bdl | 0.15 | bdl | bdl | 100.23 | |
| 98.82 | 0.41 | 0.41 | bdl | bdl | 0.15 | 0.05 | bdl | 99.93 | |
| 98.67 | 0.33 | 0.41 | bdl | bdl | 0.13 | bdl | bdl | 99.76 | |
| 98.33 | 0.46 | 0.44 | bdl | bdl | 0.06 | 0.04 | bdl | 99.42 | |
| 98.05 | 0.33 | 0.49 | bdl | bdl | 0.06 | bdl | 0.12 | 99.08 | |
| 96.61 | 0.43 | 0.35 | bdl | bdl | bdl | 0.05 | bdl | 97.66 | |
| 98.01 | 0.49 | 0.41 | bdl | bdl | 0.07 | 0.07 | bdl | 99.18 | |
| 98.81 | 0.43 | 0.39 | bdl | 0.04 | bdl | 0.07 | 0.11 | 99.89 | |
| 99.44 | 0.40 | 0.34 | bdl | bdl | 0.05 | 0.06 | bdl | 100.42 | |
| Quartz after tridymite (Qtz II) | 97.68 | 0.40 | 0.26 | bdl | bdl | 0.09 | 0.09 | bdl | 98.82 |
| 96.71 | 0.50 | 0.35 | bdl | bdl | 0.18 | 0.10 | 0.12 | 98.01 | |
| 97.43 | 0.68 | 0.92 | 0.07 | 0.09 | 0.16 | 0.05 | bdl | 99.50 | |
| 95.16 | 1.62 | 0.89 | 0.30 | 0.09 | 0.25 | 0.42 | bdl | 98.78 | |
| 97.79 | 0.39 | 0.33 | bdl | 0.05 | 0.16 | 0.06 | bdl | 98.88 | |
| 96.53 | 0.83 | 0.48 | 0.12 | bdl | 0.18 | 0.07 | bdl | 98.34 | |
| 96.03 | 0.74 | 0.44 | 0.09 | 0.09 | 0.10 | 0.05 | 0.15 | 97.72 | |
| 96.44 | 0.77 | 0.47 | 0.08 | 0.20 | 0.10 | 0.07 | bdl | 98.20 | |
| 98.36 | 0.43 | bdl | bdl | bdl | 0.07 | 0.10 | 0.10 | 99.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamiatina, D.A.; Zamyatin, D.A.; Mikhalevskii, G.B.; Chebikin, N.S. Silica Polymorphs Formation in the Jänisjärvi Impact Structure: Tridymite, Cristobalite, Quartz, Trace Stishovite and Coesite. Minerals 2023, 13, 686. https://doi.org/10.3390/min13050686
Zamiatina DA, Zamyatin DA, Mikhalevskii GB, Chebikin NS. Silica Polymorphs Formation in the Jänisjärvi Impact Structure: Tridymite, Cristobalite, Quartz, Trace Stishovite and Coesite. Minerals. 2023; 13(5):686. https://doi.org/10.3390/min13050686
Chicago/Turabian StyleZamiatina, Daria A., Dmitry A. Zamyatin, Georgii B. Mikhalevskii, and Nikolai S. Chebikin. 2023. "Silica Polymorphs Formation in the Jänisjärvi Impact Structure: Tridymite, Cristobalite, Quartz, Trace Stishovite and Coesite" Minerals 13, no. 5: 686. https://doi.org/10.3390/min13050686





