Gas Barrier Properties of Multilayer Polymer–Clay Nanocomposite Films: A Multiscale Simulation Approach
Abstract
:1. Introduction
2. Model and Methods
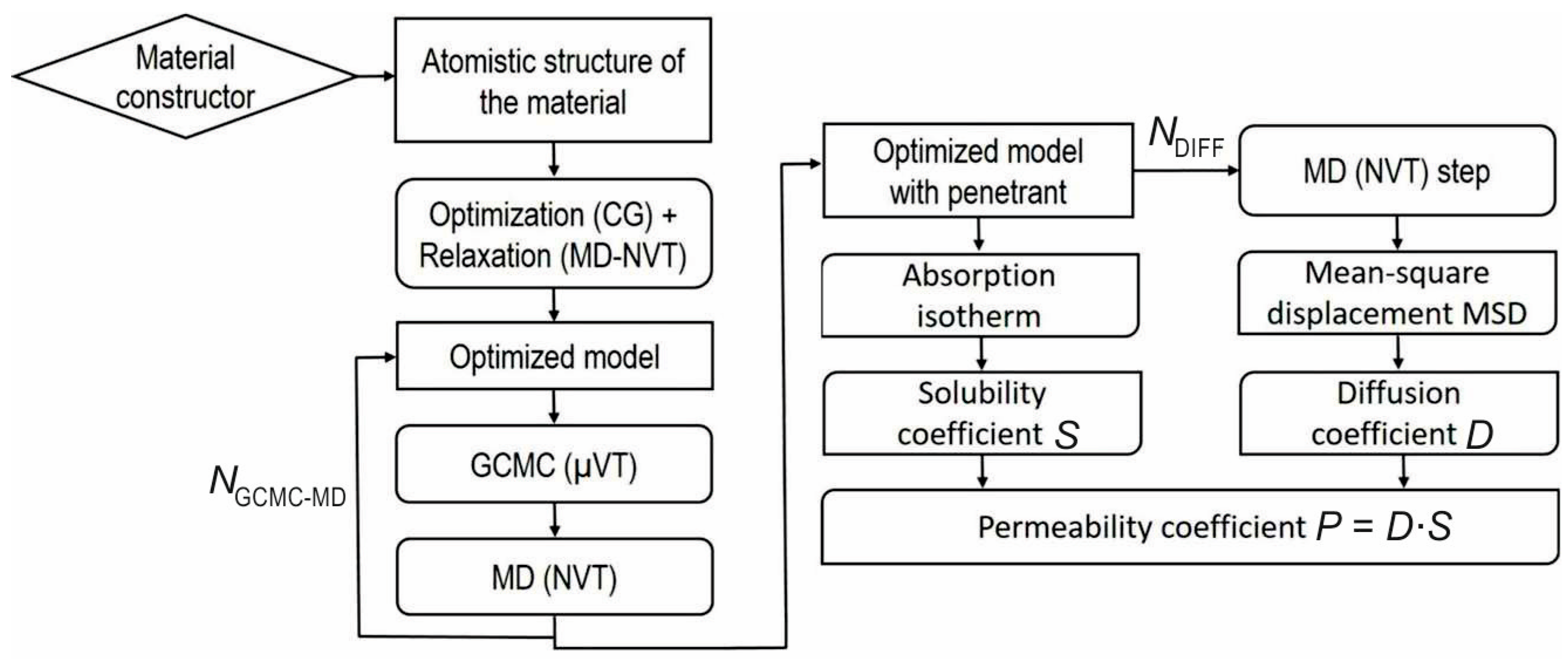
2.1. Multiscale Model Description
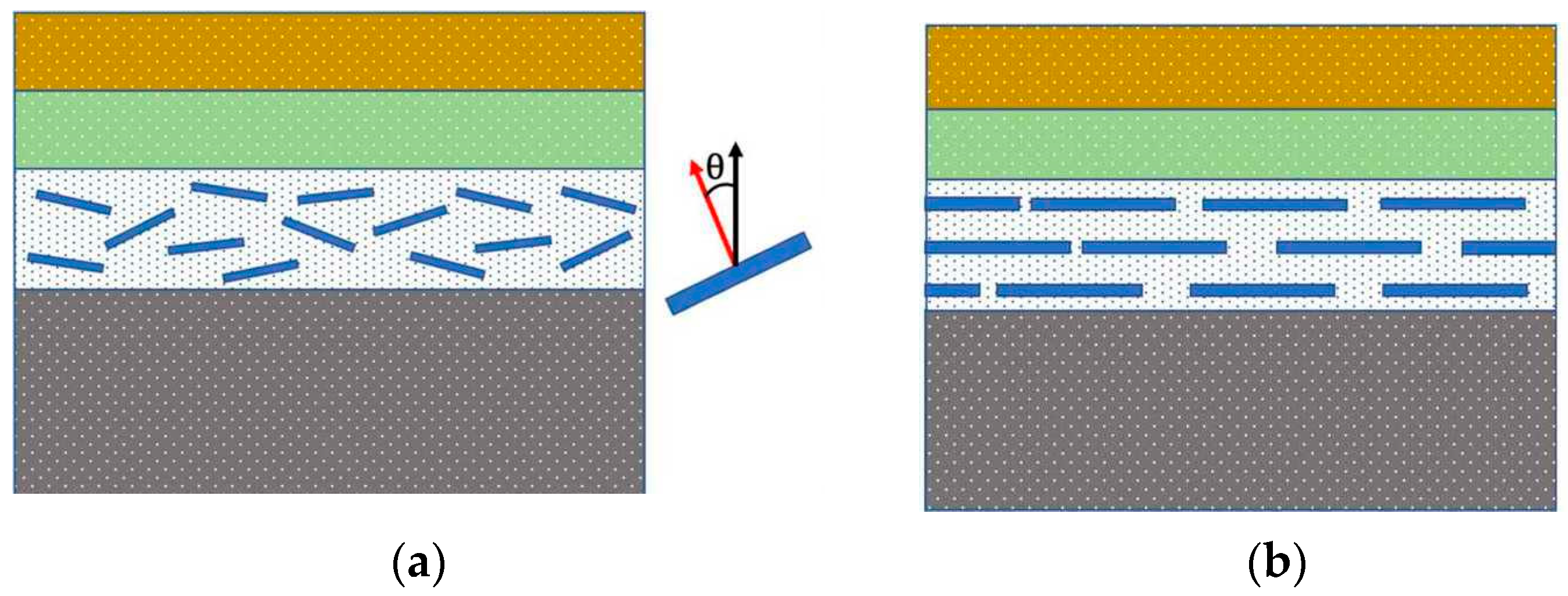
2.2. Continuous Level
2.3. The Quantitative Structure–Property Correlations
2.4. Full Atomic Level
3. Results of Full Atomistic Calculations
3.1. Generation of Polymer Matrices
3.2. Gas Permeability of Selected Polymers
3.2.1. Calculations of the Diffusion Coefficients
| D(O2) (cm2/s) | D(H2O) (cm2/s) | |||
|---|---|---|---|---|
| Material | Calculation | Experiment [84] | Calculation | Experiment [84] |
| PET | 2.0 ± 0.4 × 10−8 | 3–80 × 10−9 | 2 ± 0.5 × 10−8 | 0.3–13 × 10−8 |
| PE | 2.7 ± 0.7 × 10−7 | 0.2–12 × 10−7 | 3.8 ± 0.7 × 10−7 | 0.6–13 × 10−7 |
| PVDF | 2.3 ± 0.4 × 10−7 | 1.7 × 10−7 | 2.5 ± 1.0 × 10−7 | 5–8 × 10−7 |
| PTFE | 2.6 ± 0.4 × 10−6 | 1.5 × 10−7 | 2.6 ± 0.6 × 10−6 | 1.5–12 × 10−7 |
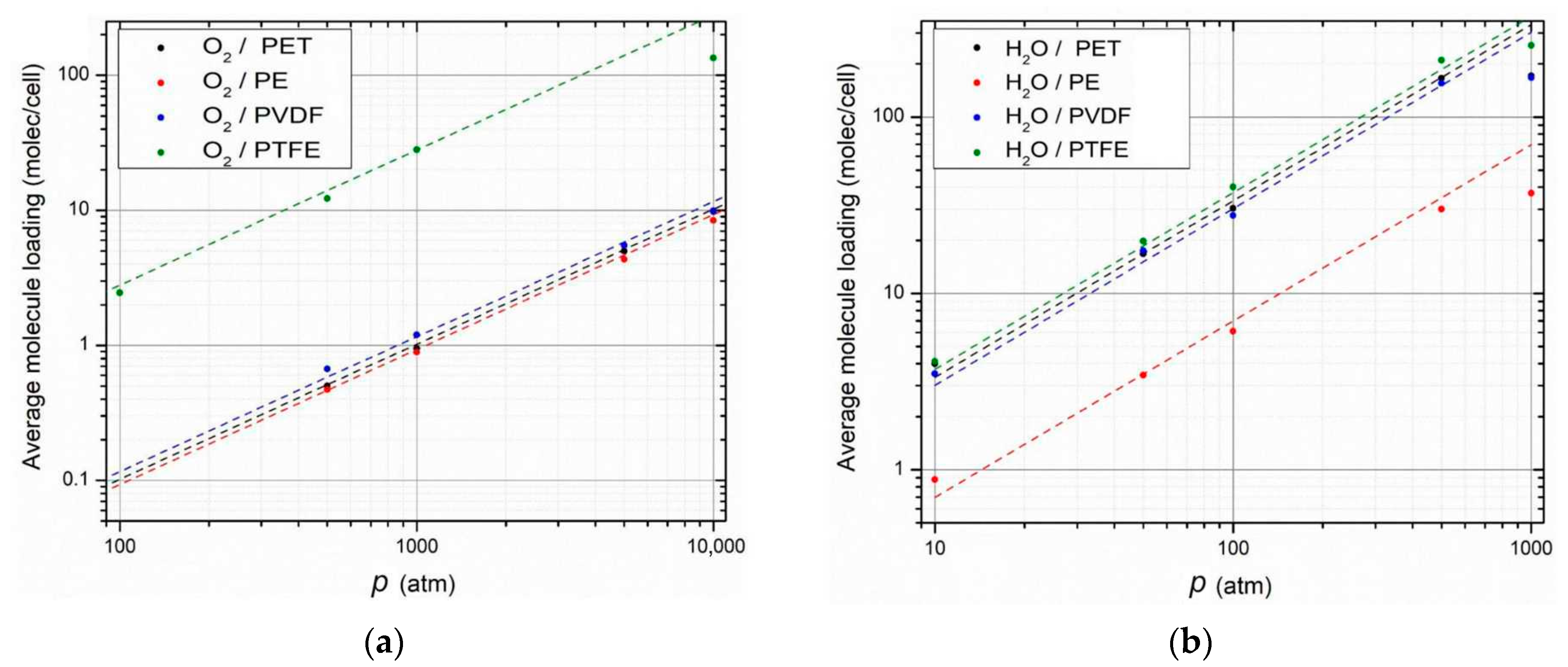
3.2.2. Calculations of the Solubility Coefficients
3.2.3. Calculations of Permeability Coefficients
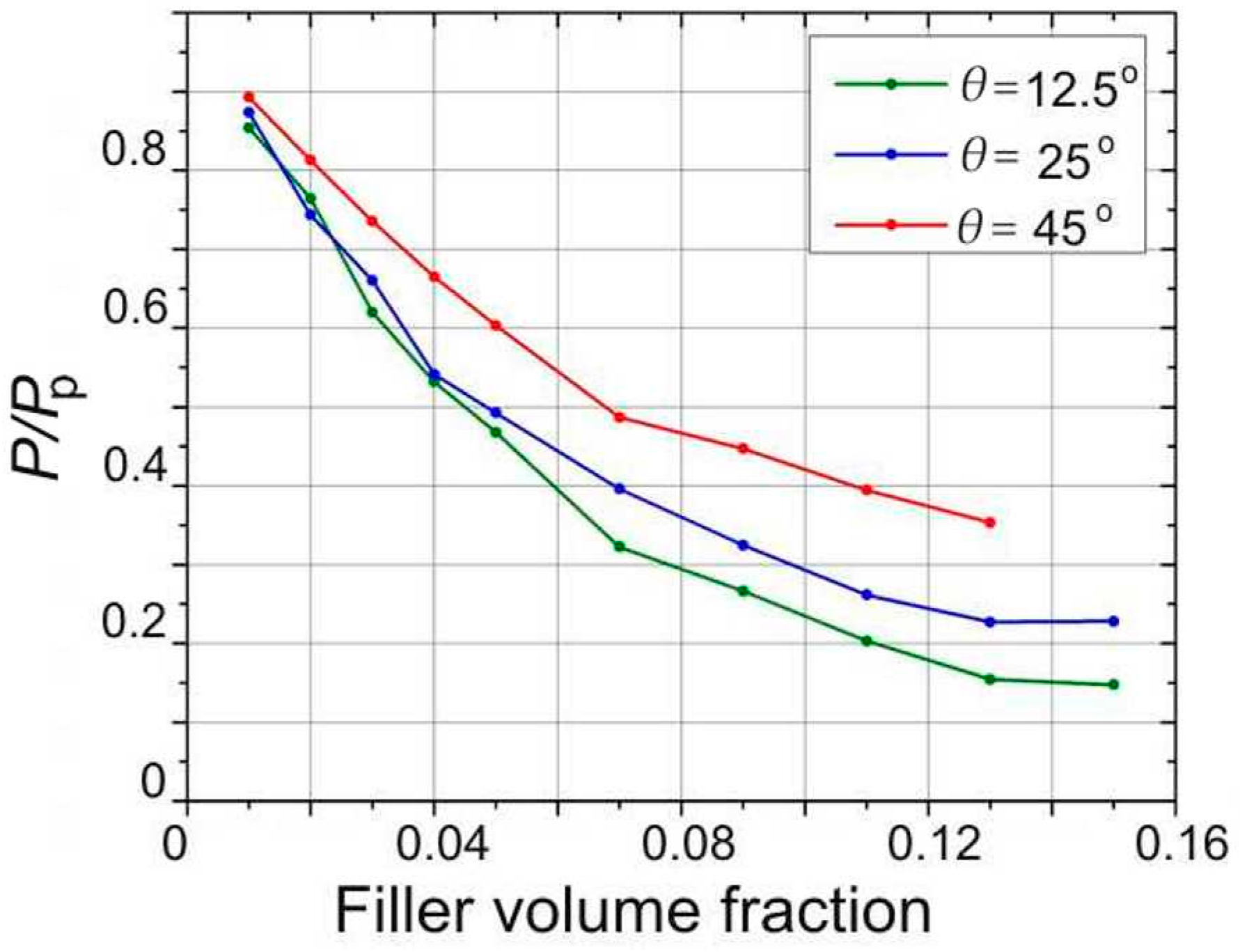
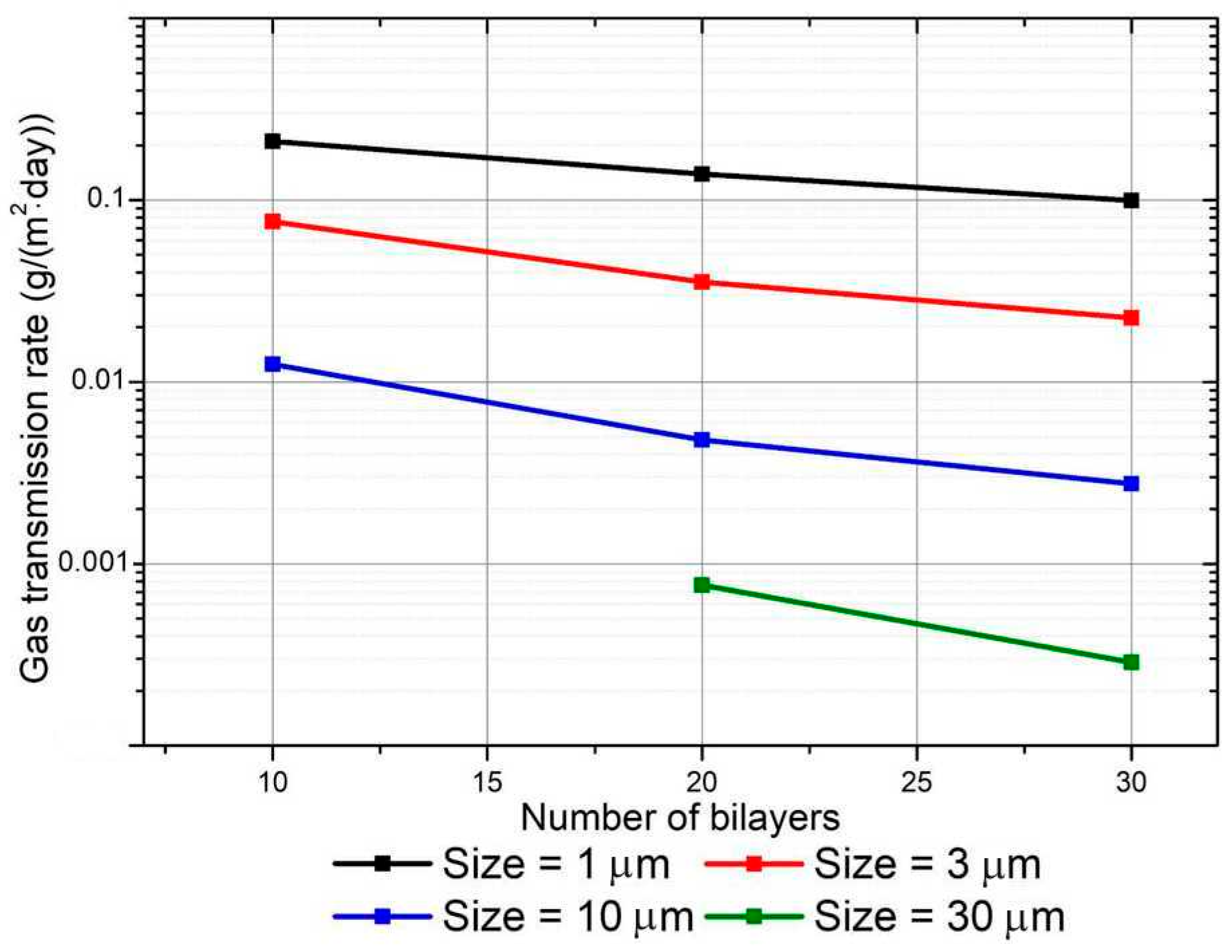
4. Results Based on the Continuous Model
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, J.; Wyser, Y. Recent Innovations in Barrier Technologies for Plastic Packaging? Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Malykh, O.V.; Golub, A.Y.; Teplyakov, V.V. Polymeric Membrane Materials: New Aspects of Empirical Approaches to Prediction of Gas Permeability Parameters in Relation to Permanent Gases, Linear Lower Hydrocarbons and Some Toxic Gases. Adv. Colloid Interface Sci. 2011, 164, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. A Review of High Performance Polymer Nanocomposites for Packaging Applications in Electronics and Food Industries. J. Plast. Film Sheeting 2020, 36, 94–112. [Google Scholar] [CrossRef]
- Pasquier, E.; Mattos, B.D.; Koivula, H.; Khakalo, A.; Belgacem, M.N.; Rojas, O.J.; Bras, J. Multilayers of Renewable Nanostructured Materials with High Oxygen and Water Vapor Barriers for Food Packaging. ACS Appl. Mater. Interfaces 2022, 14, 30236–30245. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Wagner, J.R., Jr. Multilayer Flexible Packaging, 2nd ed.; Elsevier: William Andrew, NY, USA, 2016. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer Packaging: Advances in Preparation Techniques and Emerging Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety-Controlled-Release Antioxidant And/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Bayer, I.S. Biopolymers in Multilayer Films for Long-lasting Protective Food Packaging: A Review. Sustainable Food Packaging Technology. In Sustainable Food Packaging Technology; Athanassiou, A., Ed.; Wiley: Weinheim, Germany, 2021; pp. 395–426. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Avila, L.B.; Schnorr, C.; Silva, L.F.O.; Morais, M.M.; Moraes, C.C.; da Rosa, G.S.; Dotto, G.L.; Lima, É.C.; Naushad, M. Trends in Bioactive Multilayer Films: Perspectives in the Use of Polysaccharides, Proteins, and Carbohydrates with Natural Additives for Application in Food Packaging. Foods 2023, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Grzebieniarz, W.; Biswas, D.; Roy, S.; Jamróz, E. Advances in Biopolymer-Based Multi-Layer Film Preparations and Food Packaging Applications. Food Packag. Shelf Life 2023, 35, 101033. [Google Scholar] [CrossRef]
- Attaran, S.A.; Hassan, A.; Wahit, M.U. Materials for Food Packaging Applications Based on Bio-Based Polymer Nanocomposites. J. Thermoplast. Compos. Mater. 2017, 30, 143–173. [Google Scholar] [CrossRef]
- Zabihzadeh Khajavi, M.; Ebrahimi, A.; Yousefi, M.; Ahmadi, S.; Farhoodi, M.; Mirza Alizadeh, A.; Taslikh, M. Strategies for Producing Improved Oxygen Barrier Materials Appropriate for the Food Packaging Sector. Food Eng. Rev. 2020, 12, 346–363. [Google Scholar] [CrossRef]
- Viana Batista, R.; Gonçalves Wanzeller, W.; Lim, L.-T.; Quast, E.; Zanella Pinto, V.; Machado de Menezes, V. Food Packaging and Its Oxygen Transfer Models in Active Multilayer Structures: A Theoretical Review. J. Plast. Film Sheeting 2022, 38, 458–488. [Google Scholar] [CrossRef]
- Kopacic, S.; Walzl, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and Chitosan as a Functional Barrier for Paper-Based Packaging Materials. Coat. World 2018, 8, 235. [Google Scholar] [CrossRef]
- Bekhta, P.; Lyutyy, P.; Hiziroglu, S.; Ortynska, G. Properties of Composite Panels Made from Tetra-Pak and Polyethylene Waste Material. J. Polym. Environ. 2016, 24, 159–165. [Google Scholar] [CrossRef]
- Mueller, K.; Schoenweitz, C.; Langowski, H.-C. Thin Laminate Films for Barrier Packaging Application—Influence of Down Gauging and Substrate Surface Properties on the Permeation Properties. Packag. Technol. Sci. 2012, 25, 137–148. [Google Scholar] [CrossRef]
- Fereydoon, M.; Ebnesajjad, S. Development of high-barrier film for food packaging. In Plastic Films in Food Packaging; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, UK, 2013; Chapter 5; pp. 71–92. [Google Scholar] [CrossRef]
- Sangaj, N.S.; Malshe, V.C. Permeability of Polymers in Protective Organic Coatings. Prog. Org. Coat. 2004, 50, 28–39. [Google Scholar] [CrossRef]
- Chung, D.; Papadakis, S.E.; Yam, K.L. Simple Models for Evaluating Effects of Small Leaks on the Gas Barrier Properties of Food Packages. Packag. Technol. Sci. 2003, 16, 77–86. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2022, 6, 117–144. [Google Scholar] [CrossRef]
- Lewis, J.S.; Weaver, M.S. Thin-Film Permeation-Barrier Technology for Flexible Organic Light-Emitting Devices. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 45–57. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Yang, H.; Cao, K.; Chen, R. Thin Film Encapsulation for the Organic Light-Emitting Diodes Display via Atomic Layer Deposition. J. Mater. Res. 2020, 35, 681–700. [Google Scholar] [CrossRef]
- Lewis, J. Material Challenge for Flexible Organic Devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Röhrl, M.; Timmins, R.L.; Rosenfeldt, S.; Schuchardt, D.D.; Uhlig, F.; Nürmberger, S.; Breu, J. Stretchable Clay Nanocomposite Barrier Film for Flexible Packaging. ACS Appl. Mater. Interfaces 2023, 15, 22524–22531. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Potscavage, W.J., Jr.; Sundaramoothi, A.; Henderson, C.; Kippelen, B.; Graham, S. A Correlation Study between Barrier Film Performance and Shelf Lifetime of Encapsulated Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2012, 101, 140–146. [Google Scholar] [CrossRef]
- Lee, S.; Han, J.-H.; Lee, S.-H.; Baek, G.-H.; Park, J.-S. Review of Organic/inorganic Thin Film Encapsulation by Atomic Layer Deposition for a Flexible OLED Display. JOM 2019, 71, 197–211. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of Inorganic Films Grown by Atomic Layer Deposition: Overview and General Trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- LeBaron, P. Polymer-Layered Silicate Nanocomposites: An Overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Meyer, J.; Schneidenbach, D.; Winkler, T.; Hamwi, S.; Weimann, T.; Hinze, P.; Ammermann, S.; Johannes, H.-H.; Riedl, T.; Kowalsky, W. Reliable Thin Film Encapsulation for Organic Light Emitting Diodes Grown by Low-Temperature Atomic Layer Deposition. Appl. Phys. Lett. 2009, 94, 233305. [Google Scholar] [CrossRef]
- Chou, C.-T.; Yu, P.-W.; Tseng, M.-H.; Hsu, C.-C.; Shyue, J.-J.; Wang, C.-C.; Tsai, F.-Y. Transparent Conductive Gas-Permeation Barriers on Plastics by Atomic Layer Deposition. Adv. Mater. 2013, 25, 1750–1754. [Google Scholar] [CrossRef]
- Jen, S.-H.; Bertrand, J.A.; George, S.M. Critical Tensile and Compressive Strains for Cracking of Al2O3 Films Grown by Atomic Layer Deposition. J. Appl. Phys. 2011, 109, 084305. [Google Scholar] [CrossRef]
- Kim, N.; Graham, S. Development of Highly Flexible and Ultra-Low Permeation Rate Thin-Film Barrier Structure for Organic Electronics. Thin Solid Films 2013, 547, 57–62. [Google Scholar] [CrossRef]
- Priolo, M.A.; Gamboa, D.; Holder, K.M.; Grunlan, J.C. Super Gas Barrier of Transparent Polymer-Clay Multilayer Ultrathin Films. Nano Lett. 2010, 10, 4970–4974. [Google Scholar] [CrossRef]
- Song, Y.; Tzeng, P.; Grunlan, J.C. Super Oxygen and Improved Water Vapor Barrier of Polypropylene Film with Polyelectrolyte Multilayer Nanocoatings. Macromol. Rapid Commun. 2016, 37, 963–968. [Google Scholar] [CrossRef]
- Moisan, J.Y. Effects of Oxygen Permeation and Stablliser Migration on Polymer Degradation. In Polymer Permeability; Comyn, J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 119–176. [Google Scholar] [CrossRef]
- Carcia, P.F. 4.19 Thin-Film Diffusion Barriers for Electronic Applications. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; Volume 4, pp. 463–498. [Google Scholar] [CrossRef]
- Solovyov, S.E.; Goldman, A.Y. Permeability of Multi-Layer Structures. E-polymers 2004, 4, 023. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Hu, K.; van Genuchten, M.T. Simulating the Gas Diffusion Coefficient in Macropore Network Images: Influence of Soil Pore Morphology. Soil Sci. Soc. Am. J. 2006, 70, 1252–1261. [Google Scholar] [CrossRef]
- Van Bree, I.; De Meulenaer, B.; Samapundo, S.; Vermeulen, A.; Ragaert, P.; Maes, K.C.; De Baets, B.; Devlieghere, F. Predicting the Headspace Oxygen Level due to Oxygen Permeation across Multilayer Polymer Packaging Materials: A Practical Software Simulation Tool. Innov. Food Sci. Emerg. Technol. 2010, 11, 511–519. [Google Scholar] [CrossRef]
- Mueller, K.; Weisser, H. Numerical Simulation of Permeation through Vacuum-Coated Laminate Films. Packag. Technol. Sci. 2002, 15, 29–36. [Google Scholar] [CrossRef]
- Bhunia, K.; Dhawan, S.; Sablani, S.S. Modeling the Oxygen Diffusion of Nanocomposite-Based Food Packaging Films. J. Food Sci. 2012, 77, N29–N38. [Google Scholar] [CrossRef]
- COMSOL. Available online: https://www.comsol.com/ (accessed on 18 July 2023).
- Vogiatzis, G.G.; Theodorou, D.N. Multiscale Molecular Simulations of Polymer-Matrix Nanocomposites: Or What Molecular Simulations Have Taught Us about the Fascinating Nanoworld. Arch. Comput. Methods Eng. 2018, 25, 591–645. [Google Scholar] [CrossRef] [PubMed]
- Akhukov, M.A.; Chorkov, V.A.; Gavrilov, A.A.; Guseva, D.V.; Khalatur, P.G.; Khokhlov, A.R.; Kniznik, A.A.; Komarov, P.V.; Okun, M.V.; Potapkin, B.V.; et al. MULTICOMP Package for Multilevel Simulation of Polymer Nanocomposites. Comput. Mater. Sci. 2023, 216, 111832. [Google Scholar] [CrossRef]
- Bicerano, J. Prediction of Polymer Properties, 3rd ed.; Marcel Dekker: New York, NY, USA, 2009. [Google Scholar]
- Askadskii, A.A. Computational Materials Science of Polymers; Cambridge Int Science Publishing: Cambridge, UK, 2003. [Google Scholar]
- Liu, Q.L.; Huang, Y. Transport Behavior of Oxygen and Nitrogen through Organasilicon-Containing Polystyrenes by Molecular Simulation. J. Phys. Chem. B 2006, 110, 17375–17382. [Google Scholar] [CrossRef] [PubMed]
- Graham, T. XVIII. On the Absorption and Dialytic Separation of Gases by Colloid Septa. Philos. Trans. R. Soc. Lond. 1866, 156, 399–439. [Google Scholar] [CrossRef]
- Mitchell, J.K. On the Penetrativeness of Fluids. J. Memb. Sci. 1995, 100, 11–16. [Google Scholar] [CrossRef]
- Lian, S.; Zhang, J.; Wang, J.; Xu, C.; Swart, H.C.; Terblans, J.J. A Model for Adsorption and Diffusion in Water Vapor Barrier Films. Phys. Status Solidi B Basic Res. 2021, 258, 2000609. [Google Scholar] [CrossRef]
- Seo, S.-W.; Chae, H.; Joon Seo, S.; Kyoon Chung, H.; Min Cho, S. Extremely Bendable Thin-Film Encapsulation of Organic Light-Emitting Diodes. Appl. Phys. Lett. 2013, 102, 161908. [Google Scholar] [CrossRef]
- Nielsen, L.E. Models for the Permeability of Filled Polymer Systems. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Cui, Y.; Kumar, S.; Rao Kona, B.; van Houcke, D. Gas Barrier Properties of Polymer/clay Nanocomposites. RSC Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas Barrier Performance of Graphene/polymer Nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Wakeham, W.A.; Mason, E.A. Diffusion through Multiperforate Laminae. Ind. Eng. Chem. Fundam. 1979, 18, 301–305. [Google Scholar] [CrossRef]
- Aris, R. On a Problem in Hindered Diffusion. Arch. Ration. Mech. Anal. 1986, 95, 83–91. [Google Scholar] [CrossRef]
- Falla, W.R.; Mulski, M.; Cussler, E.L. Estimating Diffusion through Flake-Filled Membranes. J. Memb. Sci. 1996, 119, 129–138. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Afanas’ev, E.S.; Matseevich, T.A.; Popova, M.N.; Kovriga, O.V.; Kondrashchenko, V.I. The Calculation Scheme for Estimation of the Water Permeability through Polymers and Copolymers. Polym. Sci. Series A 2015, 57, 924–945. [Google Scholar] [CrossRef]
- Matseevich, T.A.; Zhdanova, T.V.; Matseevich, A.V.; Askadskii, A.A. Relationship between Water Permeability and Physical Characteristics of Polyolefins, Vinyl Polymers, and Polycarbonates. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1015, 012065. [Google Scholar] [CrossRef]
- InChI Trust. Available online: https://www.inchi-trust.org/ (accessed on 18 July 2023).
- InChI Encoding of Polymers Current Results and Further Tasks. Available online: https://www.inchi-trust.org/wp/wp-content/uploads/2017/11/23.-InChI-Polymer-Yerin-201708.pdf (accessed on 18 July 2023).
- Cozmuta, I.; Blanco, M.; Goddard, W.A., 3rd. Gas Sorption and Barrier Properties of Polymeric Membranes from Molecular Dynamics and Monte Carlo Simulations. J. Phys. Chem. B 2007, 111, 3151–3166. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M. Diffusion Coefficients of Small Gas Molecules in Amorphous Cis-1,4-Polybutadiene Estimated by Molecular Dynamics Simulations. J. Chem. Phys. 2005, 123, 134906. [Google Scholar] [CrossRef]
- Zhao, L.; Zhai, D.; Liu, B.; Liu, Z.; Xu, C.; Wei, W.; Chen, Y.; Gao, J. Grand Canonical Monte Carlo Simulations for Energy Gases on PIM-1 Polymer and Silicalite-1. Chem. Eng. Sci. 2012, 68, 101–107. [Google Scholar] [CrossRef]
- Neyertz, S.; Brown, D. Single- and Mixed-Gas Sorption in Large-Scale Molecular Models of Glassy Bulk Polymers. Competitive Sorption of a Binary CH4/N2 and a Ternary CH4/N2/CO2 Mixture in a Polyimide Membrane. J. Memb. Sci. 2020, 614, 118478. [Google Scholar] [CrossRef]
- Tocci, E.; Caravella, A.; Rizzuto, C.; Barbieri, G.; Moo Lee, Y.; Drioli, E. CHAPTER 1. Modelling of Gas Separation in Thermally Rearranged Polymeric Membranes. In Membrane Engineering for the Treatment of Gases: Gas-Separation Issues with Membranes; Drioli, E., Barbieri, G., Brunetty, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Sava, D.F.; Rodriguez, M.A.; Chapman, K.W.; Chupas, P.J.; Greathouse, J.A.; Crozier, P.S.; Nenoff, T.M. Capture of Volatile Iodine, a Gaseous Fission Product, by Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2011, 133, 12398–12401. [Google Scholar] [CrossRef]
- Sava, D.F.; Chapman, K.W.; Rodriguez, M.A.; Greathouse, J.A.; Crozier, P.S.; Zhao, H.; Chupas, P.J.; Nenoff, T.M. Competitive I2 Sorption by Cu-BTC from Humid Gas Streams. Chem. Mater. 2013, 25, 2591–2596. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Z.; Wang, H.; Yang, X. Molecular Investigation of CO/CH Competitive Adsorption and Confinement in Realistic Shale Kerogen. Nanomaterials 2019, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Chen, Z.; Rahman, S.S. Insights into the Molecular Competitive Adsorption Mechanism of CH/CO in a Kerogen Matrix in the Presence of Moisture, Salinity, and Ethane. Langmuir 2021, 37, 12732–12745. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Ge, X.; Starnes, W.H., Jr. Potential Synthesis of poly(1,2-Dichloroethylene) via a Ring-Opening Metathesis Polymerization. J. Vinyl Addit. Technol. 2011, 17, 120–124. [Google Scholar] [CrossRef]
- Sun, H. Ab Initio Calculations and Force Field Development for Computer Simulation of Polysilanes. Macromolecules 1995, 28, 701–712. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Shishatskii, S.; Alentiev, A.; Loza, K. Group Contribution Method for Transport Property Predictions of Glassy Polymers: Focus on Polyimides and Polynorbornenes. J. Memb. Sci. 1998, 149, 203–220. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular Dynamics Simulations at Constant Pressure And/or Temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A Generic Force Field for Molecular Simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Celebi, A.T.; Jamali, S.H.; Bardow, A.; Vlugt, T.J.H.; Moultos, O.A. Finite-Size Effects of Diffusion Coefficients Computed from Molecular Dynamics: A Review of What We Have Learned so Far. Mol. Simul. 2021, 47, 831–845. [Google Scholar] [CrossRef]
- NIMS Materials Database (MatNavi). Available online: https://mits.nims.go.jp/en/ (accessed on 3 July 2023).
- Lightfoot, J.C.; Buchard, A.; Castro-Dominguez, B.; Parker, S.C. Comparative Study of Oxygen Diffusion in Polyethylene Terephthalate and Polyethylene Furanoate Using Molecular Modeling: Computational Insights into the Mechanism for Gas Transport in Bulk Polymer Systems. Macromolecules 2022, 55, 498–510. [Google Scholar] [CrossRef]
- Berens, A.R.; Hopfenberg, H.B. Diffusion of Organic Vapors at Low Concentrations in Glassy PVC, Polystyrene, and PMMA. J. Memb. Sci. 1982, 10, 283–303. [Google Scholar] [CrossRef]
- Börjesson, A.; Erdtman, E.; Ahlström, P.; Berlin, M.; Andersson, T.; Bolton, K. Molecular Modelling of Oxygen and Water Permeation in Polyethylene. Polymer 2013, 54, 2988–2998. [Google Scholar] [CrossRef]
- Lu, C.; Ni, S.; Chen, W.; Liao, J.; Zhang, C. A Molecular Modeling Study on Small Molecule Gas Transportation in Poly (chloro-P-Xylylene). Comput. Mater. Sci. 2010, 49, S65–S69. [Google Scholar] [CrossRef]
- Tung, K.-L.; Lu, K.-T.; Ruaan, R.-C.; Lai, J.-Y. MD and MC Simulation Analyses on the Effect of Solvent Types on Accessible Free Volume and Gas Sorption in PMMA Membranes. Desalination 2006, 192, 391–400. [Google Scholar] [CrossRef]
- Teleman, O.; Jönsson, B.; Engström, S. A Molecular Dynamics Simulation of a Water Model with Intramolecular Degrees of Freedom. Mol. Phys. 1987, 60, 193–203. [Google Scholar] [CrossRef]
- Kulasinski, K.; Guyer, R.; Keten, S.; Derome, D.; Carmeliet, J. Impact of Moisture Adsorption on Structure and Physical Properties of Amorphous Biopolymers. Macromolecules 2015, 48, 2793–2800. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Shomali, A.; Coasne, B.; Carmeliet, J.; Derome, D. Wood–moisture Relationships Studied with Molecular Simulations: Methodological Guidelines. Forests 2019, 10, 628. [Google Scholar] [CrossRef]
- Thomas, N.L. The Barrier Properties of Paint Coatings. Prog. Org. Coat. 1991, 19, 101–121. [Google Scholar] [CrossRef]
- Rogers, C.E. Permeation of Gases and Vapours in Polymers. In Polymer Permeability; Springer: Dordrecht, The Netherlands, 1985; pp. 11–73. [Google Scholar] [CrossRef]
- Rogers, C.E. Permeability and Chemical Resistance. In Engineering Design for Plastics; Baer, E., Ed.; Reinhold Publ. Corp.: New York, NY, USA, 1964; pp. 608–688. [Google Scholar]
- Rovera, C.; Ghaani, M.; Farris, S. Nano-Inspired Oxygen Barrier Coatings for Food Packaging Applications: An Overview. Trends Food Sci. Technol. 2020, 97, 210–220. [Google Scholar] [CrossRef]
- Ammala, A.; Pas, S.J.; Lawrence, K.A.; Stark, R.; Webb, R.I.; Hill, A.J. Poly(m-xylene adipamide)-montmorillonite nanocomposites: Effect of organo-modifier structure on free volume and oxygen barrier properties. J. Mater. Chem. 2008, 18, 911–916. [Google Scholar] [CrossRef]
- Rajasekar, R.; Kim, N.H.; Jung, D.; Kuila, T.; Lim, J.K.; Park, M.J.; Lee, J.H. Electrostatically Assembled Layer-by-Layer Composites Containing Graphene Oxide for Enhanced Hydrogen Gas Barrier Application. Compos. Sci. Technol. 2013, 89, 167–174. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, Y.W.; Park, T.H.; Song, E.H.; Lee, H.J.; Kim, H.; Shin, S.J.; Fai, V.L.C.; Ju, B.-K. Fuzzy Nanoassembly of Polyelectrolyte and Layered Clay Multicomposite toward a Reliable Gas Barrier. Langmuir 2012, 28, 6826–6831. [Google Scholar] [CrossRef]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar]


| Material | ||||
|---|---|---|---|---|
| Property | PET | PE | PVDF | PTFE |
| Density (g/cm3) | 1.4 ± 0.05 | 0.9 ± 0.05 | 1.8 ± 0.1 | 2.1 ± 0.1 |
| Free volume fraction | 0.13 ± 0.05 | 0.30 ± 0.05 | 0.23 ± 0.09 | <0.25 |
| S(O2) (cm3(STP)/(cm3Pa)) | S(H2O) (cm3(STP)/(cm3Pa)) | |||
|---|---|---|---|---|
| Material | Calculation | Experiment [84] | Calculation | Experiment [84] |
| PET | 2.2 ± 0.8 × 10−8 | 6–10 × 10−7 | 7.2 ± 0.7 × 10−6 | 3–8 × 10−9 |
| PE | 2.0 ± 1.0 × 10−8 | 2–5 × 10−7 | 1.5 ± 2 × 10−6 | 5.8 × 10−8 |
| PVDF | 2.5 ± 1.5 × 10−8 | 3.6 × 10−7 | 6.5 ± 0.6 × 10−6 | – |
| PTFE | 6.0 ± 1.0 × 10−7 | 6–9 × 10−7 | 8 ± 1.5 × 10−6 | – |
| P(O2) (Barrer) | P(H2O) (Barrer) | |||||
|---|---|---|---|---|---|---|
| Material | MD-GCMC Simulations | Bicerano Model | Experiment [39,84] | MD-GCMC Simulations | Askadskii Model | Experiment [39,84] |
| PET | 0.01–0.02 | 0.04 | 0.013–5 | 1–2 | 87 | 100–1300 |
| PE | 0.2–0.6 | 4.47 | 0.04–5 | 2–12 | 60 | 20–75 |
| PVDF | 0.07–0.2 | 8.91 | 0.02–1.8 | 8–30 | 193 | – |
| PTFE | 17–22 | 7.65 | 2.5–6 | 10–50 | 328 | 3–8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knizhnik, A.; Komarov, P.; Potapkin, B.; Shirabaykin, D.; Sinitsa, A.; Trepalin, S. Gas Barrier Properties of Multilayer Polymer–Clay Nanocomposite Films: A Multiscale Simulation Approach. Minerals 2023, 13, 1151. https://doi.org/10.3390/min13091151
Knizhnik A, Komarov P, Potapkin B, Shirabaykin D, Sinitsa A, Trepalin S. Gas Barrier Properties of Multilayer Polymer–Clay Nanocomposite Films: A Multiscale Simulation Approach. Minerals. 2023; 13(9):1151. https://doi.org/10.3390/min13091151
Chicago/Turabian StyleKnizhnik, Andrey, Pavel Komarov, Boris Potapkin, Denis Shirabaykin, Alexander Sinitsa, and Sergey Trepalin. 2023. "Gas Barrier Properties of Multilayer Polymer–Clay Nanocomposite Films: A Multiscale Simulation Approach" Minerals 13, no. 9: 1151. https://doi.org/10.3390/min13091151





