A Review on the Removal of Heavy Metals from Water by Phosphorus-Enriched Biochar
Abstract
:1. Introduction
2. Phosphorus in PBC
2.1. Precursor and Endogenous Phosphorus
2.2. Additives and Exogenous Phosphorus
3. Carbonization Process and Influencing Parameters
3.1. Conventional Pyrolysis
3.2. Hydrothermal Carbonization
- 323–433 K, intracellular or extracellular phosphate-containing compounds are transformed into soluble phosphates such as phosphate monoester or Al-P, Fe-P, Ca-P through a complex process, and most of the former are released into the liquid phase rather than deposited in the solid phase;
- 393–453 K, the phosphate monoester is completely converted into inorganic phosphorus in the liquid phase. The proportion of soluble inorganic phosphorus compounds entering the liquid phase increases;
4. PBC Adsorbs Heavy Metals in Water
4.1. Advantages and Research Status of PBC as Adsorbent
4.2. Adsorption Mechanism of PBC on Heavy Metals Wastewater
4.2.1. Mineral Precipitation
4.2.2. Ion Exchange
4.2.3. Surface Complexation
4.2.4. Electrostatic Interaction
4.2.5. Physical Adsorption
4.2.6. Cation-π Interaction
4.2.7. Redox
5. Challenge and Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiang, M.; Li, Y.; Yang, J.; Li, Y.; Li, F.; Hu, B.; Cao, Y. Assessment of Heavy Metal Pollution in Soil and Classification of Pollution Risk Management and Control Zones in the Industrial Developed City. Environ. Manag. 2020, 66, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.; Valente, A.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, F.; Feng, H.; Wei, C.; Wu, F. Assessment of Potential Heavy Metal Contamination in the Peri-urban Agricultural Soils of 31 Provincial Capital Cities in China. Environ. Manag. 2019, 64, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Shikha, D.; Singh, P. In situ phytoremediation of heavy metal-contaminated soil and groundwater: A green inventive approach. Environ. Sci. Pollut. Res. 2021, 28, 4104–4124. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Sindhu, M.; Kumar, A.; Dhaka, R.; Chahar, M.; Singh, S.; Nain, L. Restoration of heavy metal-contaminated soil and water through biosorbents: A review of current understanding and future challenges. Physiol. Plant. 2021, 173, 394–417. [Google Scholar] [CrossRef] [PubMed]
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2018, 17, 629–654. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, B.; Gao, X.; Cui, S.; Guan, C.Y.; Zhang, Y.; Zhang, B.; Peng, Y. Recent progresses, challenges, and opportunities of carbon-based materials applied in heavy metal polluted soil remediation. Sci. Total Environ. 2023, 856, 158810. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539–126552. [Google Scholar] [CrossRef]
- Bao, Z.; Feng, H.; Tu, W.; Li, L.; Li, Q. Method and mechanism of chromium removal from soil: A systematic review. Environ. Sci. Pollut. Res. 2022, 29, 35501–35517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.; Zhao, M.; Rong, H.; Xu, Y. The environmental characteristics and applications of biochar. Environ. Sci. Pollut. Res. 2018, 25, 21525–21534. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Y.; Ma, R.; Luo, J.; Sun, S.; Lin, J.; Wang, Y. Preparation of high performance porous carbon by microwave synergistic nitrogen/phosphorus doping for efficient removal of Cu(2+) via capacitive deionization. Environ. Res. 2023, 222, 115342–115354. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Ji, Y.; Liu, L.; Li, L.; Li, L.; Feng, X.; Zhu, J.; Lu, X.; Mu, L. A facile and green strategy to synthesize N/P co-doped bio-char as VOCs adsorbent: Through efficient biogas slurry treatment and struvite transform. Fuel 2022, 322, 124156–124164. [Google Scholar] [CrossRef]
- Ma, L.; Hu, X.; Liu, W.; Li, H.; Lam, P.; Zeng, R.; Yu, H. Constructing N, P-dually doped biochar materials from biomass wastes for high-performance bifunctional oxygen electrocatalysts. Chemosphere 2021, 278, 130508–130515. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Zhang, T.; Becker, G.; Sachs, S.; He, X.; Xue, Q. The current phosphate recycling situation in China and Germany: A comparative review. Front. Agric. Sci. Eng. 2019, 6, 403–418. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, K.; Li, G.; Zhang, W.; Zhang, T.; Hou, Y.; Ma, L.; Yuan, L.; Zhang, J.; Feng, G.; et al. Innovations of phosphorus sustainability: Implications for the whole chain. Front. Agric. Sci. Eng. 2019, 6, 321–332. [Google Scholar] [CrossRef]
- Shao, J.; Gao, C.; Afi Seglah, P.; Xie, J.; Zhao, L.; Bi, Y.; Wang, Y. Analysis of the Available Straw Nutrient Resources and Substitution of Chemical Fertilizers with Straw Returned Directly to the Field in China. Agriculture 2023, 13, 1187. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Li, D.; Li, J.; Guo, W. Performance comparison of phosphorus recovery from different sludges in sewage treatment plants through pyrolysis. J. Clean. Prod. 2022, 372, 133728–133738. [Google Scholar] [CrossRef]
- Müller-Stöver, D.; Jakobsen, I.; Grønlund, M.; Rolsted, M.; Magid, J.; Hauggaard-Nielsen, H.; Goss, M. Phosphorus bioavailability in ash from straw and sewage sludge processed by low-temperature biomass gasification. Soil Use Manag. 2018, 34, 9–17. [Google Scholar] [CrossRef]
- Xiong, Q.; Wu, X.; Lv, H.; Liu, S.; Hou, H.; Wu, X. Influence of rice husk addition on phosphorus fractions and heavy metals risk of biochar derived from sewage sludge. Chemosphere 2021, 280, 130566–130576. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, B.; Kwapinski, W.; Leahy, J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kwapinski, W.; Kolinovic, I.; Leahy, J. Sewage Sludge Thermal Treatment Technologies with a Focus on Phosphorus Recovery: A Review. Waste Biomass Valorization 2021, 12, 5837–5852. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, W.W.; Jiang, H.; Yu, H.Q. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fang, C.; Lu, X.; Jiang, R.; Tang, Y. Transformation of Phosphorus during (Hydro)thermal Treatments of Solid Biowastes: Reaction Mechanisms and Implications for P Reclamation and Recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef] [PubMed]
- Peiris, C.; Alahakoon, Y.; Malaweera Arachchi, U.; Mlsna, T.; Gunatilake, S.; Zhang, X. Phosphorus-enriched biochar for the remediation of heavy metal contaminated soil. J. Agric. Food Res. 2023, 12, 100546–100553. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Yu, F.; Wang, X.; Xiao, H.; Yan, B.; Cui, X. A review on the production of P-enriched hydro/bio-char from solid waste: Transformation of P and applications of hydro/bio-char. Chemosphere 2022, 301, 134646–134655. [Google Scholar] [CrossRef]
- Arias, C.; da Silva, L.; Soares, M.; Forti, V. A bibliometric analysis on the agricultural use of biochar in Brazil from 2003 to 2021: Research status and promising raw materials. Renew. Agric. Food Syst. 2023, 38, e19. [Google Scholar] [CrossRef]
- Wieczorek, D.; Zyszka-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorus compounds in plant tissues: From colourimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22–38. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, Z.; Fang, J.; Li, H. Effect of Pyrolysis Treatment on Phosphorus Migration and Transformation of Pig, Cow and Sheep Manure. Sustainability 2023, 15, 9215. [Google Scholar] [CrossRef]
- Bee, S.; Hamid, Z. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Fang, Z.; Zhuang, X.; Zhang, X.; Li, Y.; Li, R.; Ma, L. Influence of paraments on the transformation behaviors and directional adjustment strategies of phosphorus forms during different thermochemical treatments of sludge. Fuel 2023, 333, 126544–126563. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Wu, Z.; Deng, Q.; Tu, W.; Dai, G.; Zeng, Z.; Deng, S. Enhanced Cr(VI) removal by polyethylenimine- and phosphorus-codoped hierarchical porous carbons. J. Colloid Interface Sci. 2018, 523, 110–120. [Google Scholar] [CrossRef]
- Mohamed, B.; Ellis, N.; Kim, C.; Bi, X. The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ. Pollut. 2017, 230, 329–338. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, W.; Zhou, X.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Coeffect of pyrolysis temperature and potassium phosphate impregnation on characteristics, stability, and adsorption mechanism of phosphorus-enriched biochar. Bioresour. Technol. 2022, 344, 126273–126282. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, P.; Wang, X.; Hu, B.; Wang, C.; Xing, X. Nano-chlorapatite modification enhancing cadmium(II) adsorption capacity of crop residue biochars. Sci. Total Environ. 2023, 865, 161097–161107. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu(2+) and Cd(2+): Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413–124422. [Google Scholar] [CrossRef]
- Yang, F.; Lv, J.; Zhou, Y.; Wu, S.; Sima, J. Co-pyrolysis of biomass and phosphate tailing to produce potential phosphorus-rich biochar: Efficient removal of heavy metals and the underlying mechanisms. Environ. Sci. Pollut. Res. 2023, 30, 17804–17816. [Google Scholar] [CrossRef]
- Huang, K.; Hu, C.; Tan, Q.; Yu, M.; Shabala, S.; Yang, L.; Sun, X. Highly efficient removal of cadmium from aqueous solution by ammonium polyphosphate-modified biochar. Chemosphere 2022, 305, 135471–135478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ke, S.; Xia, M.; Bi, X.; Shao, J.; Zhang, S.; Chen, H. Effects of phosphorous precursors and speciation on reducing bioavailability of heavy metal in paddy soil by engineered biochars. Environ. Pollut. 2021, 285, 117459–117469. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, F.; Shen, X.; Hu, D.; Huang, Q. Pyrolyzed fabrication of N/P co-doped biochars from (NH(4))(3)PO(4)-pretreated coffee shells and appraisement for remedying aqueous Cr(VI) contaminants. Bioresour. Technol. 2020, 315, 123840–123847. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Deng, R.; Wan, J.; Zeng, G.; Xue, W.; Wen, X.; Zhou, C.; Hu, L.; Liu, X.; Xu, P.; et al. Remediation of lead-contaminated sediment by biochar-supported nano-chlorapatite: Accompanied with the change of available phosphorus and organic matters. J. Hazard. Mater. 2018, 348, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Yuan, H.; Ying, D.; Zhu, N. Improved removal of phosphorus from incinerated sewage sludge ash by thermo-chemical reduction method with CaCl2 application. J. Clean. Prod. 2020, 258, 120779–120786. [Google Scholar] [CrossRef]
- Gbouri, I.; Yu, F.; Wang, X.; Wang, J.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Co-Pyrolysis of Sewage Sludge and Wetland Biomass Waste for Biochar Production: Behaviors of Phosphorus and Heavy Metals. Int. J. Environ. Res. Public Health 2022, 19, 2818. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Hamid, S.; Matthias, K.; Nils, S.; Marcus, Ö. Thermochemical equilibrium study of ash transformation during combustion and gasification of sewage sludge mixtures with agricultural residues with focus on the phosphorus speciation. Biomass Convers. Biorefin. 2021, 11, 57–68. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; Sheeba Narayanan, K.; McKay, G. A review on prominent animal and municipal wastes as potential feedstocks for solar pyrolysis for biochar production. Fuel 2022, 316, 123378–123392. [Google Scholar] [CrossRef]
- Uchimiya, M.; Hiradate, S.; Antal, M. Dissolved Phosphorus Speciation of Flash Carbonization, Slow Pyrolysis, and Fast Pyrolysis Biochars. ACS Sustain. Chem. Eng. 2015, 3, 1642–1649. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Shao, H.; Sun, J. Pyrolysis temperature affects phosphorus transformation in biochar: Chemical fractionation and 31P NMR analysis. Sci. Total Environ. 2016, 569–570, 65–72. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Huang, H.; Xiao, R.; Li, R.; Zhang, Z. Influence of temperature and residence time on characteristics of biochars derived from agricultural residues: A comprehensive evaluation. Process Saf. Environ. Prot. 2020, 139, 218–229. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Liu, F.; Zhang, X.; Song, M.; Li, R. Pyrolysis of sewage sludge to biochar: Transformation mechanism of phosphorus. J. Anal. Appl. Pyrolysis 2023, 173, 106065–106074. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, F.; Li, Y.; Li, B.; Yang, T.; Li, R. Influence of microwave-assisted pyrolysis parameters and additives on phosphorus speciation and transformation in phosphorus-enriched biochar derived from municipal sewage sludge. J. Clean. Prod. 2021, 287, 125550–125563. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from microwave pyrolysis of biomass: A review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Chen, C.; Wei, R.; Shi, F.; Ma, X.; Zhou, X.; Xiong, Q. Analysis of the effects of prepyrolysis hydrothermal treatment on phosphorus recovery from sewage sludge using a life cycle assessment. J. Clean. Prod. 2022, 377, 134312–134319. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, H.; Cai, S.; Zhang, Y.; Wang, D.; Zhang, W. Molecular transformation pathway and bioavailability of organic phosphorus in sewage sludge under hydrothermal treatment: Importance of biopolymers interactions. J. Clean. Prod. 2023, 385, 135746–135754. [Google Scholar] [CrossRef]
- Tangredi, A.; Barca, C.; Ferrasse, J.; Boutin, O. Effect of process parameters on phosphorus conversion pathways during hydrothermal treatment of sewage sludge: A review. Chem. Eng. J. 2023, 463, 142342–142358. [Google Scholar] [CrossRef]
- Cui, X.; Yang, X.; Sheng, K.; He, Z.; Chen, G. Transformation of Phosphorus in Wetland Biomass during Pyrolysis and Hydrothermal Treatment. ACS Sustain. Chem. Eng. 2019, 7, 16520–16528. [Google Scholar] [CrossRef]
- Xiong, J.; Pan, Z.; Xiao, X.; Huang, H.; Lai, F.; Wang, J.; Chen, S. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water. J. Anal. Appl. Pyrolysis 2019, 144, 104692–104701. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Li, X.; Liu, Y. Distribution and transformation behaviors of heavy metals and phosphorus during hydrothermal carbonization of sewage sludge. Environ. Sci. Pollut. Res. 2020, 27, 17109–17122. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Z.; Zhu, K.; Fan, J.; Clark, J.; Luo, G.; Zhang, S. Speciation evolution and transformation mechanism of P during microwave hydrothermal process of sewage sludge. Sci. Total Environ. 2022, 815, 152801–152812. [Google Scholar] [CrossRef] [PubMed]
- Soroush, S.; Ronsse, F.; Park, J.; Ghysels, S.; Wu, D.; Kim, K.; Heynderickx, P. Microwave assisted and conventional hydrothermal treatment of waste seaweed: Comparison of hydrochar properties and energy efficiency. Sci. Total Environ. 2023, 878, 163193–163205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Zhai, F.; Hou, Y.; Hu, R. Phosphate modified hydrochars produced via phytic acid-assisted hydrothermal carbonization for efficient removal of U(VI), Pb(II) and Cd(II). J. Environ. Manag. 2021, 298, 113487–113499. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xiao, J.; Wang, T.; Chen, G.; Chen, L.; Tian, X. Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem. Eng. J. 2020, 379, 122388–122397. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Wan, J.; Xue, W.; Lei, L.; Wen, X.; Liu, X.; Chen, S.; Yang, Y.; Li, Z.; et al. Chloro-phosphate impregnated biochar prepared by co-precipitation for the lead, cadmium and copper synergic scavenging from aqueous solution. Bioresour. Technol. 2019, 293, 122102–122109. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Wang, G.; Wang, B.; Yin, Q.; Li, Y.; Deng, N. Adsorption and interaction mechanism of uranium (VI) from aqueous solutions on phosphate-impregnation biochar cross-linked Mg Al layered double-hydroxide composite. Appl. Clay Sci. 2021, 209, 106146–106157. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, N.; Ge, Y.; Ye, T.; Yang, Z.; Zou, L.; Ma, W.; Lu, L. Adsorption behavior and mechanism of U(VI) onto phytic Acid-modified Biochar/MoS2 heterojunction materials. Sep. Purif. Technol. 2022, 294, 121158–121169. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Xie, S.; Dong, Z.; Zhang, L.; Zhang, Z.; Song, J.; Deng, Y. Phosphorus-rich biochar modified with Alcaligenes faecalis to promote U(VI) removal from wastewater: Interfacial adsorption behavior and mechanism. J. Hazard. Mater. 2023, 454, 131484. [Google Scholar] [CrossRef]
- Tang, Z.; Dai, Z.; Gong, M.; Chen, H.; Zhou, X.; Wang, Y.; Jiang, C.; Yu, W.; Li, L. Efficient removal of uranium(VI) from aqueous solution by a novel phosphate-modified biochar supporting zero-valent iron composite. Environ. Sci. Pollut. Res. 2023, 30, 40478–40489. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030–110040. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, C.; Xu, C.Y.; Geng, Z. Efficient removal of Cd(II) by phosphate-modified biochars derived from apple tree branches: Processes, mechanisms, and application. Sci. Total Environ. 2022, 819, 152876–152887. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ning, P.; Miao, R.; He, L.; Guan, Q. Resource utilization of agricultural residues: One-step preparation of biochar derived from Pennisetum giganteum for efficiently removing chromium from water in a wide pH range. Environ. Sci. Pollut. Res. 2021, 28, 69381–69392. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Xia, H.; Ren, Q.; Li, Y.; Xu, L.; Xie, C.; Wang, Y. “One-can” strategy for the synthesis of hydrothermal biochar modified with phosphate groups and efficient removal of uranium(VI). J. Environ. Radioact. 2023, 263, 107182–107194. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Zong, Y.; Yu, J.; Ding, H.; Kong, Y.; Ma, J.; Ding, L. Efficient removal of cadmium by salts modified-biochar: Performance assessment, theoretical calculation, and quantitative mechanism analysis. Bioresour. Technol. 2022, 361, 127717–127728. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Tian, Q.; Wang, S.; Zhang, J.; Hou, R. Highly Efficient Removal of Lead/Cadmium by Phosphoric Acid-Modified Hydrochar Prepared from Fresh Banana Peels: Adsorption Mechanisms and Environmental Application. Langmuir 2022, 38, 15394–15403. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gong, P.; Sun, Y.; Qin, Q.; Song, K.; Ye, J.; Zhang, H.; Zhou, B.; Xue, Y. Modified chicken manure biochar enhanced the adsorption for Cd2+ in aqueous and immobilization of Cd in contaminated agricultural soil. Sci. Total Environ. 2022, 851, 158252–158260. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Lin, S. Advances in the Study of Heavy Metal Adsorption from Water and Soil by Modified Biochar. Water 2022, 14, 3894. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, T.; Li, Q.; Yang, H.; Yan, Y.; Sarkar, B.; Lam, S.S.; Bolan, N. Influence of pyrolysis temperature on the characteristics and lead(II) adsorption capacity of phosphorus-engineered poplar sawdust biochar. J. Anal. Appl. Pyrolysis 2021, 154, 105010. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Liu, H.; Cai, Y.; Qi, X.; Dang, Z. Multicomponent adsorption of heavy metals onto biogenic hydroxyapatite: Surface functional groups and inorganic mineral facilitating stable adsorption of Pb(II). J. Hazard. Mater. 2023, 443, 130167–130177. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Yang, J.; Yan, Z.; Zhang, Z.; Zhong, B.; Wang, X. Treatment of distiller grain with wet-process phosphoric acid leads to biochar for the sustained release of nutrients and adsorption of Cr(VI). J. Hazard. Mater. 2023, 441, 129949. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xue, Y.; Gao, F.; Mosa, A. Sorption of heavy metal ions onto crayfish shell biochar: Effect of pyrolysis temperature, pH and ionic strength. J. Taiwan Inst. Chem. Eng. 2017, 80, 114–121. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Xie, S.; Wang, J.; Guo, Y. Removal behavior and mechanisms of U(VI) in aqueous solution using aloe vera biochar with highly developed porous structure. J. Radioanal. Nucl. Chem. 2022, 331, 2273–2283. [Google Scholar] [CrossRef]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Du, C.; Guo, H.; Wang, Z. Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J. Hazard. Mater. 2009, 169, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Coordination Chemistry: Principles and Applications; Chemical Industry Press: Beijing, China, 2008; pp. 334–335. [Google Scholar]
- Yi, W. Research on Heavy Metal Removal from Stormwater Runoff in Constructed Wetlands with Media of Zeolite; Zhejiang University: Hangzhou, China, 2015. [Google Scholar]
- Li, X. Remediation of Cd and Ni Contaminated Soil by Cattle-Manure-Derived Biochar and Zeolite; Huazhong Agricultual University: Wuhan, China, 2017. [Google Scholar]
- Zhao, G.; Zhu, H. Cation-pi Interactions in Graphene-Containing Systems for Water Treatment and Beyond. Adv. Mater. 2020, 32, 1905756–1905777. [Google Scholar] [CrossRef] [PubMed]
- Harvey, O.; Herbert, B.; Rhue, R.; Kuo, L. Metal interactions at the biochar-water interface: Energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Chu, G. Comparison of the Sorption of Cu(II) and Pb(II) by Bleached and Activated Biochars: Insight into Complexation and Cation–π Interaction. Agronomy 2023, 13, 1282. [Google Scholar] [CrossRef]
- Chen, M.; He, F.; Hu, D.; Bao, C.; Huang, Q. Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem. Eng. J. 2020, 381, 122739–122748. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Zhang, Y.; Yang, K.; Ma, J.; Ning, P. Formation and mechanism of nanoscale zerovalent iron supported by phosphoric acid modified biochar for highly efficient removal of Cr(VI). Adv. Powder Technol. 2023, 34, 103826–103838. [Google Scholar] [CrossRef]
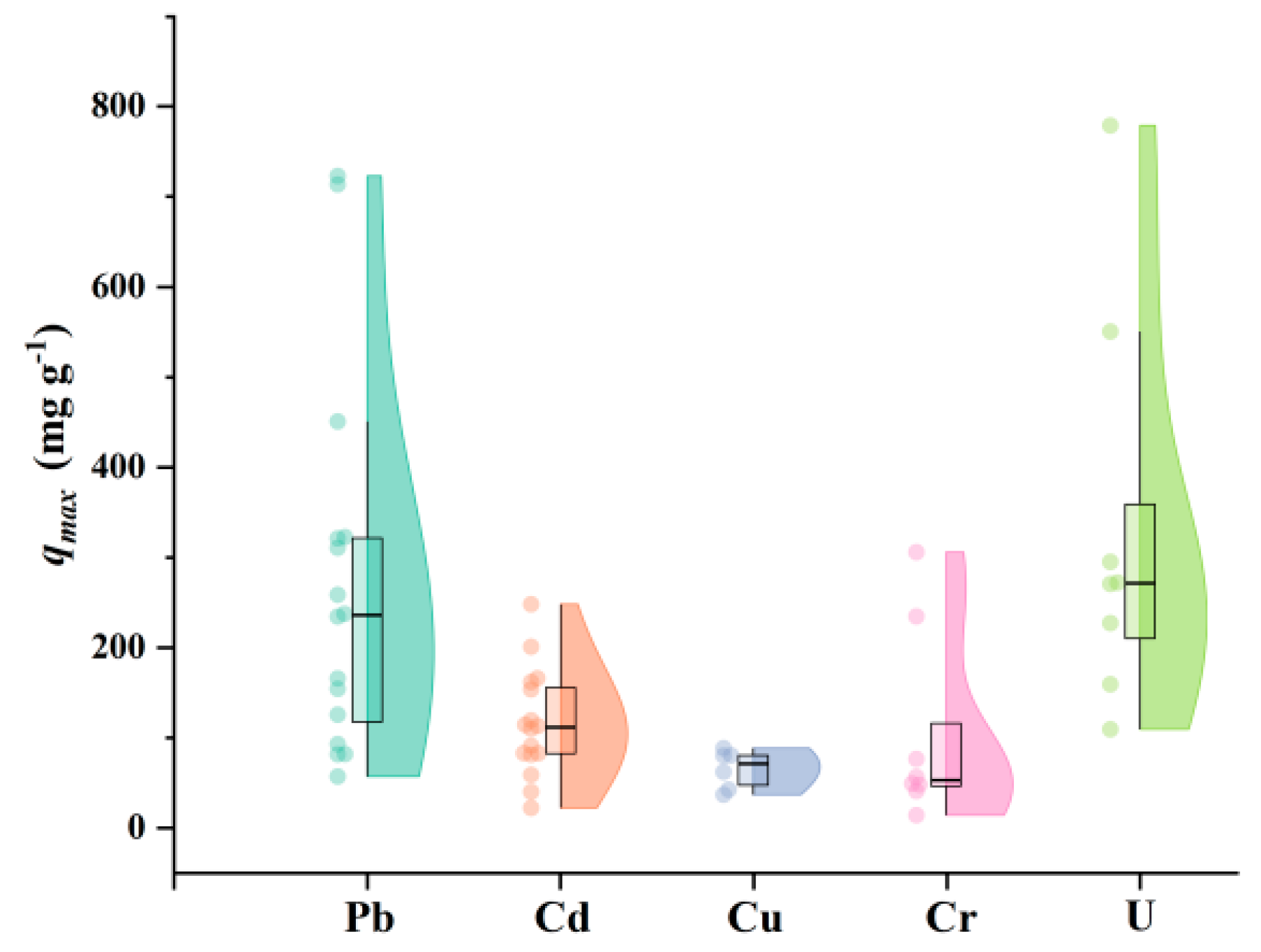

| HMs | PBC Precursor | Adsorption Conditions | qmax (mg g−1) | Biochar Features on Adsorption | Ref. |
|---|---|---|---|---|---|
| Pb | Camellia oleifera shells, (NH4, H)n 2PnO3n+1 | C0: 100–2000 mg L−1, dose = 1 g L−1, pH = 6, T = 298 K, t = 360 min | 723.6 | High surface area and pore volume, rich P- and N-containing functional groups | [70] |
| Bamboo sawdust, C6H18O24P6 | C0: 50–300 mg L−1, dose = 0.4 g L−1, pH = 5, T = 293 K, t = 1440 min | 234.8 | Low ZPC, great surface modification (maximum of 2.1 wt% of P), | [63] | |
| Cd | Raw bamboo scraps, K3PO4 3H2O | C0: 10–500 mg L−1, dose = 1 g L−1, pH = 6.8, T = 298 K, t = 720 min | 249.8 | Great pore structure (mainly micropores), high aromatization and graphitization, more P compounds (mainly PO43− and P2O74−) | [37] |
| Apple tree branches, K3PO4 3H2O | C0: 2–250 mg L−1, dose = 1 g L−1, pH = 6, T = 298 K, t = 1440 min | 114.7 | Higher P, C retention and electron density, High absolute value of zeta potential | [71] | |
| Cu | Sewage sludge, CaCl2, (NH4)2HPO4 | C0: NM, dose = 1 g L−1, pH = 5, T = 298 K, t = 1440 min | 90.0 | More dispersed HAP, High P loading | [39] |
| Wood waste, CaCl2 2H2O, Na3PO4 12H2O | C0: 2–200 mg L−1, dose = 0.5 g L−1, pH = 7, T = 298 K, t = 1440 min | 63.8 | More inserted chlorophosphates, very small amount of AP | [65] | |
| Cr | Oil-tea shells, H3PO4, PEI/Methanol, Glutaraldehyde | C0: 200–600 mg L−1, dose = 1 g L−1, pH = 2, T = 313 K, t = 1440 min | 320.5 | More N- and P- containing groups that can act as electron donors (P also acts as adsorption sites of Cr and the adhesive sites of PEI); multi-scales and hierarchical with micro-, meso- and macropores | [35] |
| Egeria najas, H3PO4, FeSO4/PEG/KBH | C0: 30–100 mg L−1, dose = 0.75 g L−1, pH = 5.5, T = 333 K, t = 1440 min | 57.5 | More dispersed nZVI, stronger antioxidant capacity (addition of H3PO4) | [72] | |
| U | Bamboo chopsticks, H3PO4, citric acid | C0: 25–300 mg L−1, dose = 0.6 g L−1, pH = 4, T = 298 K, t = 100 min | 781.0 | Great affinity and selectivity for U (P-O, P=O provide a lone electron-pairs to form coordination bonds) | [73] |
| Bamboo, KH2PO4, AlCl3 6H2O, MgCl2 6H2O | C0: 5–250 mg L−1, dose = 1 g L−1, pH = 4, T = 298 K, t = NM | 274.2 | High amounts of functional groups and ions (reduction and complexation: P-O, Mg-O-H and -OH, co-precipitation: polyhydroxy aluminum cations) | [66] |
| Heavy Metal Ion | Atomic Electronegativity (Pauling) | Ionic Radius | Hydrated Radius | Hydration Energy | Hydrolysis Constant |
|---|---|---|---|---|---|
| Å | kJ/mol | ||||
| Pb | 2.33 | 1.19 | 4.01 | 1481 | 7.71 |
| Cu | 1.90 | 0.73 | 4.10 | 2100 | 10.10 |
| Cd | 1.69 | 0.95 | 4.26 | 1828 | 7.70 |
| Zn | 1.65 | 0.74 | 4.30 | 2056 | 9.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Lin, Y.; Ma, M.; Chen, H. A Review on the Removal of Heavy Metals from Water by Phosphorus-Enriched Biochar. Minerals 2024, 14, 61. https://doi.org/10.3390/min14010061
Zeng Y, Lin Y, Ma M, Chen H. A Review on the Removal of Heavy Metals from Water by Phosphorus-Enriched Biochar. Minerals. 2024; 14(1):61. https://doi.org/10.3390/min14010061
Chicago/Turabian StyleZeng, Yang, Yuhan Lin, Ming Ma, and Hong Chen. 2024. "A Review on the Removal of Heavy Metals from Water by Phosphorus-Enriched Biochar" Minerals 14, no. 1: 61. https://doi.org/10.3390/min14010061





