The Mineral Chemistry of Magnetite and Its Constraints on Ore-Forming Mechanism in the Sandaozhuang Skarn-Type W-Mo Deposit in East Qinling, China
Abstract
:1. Introduction
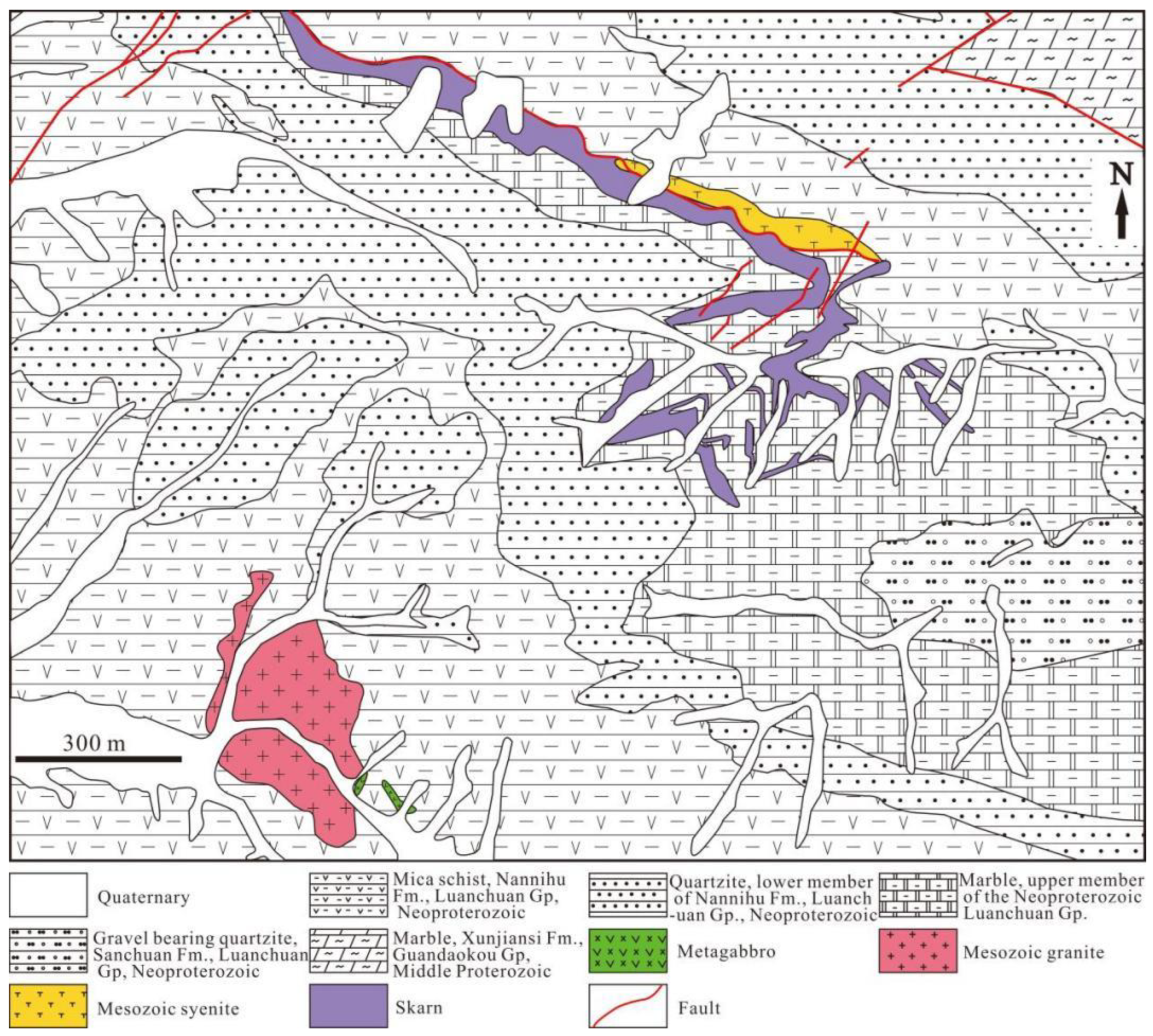
2. Regional Geology
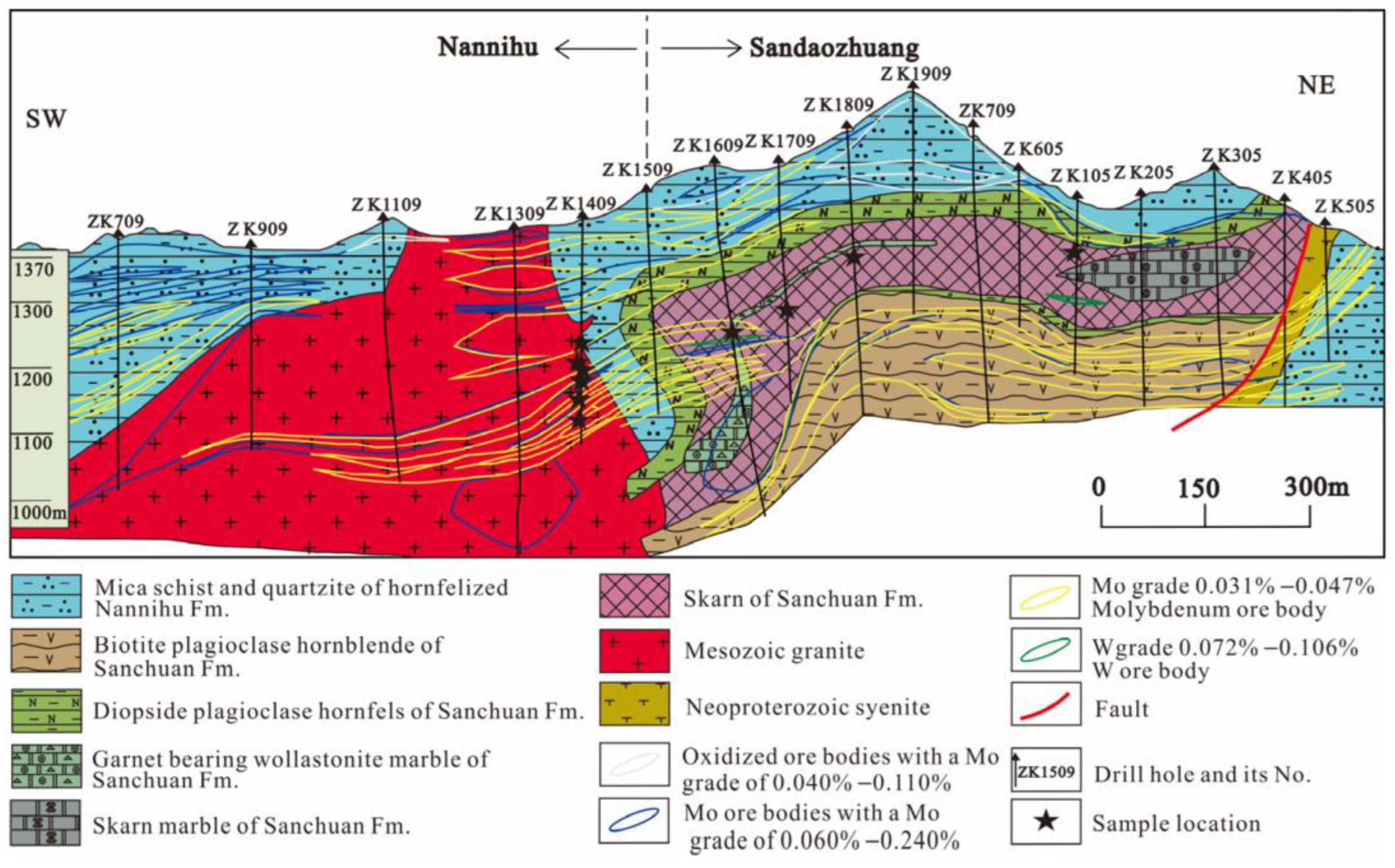
3. Ore Deposit Geology
- (1)
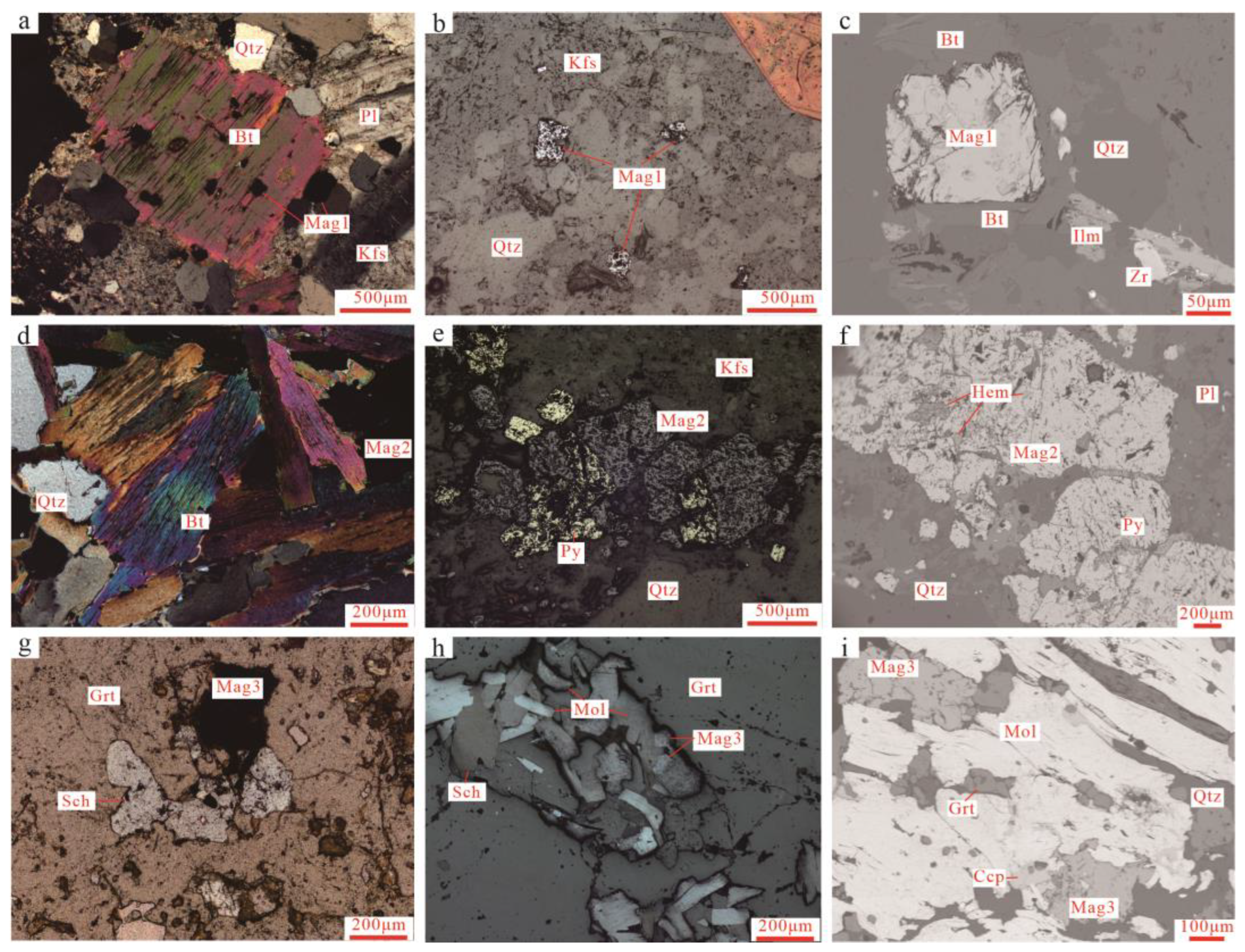
- The magmatic stage (I) is the stage of granitic porphyry formation associated with mineralization. During this stage, minerals such as biotite, plagioclase, K-feldspar, quartz, apatite, and magnetite are formed (Figure 5a,b and Figure 6a–c). Mag1 occurs as an accessory mineral in the granitic porphyry, and magnetite is commonly enclosed within biotite and K-feldspar phenocrysts, as a result of early magmatic crystallization, with a small grain size ranging from 0.1 to 0.2 mm, mostly in ovoid or ellipsoid shapes (Figure 6a–c).
- (2)
- The stage (II) with quartz-K-feldspar-biotite-magnetite assemblage (potassic alteration stage) is characterized by the formation of minerals such as quartz, K-feldspar, biotite, magnetite, and pyrite. K-feldspar includes K-feldspar altered with a sheet-like or linear distribution, and K-feldspar associated with quartz and biotite (Figure 5b and Figure 6d–f). Magnetite is common in this stage, usually appearing as aggregates, forming intergrowths with pyrite. A small amount of molybdenite occurred in thin veins or impregnations, appearing with K-feldspar alteration. Magnetite is commonly distributed on both sides of the veins, appearing in granular aggregates (Figure 5b).
- (3)
- The early silicate skarn stage (III) is mainly characterized by the formation of anhydrous silicate minerals, predominantly garnet and diopside (Figure 5c), as well as minor amounts of wollastonite and vesuvianite, and the formation of small amounts of scheelite.
- (4)
- The late silicate skarn stage (retrograde stage) (IV) is mainly characterized by the formation of a large number of hydrous silicate minerals, such as vesuvianite, chlorite, tremolite, epidote, zoisite, and minor amounts of quartz and apatite. At the same time, this stage also formed a large amount of scheelite and magnetite (Figure 5d–f), which is the main mineralization stage for scheelite. The scheelite occurs as subhedral to euhedral grains intermittently distributed in the interstices between garnet and diopside or intercalated in quartz veins in the skarn, often forming intergrowths, inclusions, or crosscutting relationships with molybdenite. It is distributed more in the middle part of the thin veins. Magnetite in the silicate skarn minerals commonly coexists with garnet, zoisite, and pyrite, appearing in granular aggregates (Figure 6g–i).
- (5)
- The quartz-sulfide stage (V) is the main stage of molybdenite formation, with a large amount of metal sulfide such as pyrite and chalcopyrite formed together with quartz, calcite, fluorite, and other minerals to form various veins, including molybdenite, quartz-molybdenite, quartz-pyrite-chalcopyrite-molybdenite, quartz-pyrite-molybdenite, and quartz-pyrite veins. These veins are filled in the fractures of silicate skarn, hornfels, and granite porphyry (Figure 5e,f).
- (6)
- The quartz-carbonate stage (VI) is the final stage of ore formation, characterized by the formation of a large amount of calcite, quartz, pyrite, with minor fluorite (Figure 5f).
4. Sampling and Analytical Methods
4.1. Sampling
4.2. EMPA Major Element Analyses
4.3. LA–ICP–MS Trace Element Analysis
5. Results
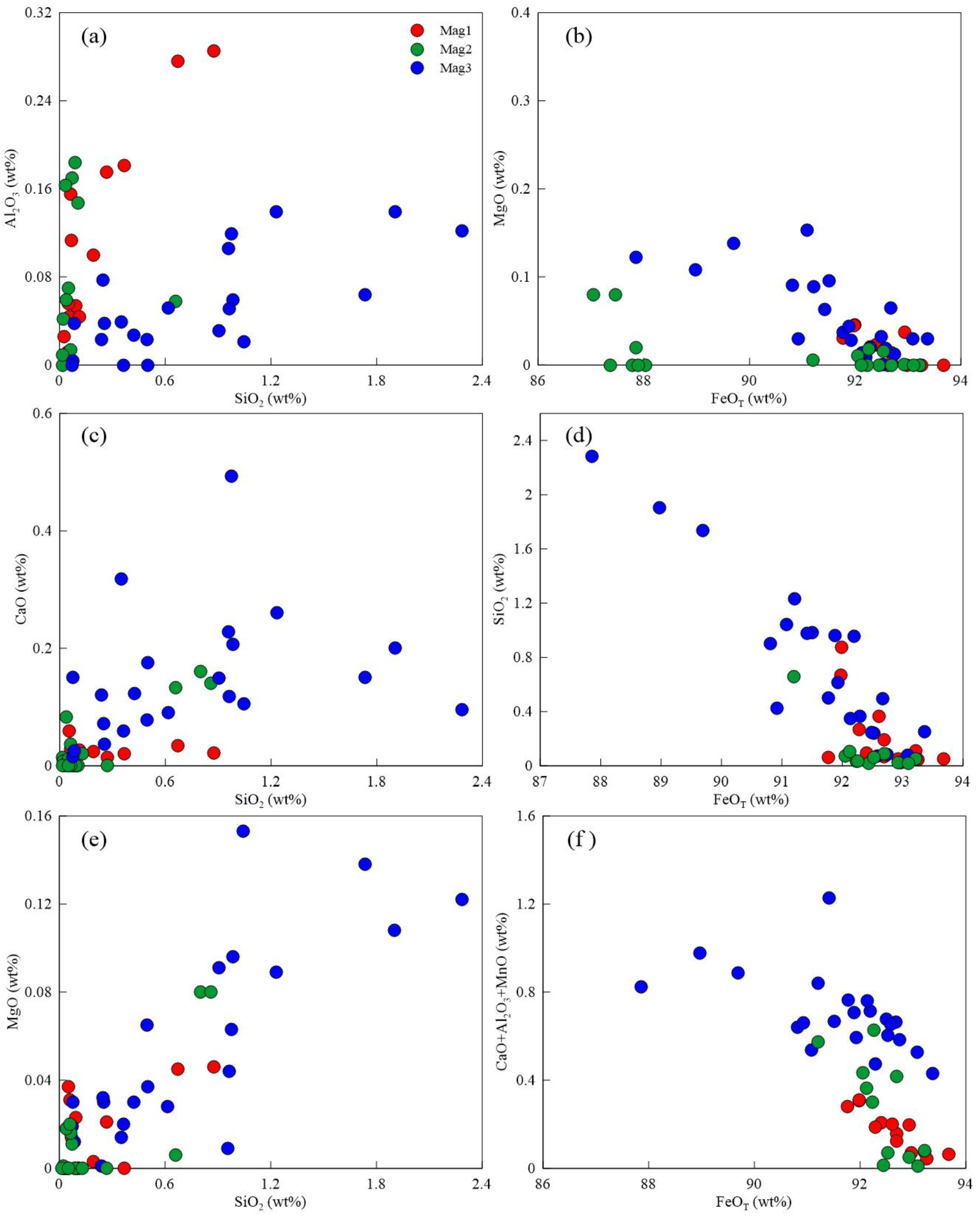
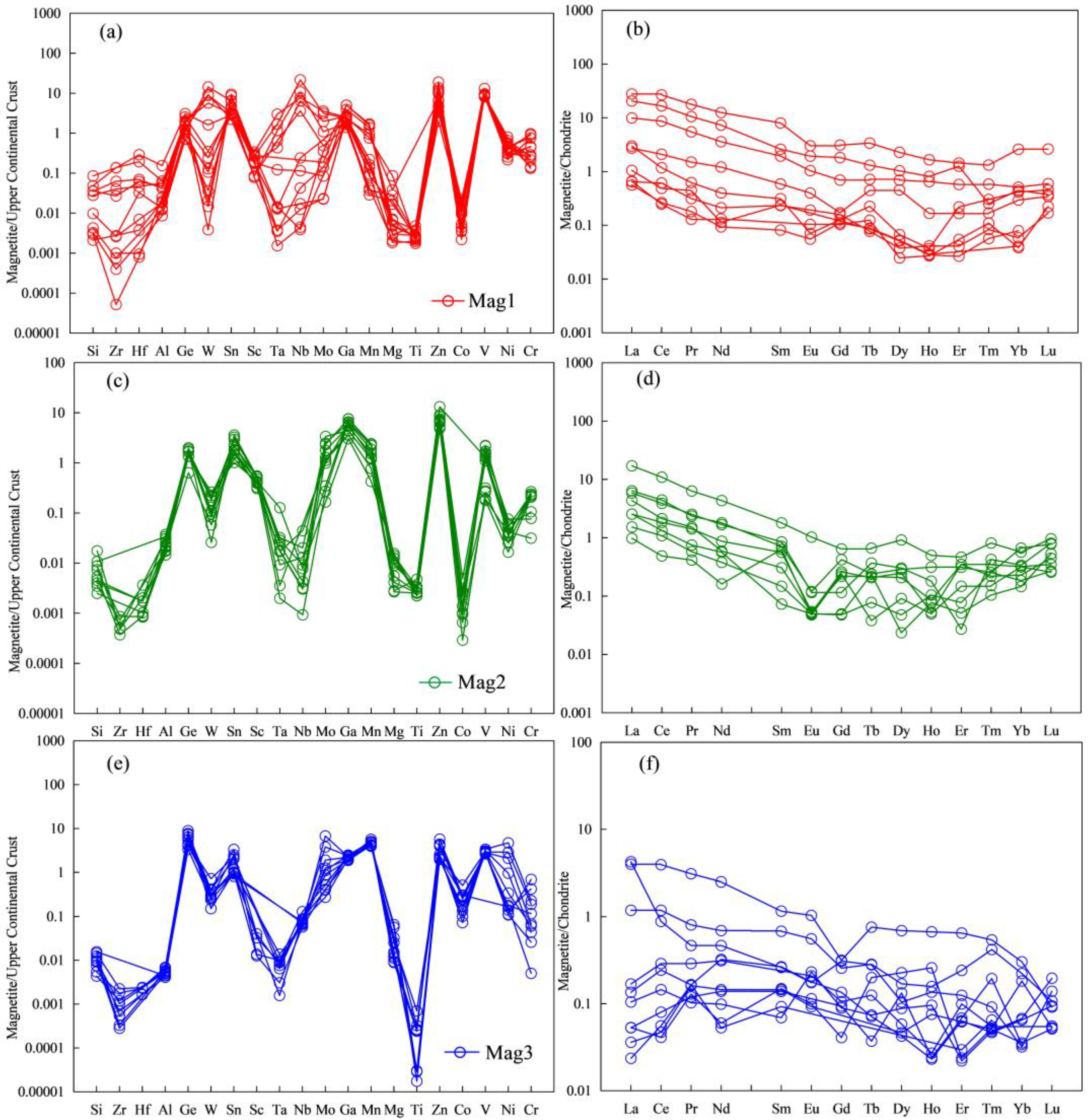
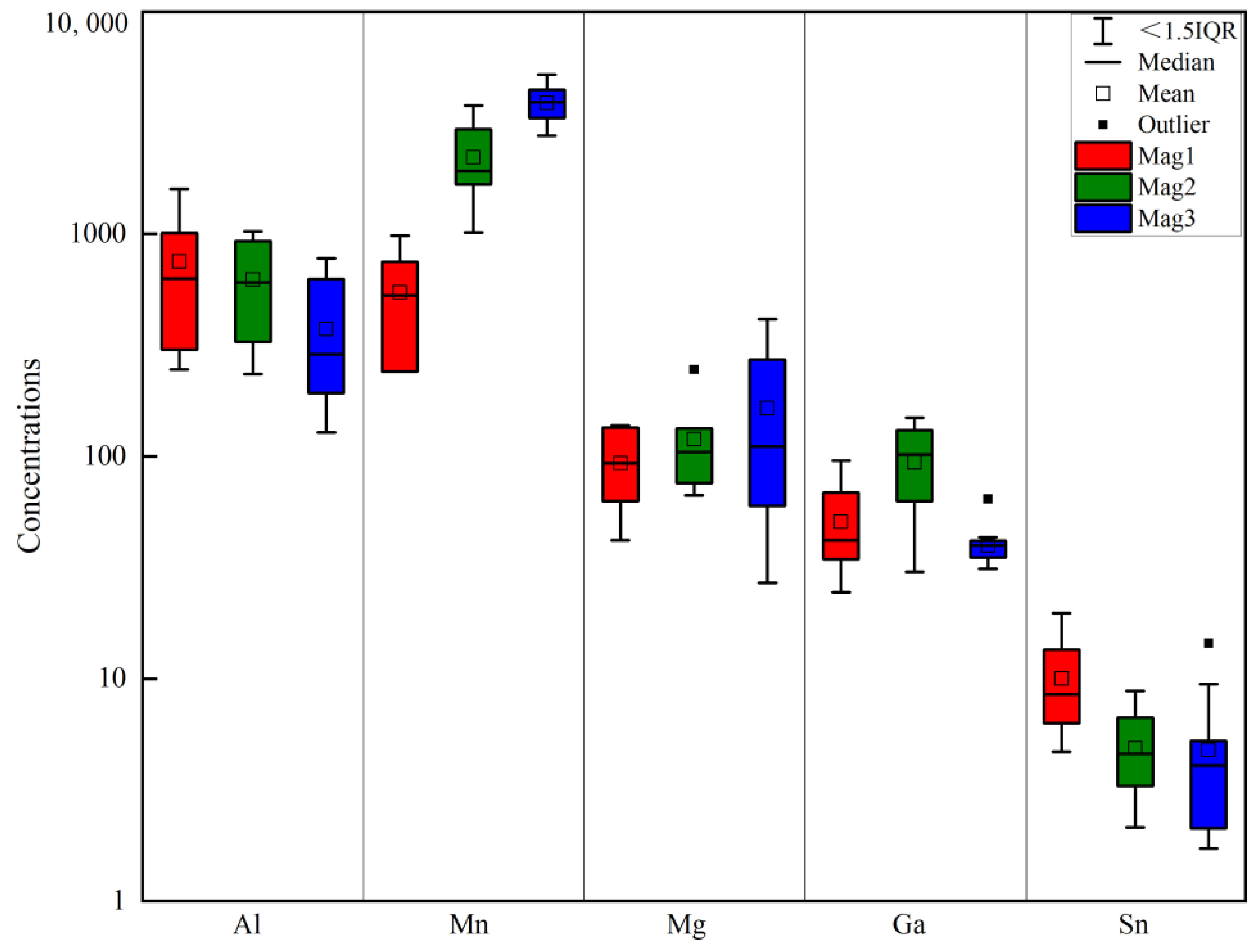
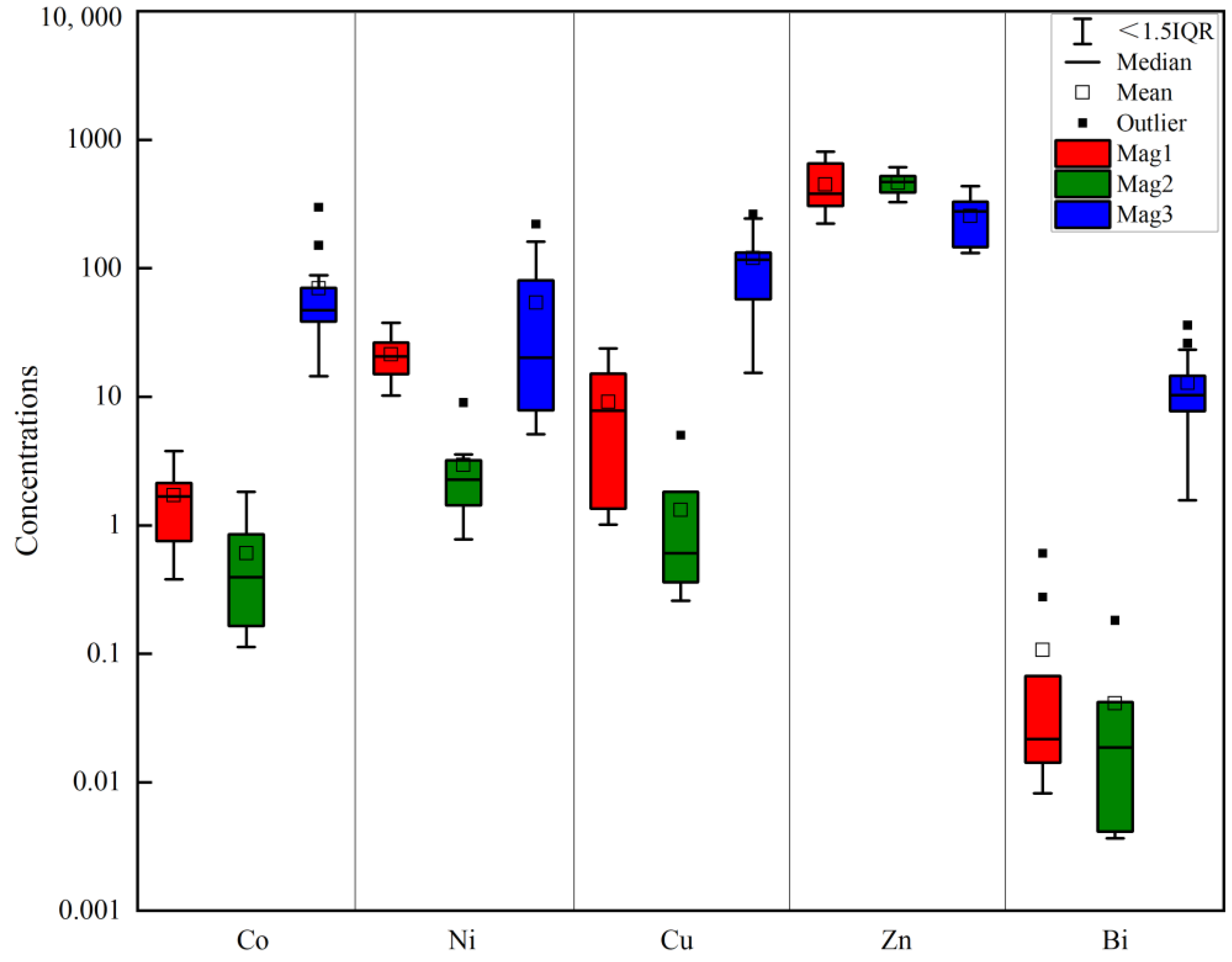
5.1. Major Elements
5.2. Trace and Rare Earth Elements
| Type | Mag1 | Mag2 | Mag3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | 10 | 8 | 11 | ||||||
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| Li | 0.44 | 12.72 | 2.60 | 0.86 | 4.56 | 2.63 | 0.34 | 4.07 | 1.30 |
| B | 0.43 | 4.64 | 2.02 | 2.31 | 6.87 | 5.20 | 0.16 | 10.43 | 3.28 |
| Sc | 1.10 | 4.76 | 2.78 | 4.31 | 9.65 | 6.86 | 0.46 | 0.95 | 0.72 |
| V | 787.50 | 1274.32 | 895.50 | 21.00 | 215.51 | 106.49 | 256.31 | 362.04 | 285.92 |
| Cr | 12.20 | 70.29 | 33.05 | 7.19 | 69.15 | 22.61 | 0.15 | 57.79 | 17.43 |
| Co | 0.38 | 3.77 | 1.90 | 0.11 | 1.82 | 0.61 | 14.51 | 298.72 | 77.69 |
| Ni | 10.21 | 37.73 | 22.09 | 0.78 | 9.01 | 2.95 | 5.12 | 220.71 | 36.01 |
| Cu | 0.13 | 23.78 | 6.75 | 0.26 | 5.02 | 1.32 | 15.41 | 264.60 | 138.38 |
| Zn | 222.39 | 807.39 | 448.50 | 326.37 | 610.59 | 402.82 | 131.44 | 435.30 | 255.50 |
| Ga | 24.45 | 68.92 | 40.97 | 53.13 | 149.48 | 100.72 | 31.26 | 43.28 | 38.65 |
| Ge | 1.00 | 4.32 | 2.31 | 1.90 | 3.57 | 2.36 | 4.90 | 12.21 | 8.68 |
| As | 7.57 | 15.52 | 10.66 | 3.37 | 7.79 | 6.16 | 2.47 | 13.55 | 8.03 |
| Rb | 0.09 | 4.08 | 1.61 | 0.35 | 1.85 | 0.97 | 0.16 | 17.57 | 3.23 |
| Sr | 0.13 | 87.76 | 10.63 | 0.30 | 4.12 | 1.28 | 0.36 | 24.62 | 5.53 |
| Y | 0.03 | 20.18 | 3.18 | 0.12 | 0.74 | 0.34 | 0.05 | 1.45 | 0.30 |
| Zr | 0.08 | 26.55 | 7.47 | 0.07 | 11.74 | 2.73 | 0.13 | 9.83 | 2.02 |
| Nb | 0.05 | 92.76 | 24.31 | 0.04 | 4.10 | 0.92 | 0.76 | 1.25 | 0.99 |
| Mo | 0.54 | 3.92 | 1.94 | 1.23 | 8.54 | 3.57 | 0.20 | 2.09 | 1.12 |
| Cd | 0.15 | 0.85 | 0.41 | 0.25 | 0.69 | 0.47 | 0.21 | 1.22 | 0.62 |
| In | 0.00 | 0.44 | 0.08 | 0.02 | 0.36 | 0.12 | 0.01 | 0.78 | 0.18 |
| Sn | 4.70 | 13.81 | 8.57 | 2.14 | 8.82 | 4.94 | 1.73 | 14.45 | 5.12 |
| Sb | 0.06 | 1.00 | 0.31 | 0.04 | 0.96 | 0.26 | 0.10 | 0.78 | 0.40 |
| Cs | 0.01 | 6.53 | 0.84 | 0.00 | 0.08 | 0.03 | 0.03 | 24.59 | 3.73 |
| Ba | 0.45 | 17.28 | 3.20 | 1.71 | 9.02 | 4.48 | 0.84 | 35.12 | 10.12 |
| Hf | 0.01 | 1.54 | 0.42 | 0.00 | 0.09 | 0.03 | 0.01 | 0.23 | 0.07 |
| Ta | 0.00 | 2.69 | 0.53 | 0.02 | 0.11 | 0.04 | 0.00 | 0.03 | 0.01 |
| W | 0.04 | 4.00 | 1.13 | 1.30 | 6.82 | 3.15 | 0.40 | 1.67 | 0.86 |
| Tl | 0.01 | 0.57 | 0.11 | 0.00 | 0.01 | 0.01 | 0.00 | 0.43 | 0.09 |
| Pb | 0.07 | 89.84 | 19.06 | 0.42 | 6.32 | 1.99 | 3.14 | 21.23 | 8.63 |
| Bi | 0.01 | 0.61 | 0.12 | 0.00 | 0.22 | 0.06 | 1.57 | 36.05 | 12.69 |
| Th | 0.02 | 66.69 | 12.62 | 0.05 | 1.37 | 0.40 | 0.01 | 0.33 | 0.09 |
| U | 0.00 | 94.76 | 13.35 | 0.01 | 0.86 | 0.18 | 0.05 | 13.46 | 2.10 |
| La | 0.04 | 6.59 | 1.23 | 0.23 | 4.05 | 1.19 | 0.01 | 1.00 | 0.24 |
| Ce | 0.09 | 16.36 | 2.75 | 0.30 | 6.67 | 1.95 | 0.02 | 2.40 | 0.53 |
| Pr | 0.01 | 1.70 | 0.28 | 0.04 | 0.60 | 0.18 | 0.00 | 0.29 | 0.07 |
| Nd | 0.03 | 5.86 | 0.95 | 0.08 | 2.03 | 0.59 | 0.02 | 1.17 | 0.31 |
| Sm | 0.01 | 1.23 | 0.22 | 0.01 | 0.28 | 0.09 | 0.01 | 0.21 | 0.07 |
| Eu | 0.00 | 3.27 | 0.51 | 0.00 | 0.06 | 0.01 | 0.00 | 0.07 | 0.02 |
| Gd | 0.02 | 39.73 | 5.80 | 0.01 | 0.13 | 0.05 | 0.02 | 0.23 | 0.06 |
| Tb | 0.00 | 4.14 | 0.54 | 0.00 | 0.02 | 0.01 | 0.00 | 0.03 | 0.01 |
| Dy | 0.01 | 26.63 | 2.76 | 0.01 | 0.23 | 0.07 | 0.01 | 0.18 | 0.05 |
| Ho | 0.00 | 6.54 | 0.67 | 0.00 | 0.03 | 0.01 | 0.00 | 0.04 | 0.01 |
| Er | 0.00 | 21.49 | 3.13 | 0.00 | 0.08 | 0.03 | 0.00 | 0.11 | 0.03 |
| Tm | 0.00 | 3.94 | 0.50 | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 | 0.00 |
| Yb | 0.01 | 32.14 | 3.28 | 0.02 | 0.11 | 0.06 | 0.01 | 0.08 | 0.03 |
| Lu | 0.00 | 5.89 | 0.75 | 0.01 | 0.02 | 0.01 | 0.00 | 0.01 | 0.00 |
| ΣREE | 0.19 | 34.13 | 7.34 | 0.91 | 14.32 | 4.38 | 0.08 | 5.52 | 1.37 |
| LREE | 0.17 | 31.91 | 6.89 | 0.75 | 13.68 | 4.12 | 0.05 | 5.04 | 1.21 |
| HREE | 0.02 | 2.22 | 0.45 | 0.08 | 0.64 | 0.26 | 0.01 | 0.60 | 0.16 |
| LREE/HREE | 7.73 | 19.39 | 12.59 | 4.51 | 22.61 | 15.16 | 1.88 | 14.41 | 7.15 |
| LaN/YbN | 5.20 | 45.94 | 15.30 | 3.08 | 30.16 | 14.07 | 1.02 | 13.11 | 4.31 |
| δEu | 0.32 | 1.02 | 0.70 | 0.11 | 0.84 | 0.38 | 0.00 | 3.95 | 1.43 |
| δCe | 0.63 | 1.27 | 0.95 | 0.70 | 1.03 | 0.88 | 0.38 | 1.62 | 1.07 |
6. Discussion
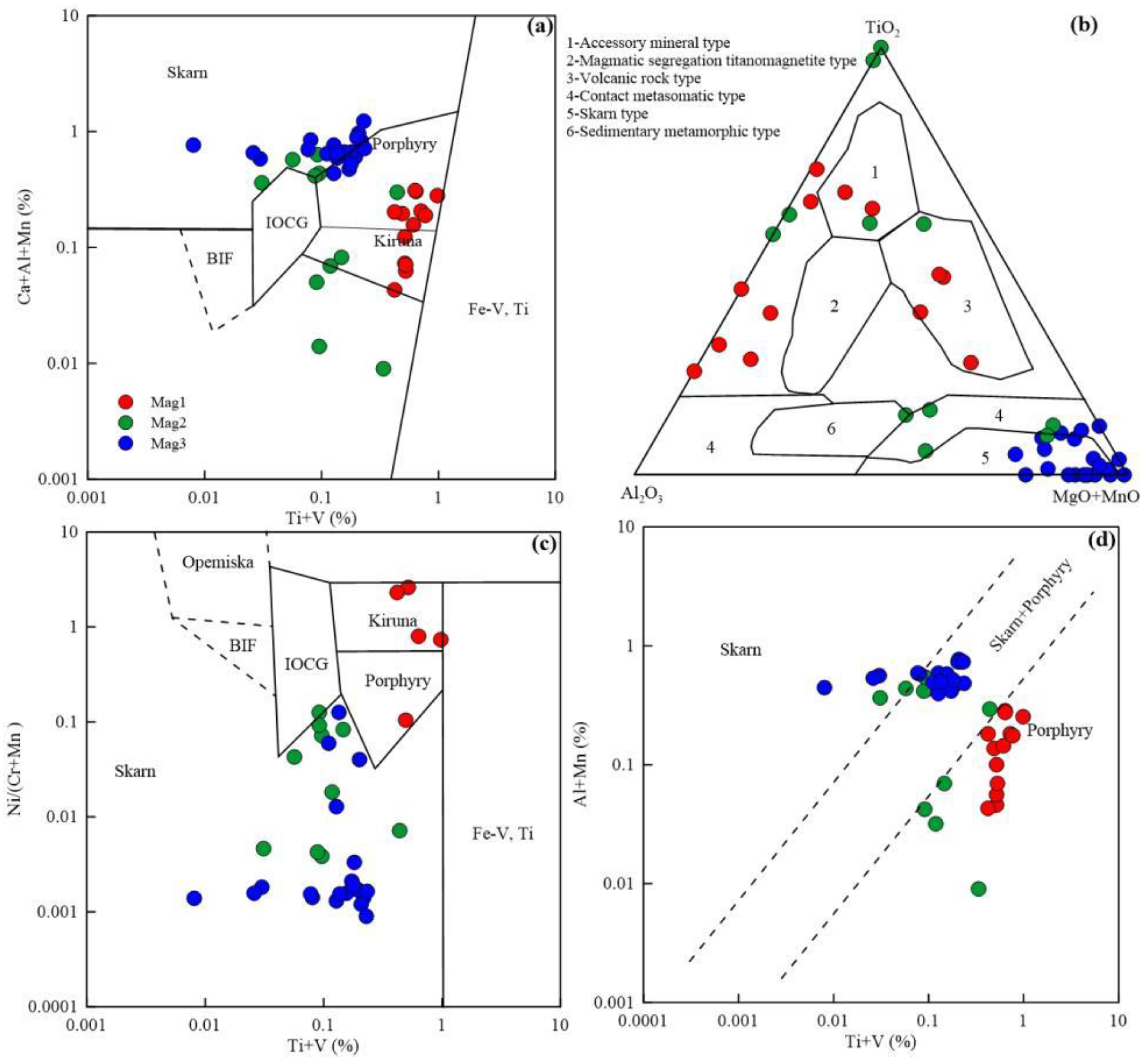
6.1. Origin of Magnetite
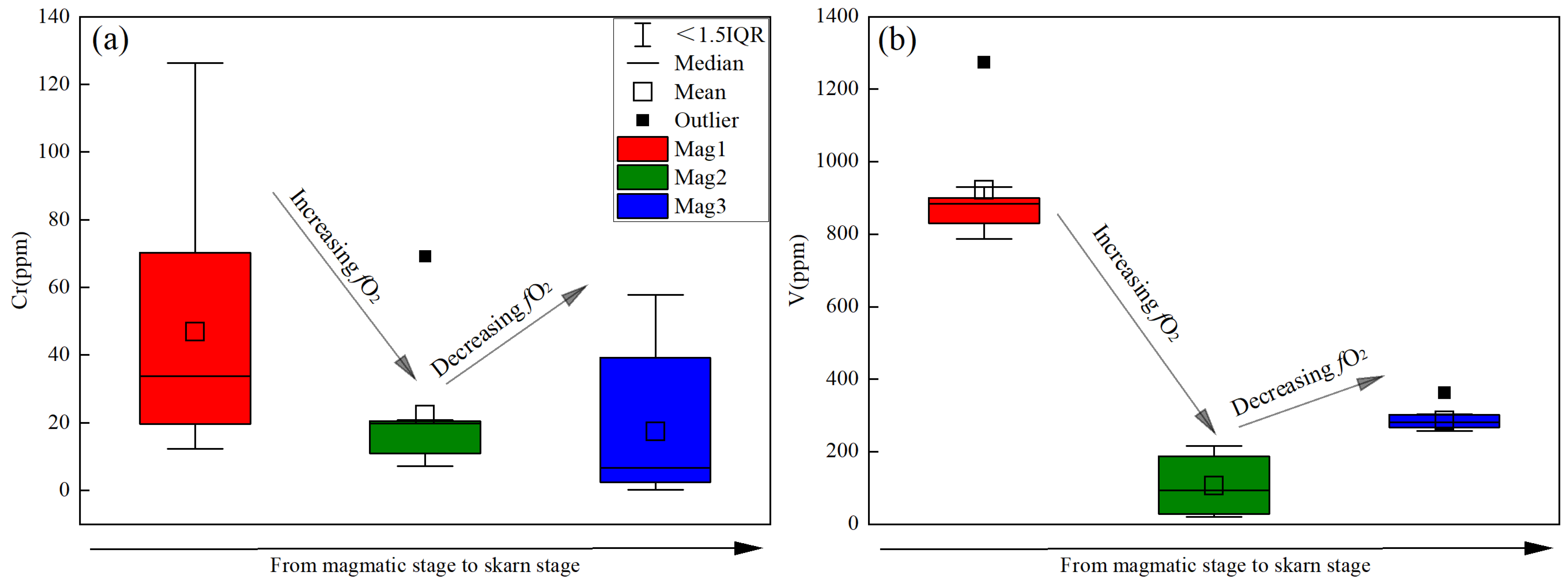
6.2. Element Behavior of Magnetite during Skarn Formation
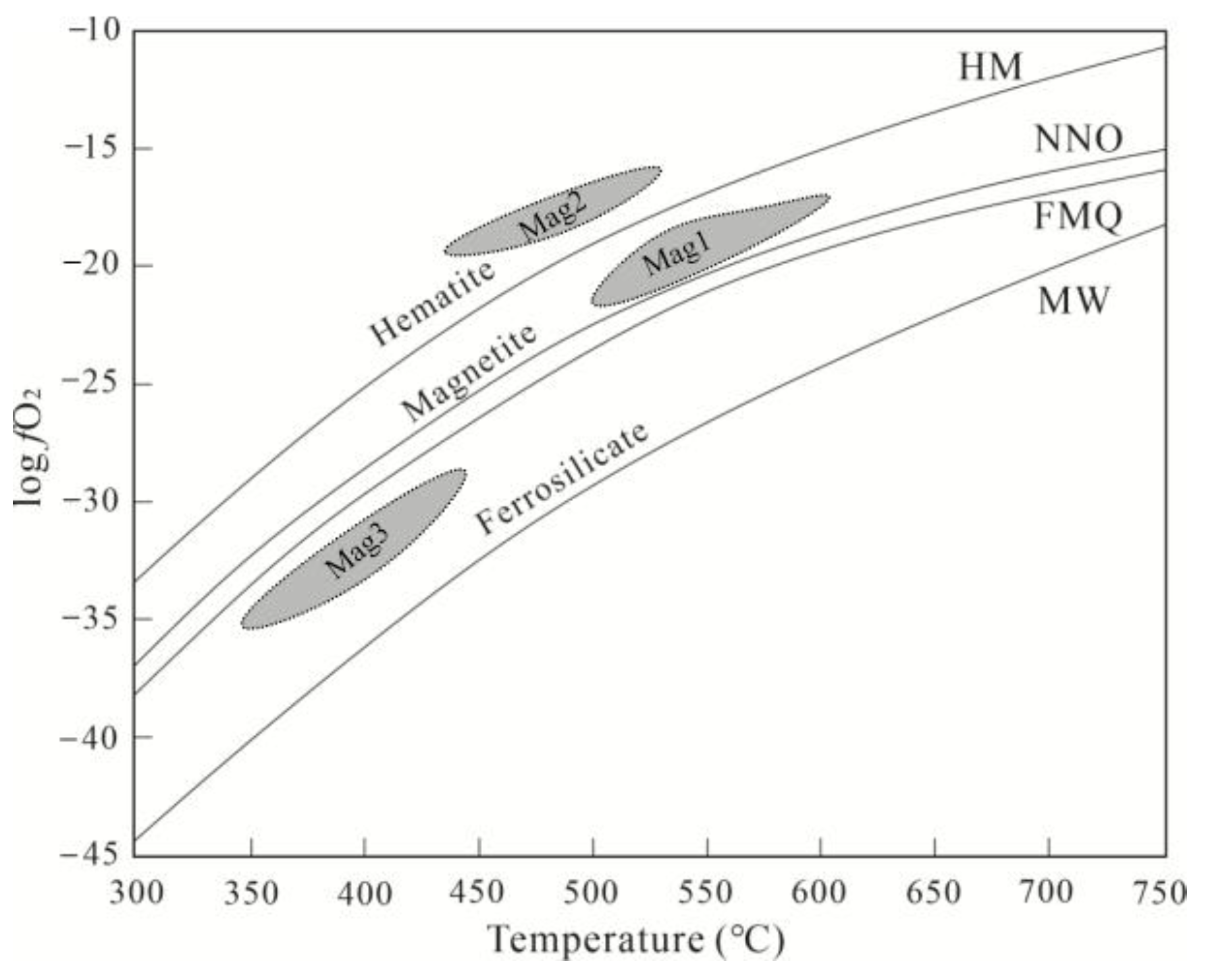
6.3. Constraints to Metallogenic Mechanism from Compositional Change of Magnetite
7. Conclusions
- (1)
- The magnetite in the Sandaozhuang deposit can be classified into three types: magmatic magnetite (Mag1), magmatic-hydrothermal magnetite (Mag2), and hydrothermal magnetite (Mag3). All three types of magnetite are depleted in high-field-strength elements (HFSEs) such as Zr, Hf, Nb, Ta, and Ti, as well as large-ion lithophile elements (LILEs) such as Rb, K, Ba, and Sr, and enriched in Sn, Mo, V, Cr, Zn, and Ga relative to the overall continental crust composition. Mag3 does not exhibit depletion in Co, Ni, Cu, and Bi, suggesting that magnetite precipitation occurred slightly earlier than sulfides. The depletion of Zn in Mag3 implies that Zn was extracted by later hydrothermal fluids during the same stage, indicating limited influence of coexisting sulfides on the composition of magnetite in the Sandaozhuang deposit.
- (2)
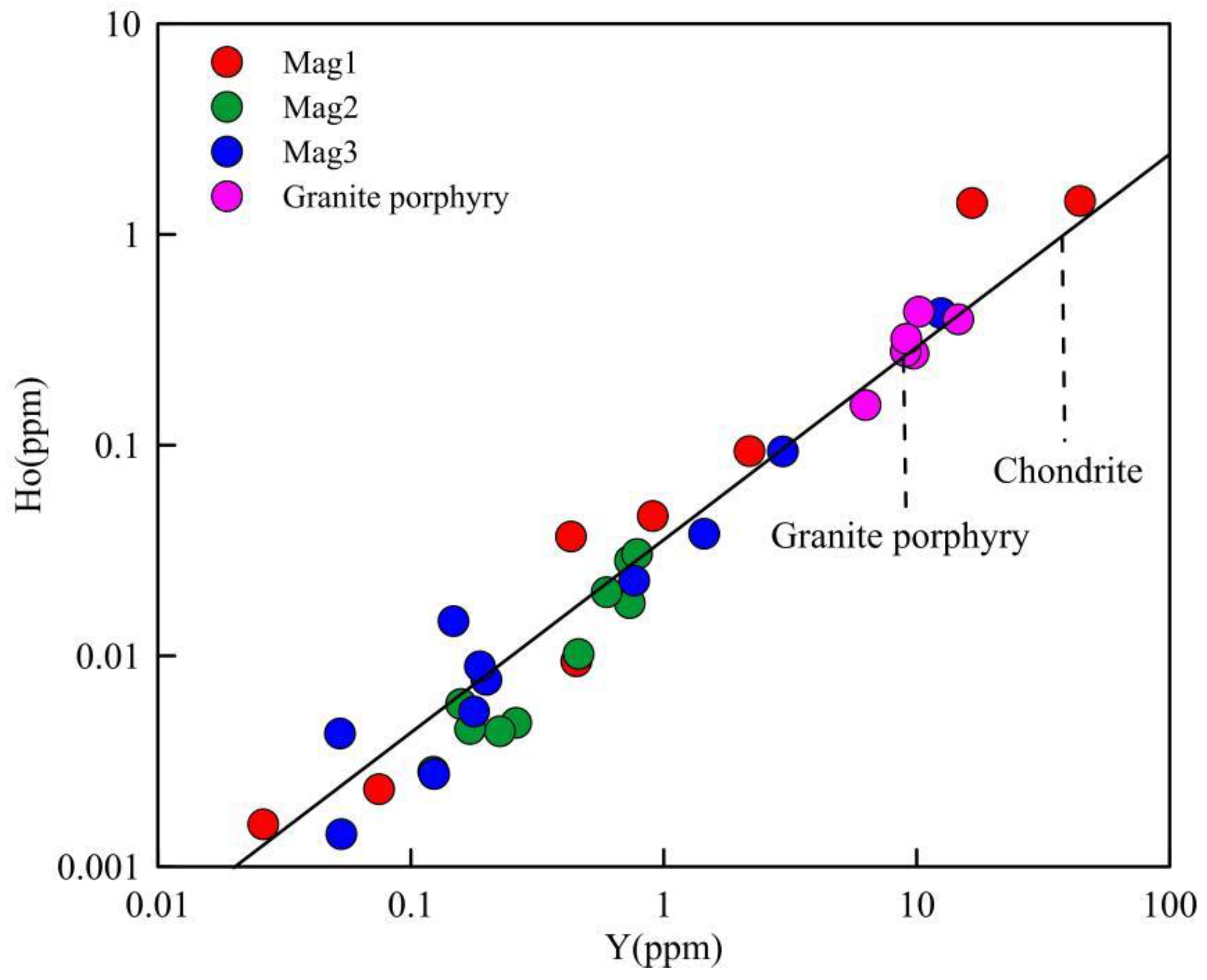
- Mag1, Mag2, and Mag3 show similar trace element spider and rare earth element distribution patterns, in addition to Y/Ho ratios, indicating a consistent magmatic source.
- (3)
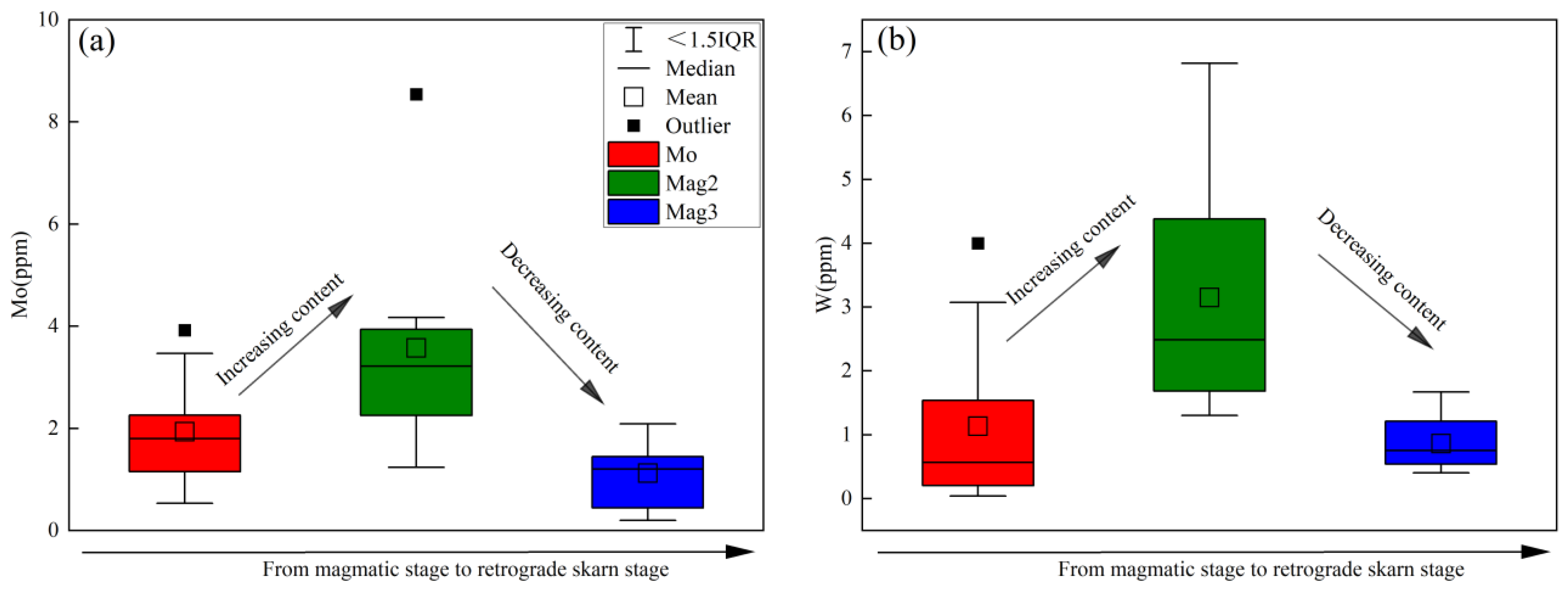
- The changes in V and Cr contents in magnetite, and the (Ti + V) and (Al + Mn) values, reflect the evolution of oxygen fugacity (increasing from Mag1 to Mag2 and decreasing from Mag2 to Mag3) and temperature (decreasing from Mag1 to Mag3) during the skarn mineralization process. The early high-temperature and high-oxygen-fugacity magmatic fluids further enriched W and Mo in the hydrothermal fluids. The rapid change in fluid properties during the retrograde alteration stage resulted in the precipitation of scheelite and molybdenite.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The Range of Spinel Compositions in Terrestrial Mafic and Ultramafic Rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Nadoll, P.; Koenig, A.E. LA-ICP-MS of magnetite: Methods and reference materials. J. Anal. Atom. Spectrom. 2011, 26, 1872–1877. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhou, M.-F.; Gao, J.-F.; Hu, R. Geochemistry of magnetite from Proterozoic Fe-Cu deposits in the Kangdian metallogenic province, SW China. Miner. Depos. 2015, 50, 795–809. [Google Scholar] [CrossRef]
- Hu, H.; Lentz, D.; Li, J.W.; McCarron, T.; Zhao, X.F.; Hall, D. Reequilibration processes in magnetite from iron skarn deposits. Econ. Geol. 2014, 110, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Han, J. Study of Magnetite: Problems and Future. Bull. Mineral. Petrol. Geochem. 2015, 34, 724–730. [Google Scholar]
- Canil, D.; Grondahl, C.; Lacourse, T.; Pisiak, L.K. Trace elements in magnetite from porphyry Cu-Mo-Au deposits in British Columbia, Canada. Ore Geol. Rev. 2016, 72, 1116–1128. [Google Scholar] [CrossRef]
- Ding, T.; Ma, D.; Lu, J.; Zhang, R. Magnetite as an indicator of mixed sources for W-Mo-Pb-Zn mineralization in the Huangshaping polymetallic deposit, southern Hunan Province, China. Ore Geol. Rev. 2018, 95, 65–78. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Yan, S. Minerals and relevant metallogeny and exploration. Acta Petrol. Sin. 2019, 35, 31–68. [Google Scholar]
- Liu, Y.; Fan, Y.; Zhou, T.; Xiao, X.; White, N.C.; Thompson, J.; Hong, H.; Zhang, L. Geochemical characteristics of magnetite in Longqiao skarn iron deposit in the Middle-Lower Yangtze Metallogenic Belt, Eastern China. Miner. Depos. 2019, 54, 1229–1242. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Hong, W.; Li, D.; Liang, P.; Fang, J.; Zhang, L.; Lai, C. Magnetite chemistry and implications for the magmatic-hydrothermal ore-forming process: An example from the Devonian Yuleken porphyry Cu system, NW China. Chem. Geol. 2019, 522, 1–15. [Google Scholar] [CrossRef]
- Guo, J.-H.; Leng, C.-B.; Zhang, X.-C.; Zafar, T.; Chen, W.T.; Zhang, W.; Tian, Z.-D.; Tian, F.; Lai, C.-K. Textural and chemical variations of magnetite from porphyry Cu-Au and Cu skarn deposits in the Zhongdian region, northwestern Yunnan, SW China. Ore Geol. Rev. 2020, 116, 103245. [Google Scholar] [CrossRef]
- Xiang, J.P.; Pei, R.F.; Xing, B.; Wang, C.Y.; Tian, Z.H.; Chen, X.D.; Ye, H.S.; Wang, H.L. The formation process and Mo (W) mineralizaton of the skarn in the NannihuSandaozhuang Mo (W) deposit. Geol. China 2016, 43, 2131–2153. [Google Scholar]
- Shi, Y.L.; Li, N.; Yang, Y. Ore geoloey and fluid inclusion geochemistry of the Sandaozhuang Mo-Wdeposit in Luanchuan county, Henan province. Acta Petrol. Sin. 2009, 25, 2575–2587. [Google Scholar]
- Zhan, Q.; Gao, X.Y.; Meng, L.; Zhao, T.P. Ore genesis and fluid evolution of the Sandaozhuang supergiant W-Mo skarn deposit, southern margin of the North China Craton: Insights from scheelite, garnet and clinopyroxene geochemistry. Ore Geol. Rev. 2021, 139, 104551. [Google Scholar] [CrossRef]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19, 145–162. [Google Scholar]
- Nie, X.; Yi, J.W.; Chen, P.P.; Wang, M.Y.; Yan, X.G.; Hou, F.J.; Zhang, S.T.; Li, L. Skarn mineral characteristics of Sandaozhuang Mo-W deposit and their geological significance. J. Chin. Electron Microsc. Soc. 2014, 33, 108–116. [Google Scholar]
- Chen, Y.F.; Fu, S.G.; Qiang, L.Z. The tectonic environment for the formation of the Xionger Group and the Xiyanghe Group. Geol. Rev. 1992, 38, 325–333. [Google Scholar]
- Mao, J.W.; Pirajno, F.; Xiang, J.F.; Gao, J.J.; Ye, H.S.; Li, Y.F.; Guo, B.J. Mesozoic molybdenum deposits in the East Qinling-Dabie orogenic belt: Characteristics and tectonic settings. Ore Geol. Rev. 2011, 43, 264–293. [Google Scholar] [CrossRef]
- Zhang, G.W.; Meng, Q.R.; Yu, Z.P.; Sun, Y.; Zhou, D.W.; Guo, A.L. Orogenic process and its dynamic characteristics of the Qinling Orogenic belt. Sci. Sin. (Terrae) 1996, 3, 193–200. [Google Scholar]
- Zhang, Z.W.; Yang, H.Z.; Zhu, B.Q. The Development of Sedimentary Buildups and Mineralization in the Eastern Qinling Mountain. Acta Geosci. Sin. 2003, 24, 293–298. [Google Scholar]
- Li, N.; Ulrich, T.; Chen, Y.-J.; Thomsen, T.B.; Pease, V.; Pirajno, F. Fluid evolution of the Yuchiling porphyry Mo deposit, East Qinling, China. Ore Geol. Rev. 2012, 48, 442–459. [Google Scholar] [CrossRef]
- Yang, Y.F.; Li, N.; Yang, Y. Fluid inclusion study of the Nannihu porphyry Mo-W deposit, Luanchuan county, Henan province. Acta Petrol. Sin. 2009, 25, 2550–2562. [Google Scholar]
- Xiang, J.; Pei, R.; Ye, H.; Wang, C.; Tian, Z. Source and evolution of the ore-forming fluid in the Nannihu-Sandaozhuang Mo (W) deposit: Constraints from C-H-O stable isotope data. Geol. China 2012, 39, 1778–1789. [Google Scholar]
- Hu, Z.; Gao, S.; Liu, Y.; Hu, S.; Chen, H.; Yuan, H. Signal enhancement in laser ablation ICP-MS by addition of nitrogen in the central channel gas. J. Anal. Atom. Spectrom. 2008, 23, 1093–1101. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Y.; Gao, S.; Liu, W.; Zhang, W.; Tong, X.; Lin, L.; Zong, K.; Li, M.; Chen, H.; et al. Improved in situ Hf isotope ratio analysis of zircon using newly designed X skimmer cone and jet sample cone in combination with the addition of nitrogen by laser ablation multiple collector ICP-MS. J. Anal. Atom. Spectrom. 2012, 27, 1391–1399. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Zong, K.; Gao, C.; Gao, S.; Xu, J.; Chen, H. Reappraisement and refinement of zircon U-Pb isotope and trace element analyses by LA-ICP-MS. Chin. Sci. Bull. 2010, 55, 1535–1546. [Google Scholar] [CrossRef]
- Rudnick, R.; Gao, S. Composition of the Continental Crust. Ref. Mod. Earth Syst. Environ. Sci. 2014, 4, 1–51. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Lin, S. Discussion on mineral chemistry, genesis and evolution of Magnetite. Acta Mineral. Sin. 1982, 3, 166–174. [Google Scholar]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Huang, X.W.; Beaudoin, G. Textures and Chemical Compositions of Magnetite from Iron Oxide Copper-Gold (IOCG) and Kiruna-Type Iron Oxide-Apatite (IOA) Deposits and Their Implications for Ore Genesis and Magnetite Classification Schemes. Econ. Geol. 2019, 114, 953–979. [Google Scholar] [CrossRef]
- Huang, X.W.; Boutroy, É.; Makvandi, S.; Beaudoin, G.; Corriveau, L.; De Toni, A.F. Trace element composition of iron oxides from IOCG and IOA deposits: Relationship to hydrothermal alteration and deposit subtypes. Miner. Depos. 2018, 54, 525–552. [Google Scholar] [CrossRef]
- Huang, X.W.; Sappin, A.-A.; Boutroy, É.; Beaudoin, G.; Makvandi, S. Trace Element Composition of Igneous and Hydrothermal Magnetite from Porphyry Deposits: Relationship to Deposit Subtypes and Magmatic Affinity. Econ. Geol. 2019, 114, 917–952. [Google Scholar] [CrossRef]
- James, B.R. Chromium. In Encyclopedia of Water Science; Stewart, B.A., Howell, T.A., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 77–82. [Google Scholar]
- Nadoll, P.; Mauk, J.L.; Leveille, R.A.; Koenig, A.E. Geochemistry of magnetite from porphyry Cu and skarn deposits in the southwestern United States. Miner. Depos. 2015, 50, 493–515. [Google Scholar] [CrossRef]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. Mineral. Petrol. 2002, 144, 22–37. [Google Scholar] [CrossRef]
- Acosta-Gongora, P.; Gleeson, S.A.; Samson, I.M. Trace Element Geochemistry of Magnetite and Its Relationship to Cu-Bi-Co-Au-Ag-U-W Mineralization in the Great Bear Magmatic Zone, NWT, Canada. Econ. Geol. 2014, 109, 1901–1928. [Google Scholar] [CrossRef]
- Broughm, S.G.; Hanchar, J.M.; Tornos, F.; Westhues, A.; Attersley, S. Mineral chemistry of magnetite from magnetite-apatite mineralization and their host rocks: Examples from Kiruna, Sweden, and El Laco, Chile. Miner. Depos. 2017, 52, 1223–1244. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Enivron. Pollut. 2004, 107, 263–283. [Google Scholar] [CrossRef]
- Righter, K.; Leeman, W.P.; Hervig, R.L. Partitioning of Ni, Co and V between spinel-structured oxides and silicate melts: Importance of spinel composition. Chem. Geol. 2006, 227, 1–25. [Google Scholar] [CrossRef]
- Balan, E.; Villiers, J.P.R.D.; Eeckhout, S.G.; Glatzel, P.; Toplis, M.J.; Fritsch, E.; Allard, T.; Galoisy, L.; Calas, G. The oxidation state of vanadium in titanomagnetite from layered basic intrusions. Am. Mineral. 2006, 91, 953–956. [Google Scholar] [CrossRef]
- Sun, X.; Lin, H.; Fu, Y.; Li, D.; Hollings, P.; Yang, T.; Liu, Z. Trace element geochemistry of magnetite from the giant Beiya gold-polymetallic deposit in Yunnan Province, Southwest China and its implications for the ore forming processes. Ore Geol. Rev. 2017, 91, 477–490. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Zhang, L.; Li, D.; Zhang, W.; Wang, C.; Yang, J.; Yan, X. Magnetite geochemistry of the Heijianshan Fe-Cu (-Au) deposit in Eastern Tianshan: Metallogenic implications for submarine volcanic-hosted Fe-Cu deposits in NW China. Ore Geol. Rev. 2018, 100, 422–440. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Li, D.; Lin, H. U-Pb Geochronology and Geochemistry of U-Rich Garnet from the Giant Beiya Gold-Polymetallic Deposit in SW China: Constraints on Skarn Mineralization Process. Minerals 2018, 8, 128. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D.H. Iron-Titanium Oxide Minerals and Synthetic Equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- McIntire, W.L. Trace element partition coefficients—A review of theory and applications to geology. Geochim. Cosmochim. Acta 1963, 27, 1209–1264. [Google Scholar] [CrossRef]
- Knipping, J.L.; Bilenker, L.D.; Simon, A.C.; Reich, M.; Barra, F.; Deditius, A.P.; Wälle, M.; Heinrich, C.A.; Holtz, F.; Munizaga, R. Trace elements in magnetite from massive iron oxide-apatite deposits indicate a combined formation by igneous and magmatic-hydrothermal processes. Geochim. Cosmochim. Acta 2015, 171, 15–38. [Google Scholar] [CrossRef]
- O’Neil, H.S.C.; Navrotsky, A. Cation distributions and thermodynamic properties of binary spinel solid solutions. Am. Mineral. 1984, 69, 733–753. [Google Scholar]
- Shannon, R.D.J. Revised Effective Ionic Radii and Systematic Study of Inter Atomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 1996, 143, 245–255. [Google Scholar] [CrossRef]
- Bao, Z.W.; Zeng, Q.S.; Zhao, T.P.; Yuan, Z.L. Geochemistry and petrogenesis of the ore-related Nannihu and Shangfanggou granite porphyries from east Qinling belt and their constaints on the molybdenum mineralization. Acta Petrol. Sin. 2009, 25, 2523–2536. [Google Scholar]
- Flynn, R.T.; Wayne Burnham, C. An experimental determination of rare earth partition coefficients between a chloride containing vapor phase and silicate melts. Geochim. Cosmochim. Acta 1978, 42, 685–701. [Google Scholar] [CrossRef]
- Ayers, J.C.; Eggler, D.H. Partitioning of elements between silicate melt and H2O-NaCl fluids at 1.5 and 2.0 GPa pressure: Implications for mantle metasomatism. Geochim. Cosmochim. Acta 1995, 59, 4237–4246. [Google Scholar] [CrossRef]
- Bai, T.B.; Van Groos, A.K. The distribution of Na, K, Rb, Sr, Al, Ge, Cu, W, Mo, La, and Ce between granitic melts and coexisting aqueous fluids. Geochim. Cosmochim. Acta 1999, 63, 1117–1131. [Google Scholar] [CrossRef]
- Ghaderi, M.; Palin, J.M.; Campbell, I.H.; Sylvester, P.J. Rare earth element systematics in scheelite from hydrothermal gold deposits in the Kalgoorlie-Norseman region, Western Australia. Econ. Geol. 1999, 94, 423–437. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The Hydrothermal Geochemistry of Tungsten in Granitoid Environments: I. Relative Solubilities of Ferberite and Scheelite as a Function of T, P, pH, and mNaCl. Econ. Geol. 2000, 95, 143–182. [Google Scholar] [CrossRef]
- Reed, M.J.; Candela, P.A.; Piccoli, P.M. The distribution of rare earth elements between monzogranitic melt and the aqueous volatile phase in experimental investigations at 800 °C and 200 MPa. Contirb. Mineral. Petrol. 2000, 140, 251–262. [Google Scholar] [CrossRef]
- Gaspar, M.; Knaack, C.; Meinert, L.D.; Moretti, R. REE in skarn systems: A LA-ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmochim. Acta 2008, 72, 185–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Shao, Y.; Li, H. Fingerprinting the Hydrothermal Fluid Characteristics from LA-ICP-MS Trace Element Geochemistry of Garnet in the Yongping Cu Deposit, SE China. Minerals 2017, 7, 199. [Google Scholar] [CrossRef]
- Allen, D.E.; Seyfried, W.E. REE controls in ultramafic hosted MOR hydrothermal systems: An experimental study at elevated temperature and pressure. Geochim. Cosmochim. Acta 2005, 69, 675–683. [Google Scholar] [CrossRef]
- Zhai, D.-G.; Liu, J.-J.; Zhang, H.-Y.; Wang, J.-P.; Su, L.; Yang, X.-A.; Wu, S.-H. Origin of oscillatory zoned garnets from the Xieertala Fe-Zn skarn deposit, northern China: In situ LA-ICP-MS evidence. Lithos 2014, 190–191, 279–291. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Zhang, D.; Carranza, E.J.M.; Zhai, D.; Ge, L.; Sun, H.; Wang, B.; Chen, Y.; Liu, P. Genesis of the Saishitang skarn type copper deposit, West Qinling, Qinghai Province: Evidence from fluid inclusions and stable isotopes. Ore Geol. Rev. 2016, 75, 268–283. [Google Scholar] [CrossRef]





| Type | Mag1 | Mag2 | Mag3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | 13 | 11 | 20 | ||||||
| Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | |
| SiO2 | 0.03 | 0.88 | 0.22 | 0.02 | 0.66 | 0.11 | 0.07 | 2.28 | 0.76 |
| TiO2 | 0.04 | 0.47 | 0.16 | 0.02 | 0.42 | 0.12 | 0.00 | 0.08 | 0.02 |
| Al2O3 | 0.01 | 0.29 | 0.12 | 0.00 | 0.18 | 0.08 | 0.00 | 0.14 | 0.05 |
| FeOT | 91.76 | 93.67 | 92.65 | 91.20 | 93.21 | 92.43 | 87.85 | 93.37 | 91.60 |
| MnO | 0.00 | 0.13 | 0.03 | 0.00 | 0.49 | 0.16 | 0.36 | 0.67 | 0.50 |
| MgO | 0.01 | 0.05 | 0.02 | 0.01 | 0.02 | 0.01 | 0.05 | 0.15 | 0.06 |
| CaO | 0.01 | 0.06 | 0.02 | 0.04 | 0.13 | 0.03 | 0.02 | 0.49 | 0.15 |
| Na2O | 0.01 | 0.09 | 0.04 | 0.02 | 0.06 | 0.03 | 0.03 | 0.07 | 0.03 |
| K2O | 0.05 | 0.17 | 0.05 | 0.04 | 0.04 | 0.02 | 0.02 | 0.05 | 0.03 |
| Cr2O3 | 0.01 | 0.13 | 0.05 | 0.02 | 0.14 | 0.06 | 0.01 | 0.13 | 0.04 |
| V2O3 | 0.30 | 0.54 | 0.44 | 0.01 | 0.06 | 0.03 | 0.01 | 0.25 | 0.12 |
| Total | 93.41 | 94.56 | 93.93 | 92.73 | 93.81 | 93.22 | 91.48 | 94.43 | 93.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Siddique, U.; Sun, M.; Gao, Q.; Chen, Y.; Chen, L.; Li, Z. The Mineral Chemistry of Magnetite and Its Constraints on Ore-Forming Mechanism in the Sandaozhuang Skarn-Type W-Mo Deposit in East Qinling, China. Minerals 2023, 13, 1091. https://doi.org/10.3390/min13081091
Zeng Z, Siddique U, Sun M, Gao Q, Chen Y, Chen L, Li Z. The Mineral Chemistry of Magnetite and Its Constraints on Ore-Forming Mechanism in the Sandaozhuang Skarn-Type W-Mo Deposit in East Qinling, China. Minerals. 2023; 13(8):1091. https://doi.org/10.3390/min13081091
Chicago/Turabian StyleZeng, Zhijie, Uzair Siddique, Miaojun Sun, Qin Gao, Yanting Chen, Lei Chen, and Zilong Li. 2023. "The Mineral Chemistry of Magnetite and Its Constraints on Ore-Forming Mechanism in the Sandaozhuang Skarn-Type W-Mo Deposit in East Qinling, China" Minerals 13, no. 8: 1091. https://doi.org/10.3390/min13081091







