Genesis of the Mahour Base Metal Deposit, Iran: Constraints from Fluid Inclusions and Sulfur Isotopes
Abstract
:1. Introduction
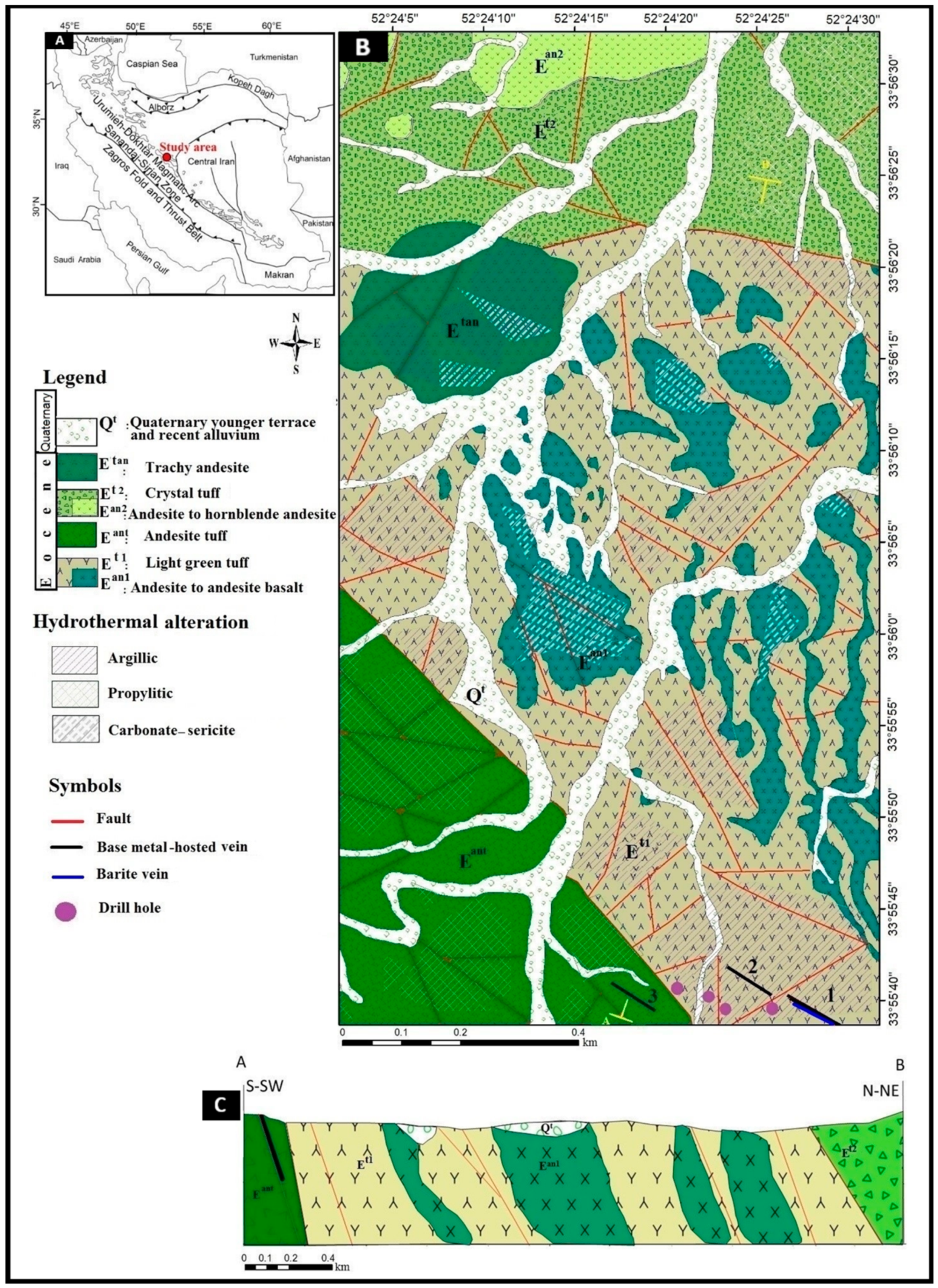
2. Regional Geology
3. Geological Features of the Mahour Deposit
3.1. Ore Deposit Geology
3.2. Mineralized Veins
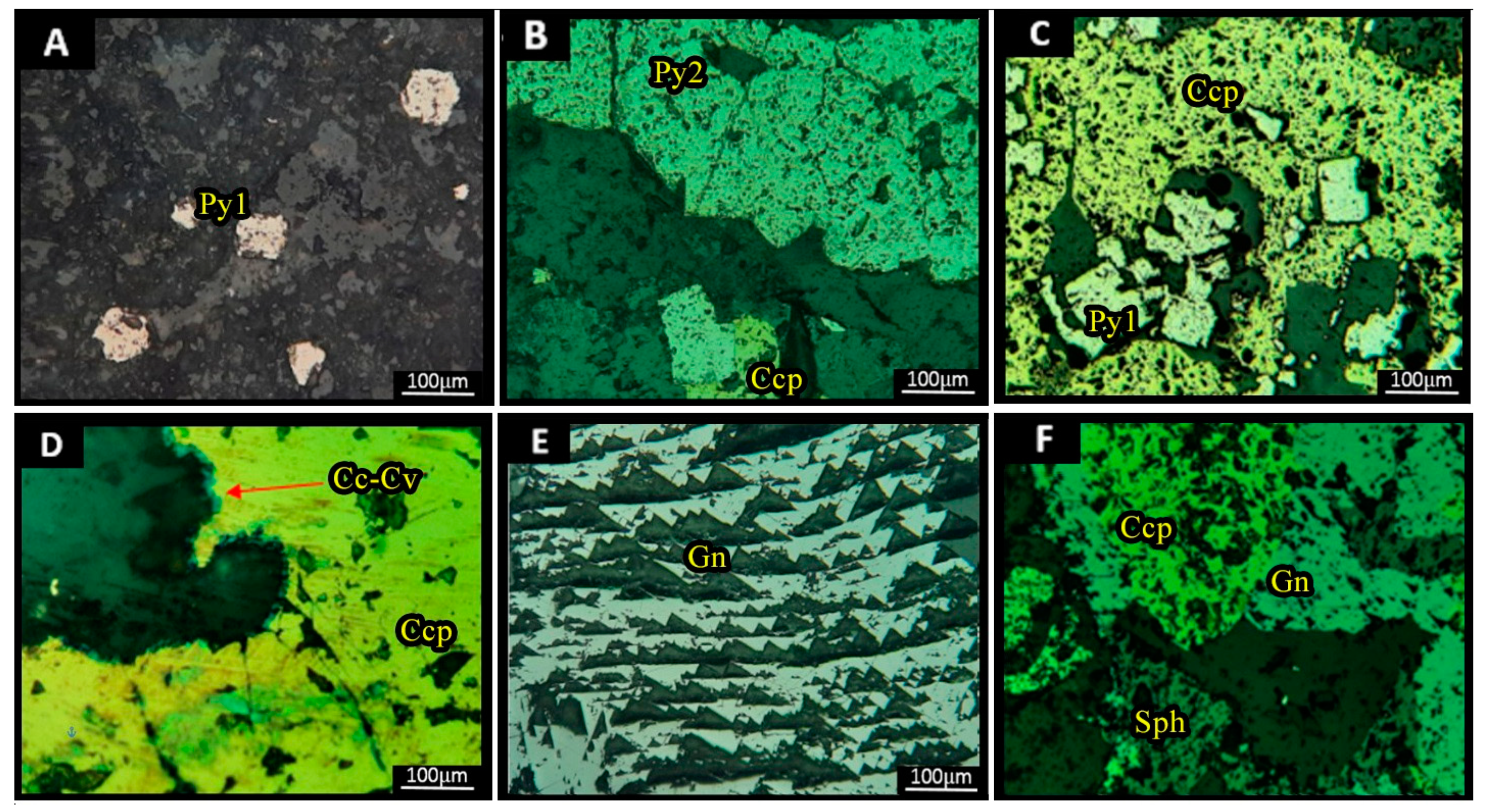
3.3. Ore Mineralogy
4. Analytical Methods
4.1. Fluid Inclusions
4.2. Sulfur Isotopes
5. Results
5.1. Fluid Inclusion Studies
5.1.1. Types and Features of Fluid Inclusions
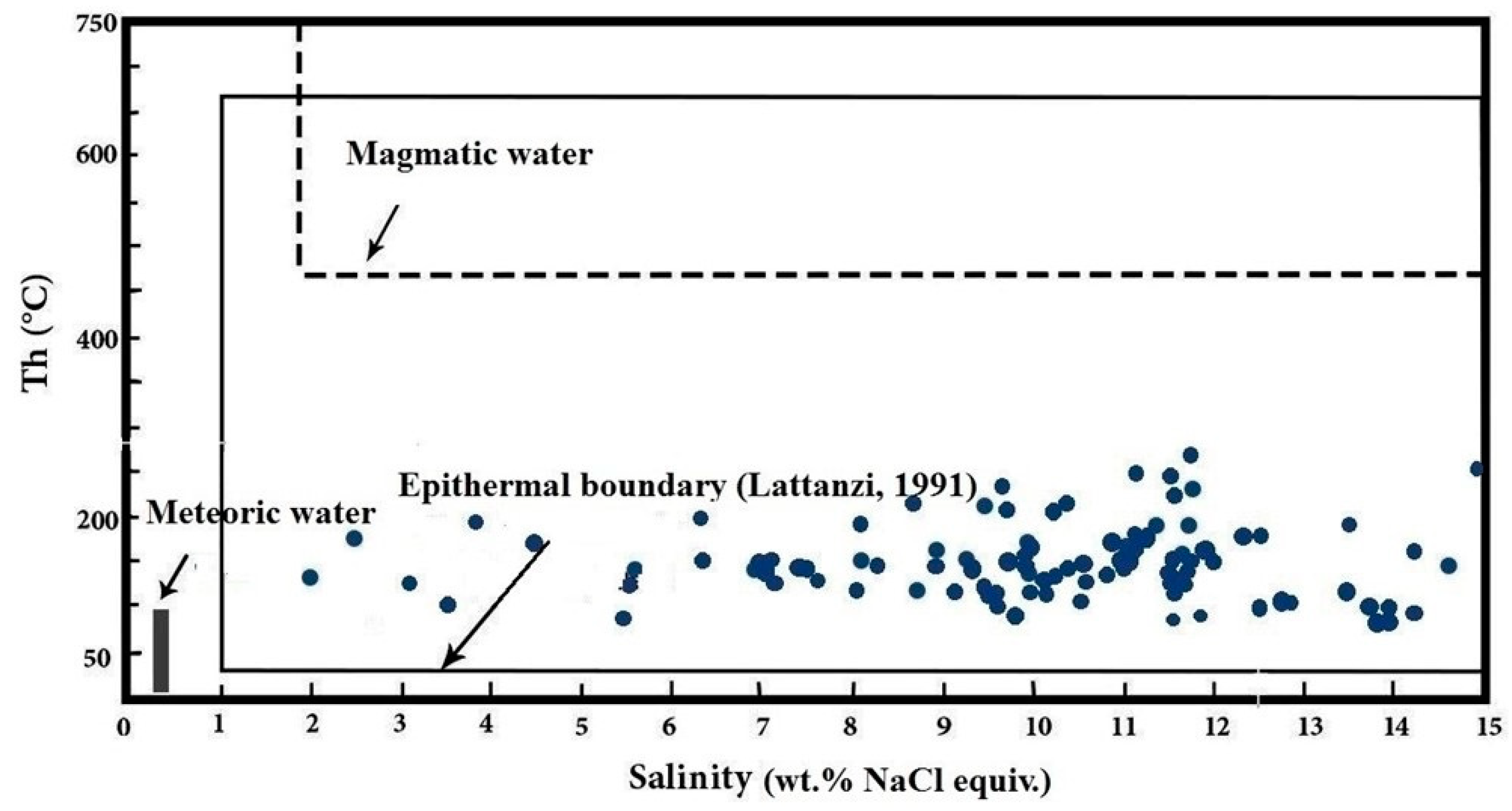
5.1.2. Microthermometry
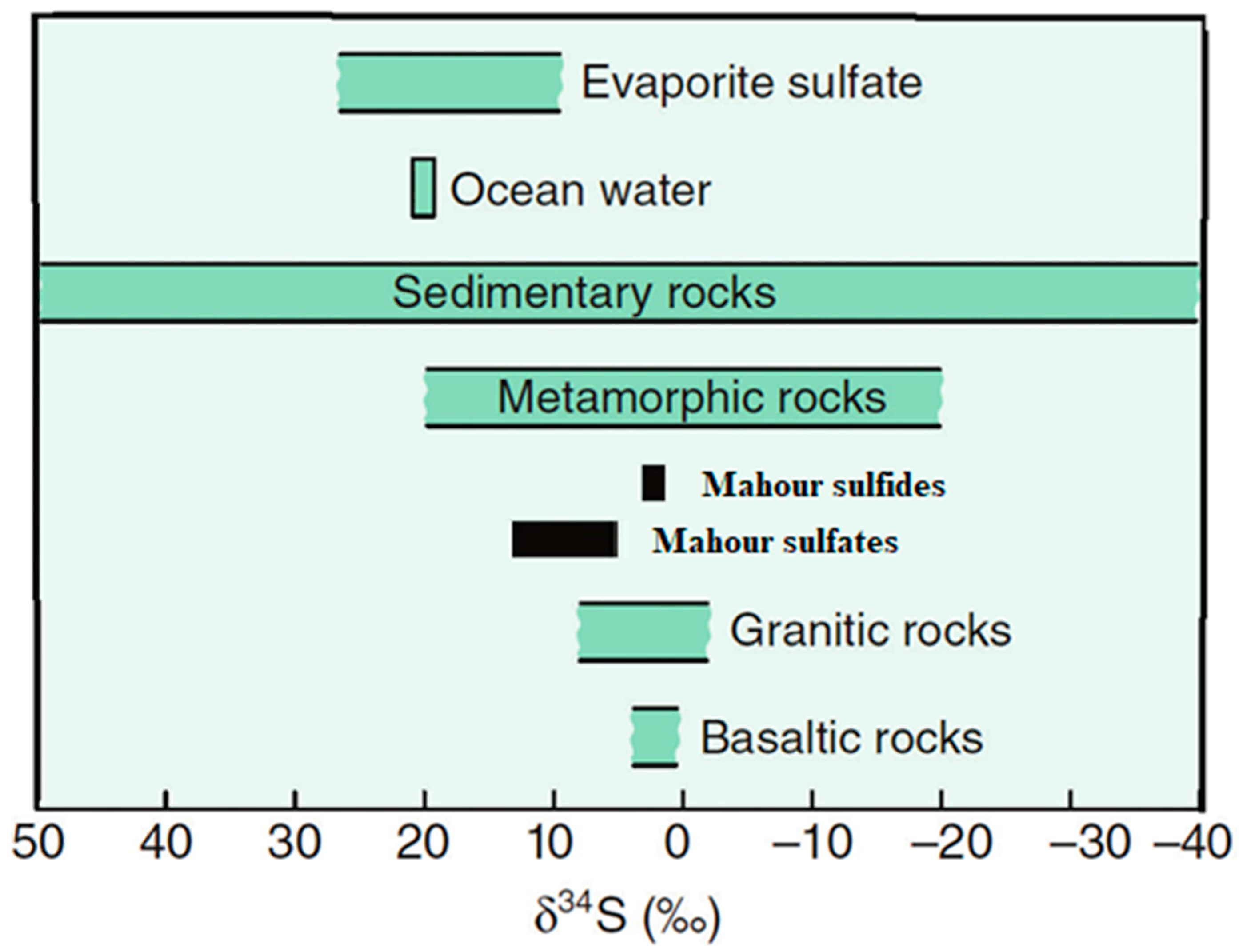
5.2. Sulfur Isotope Studies
6. Discussion
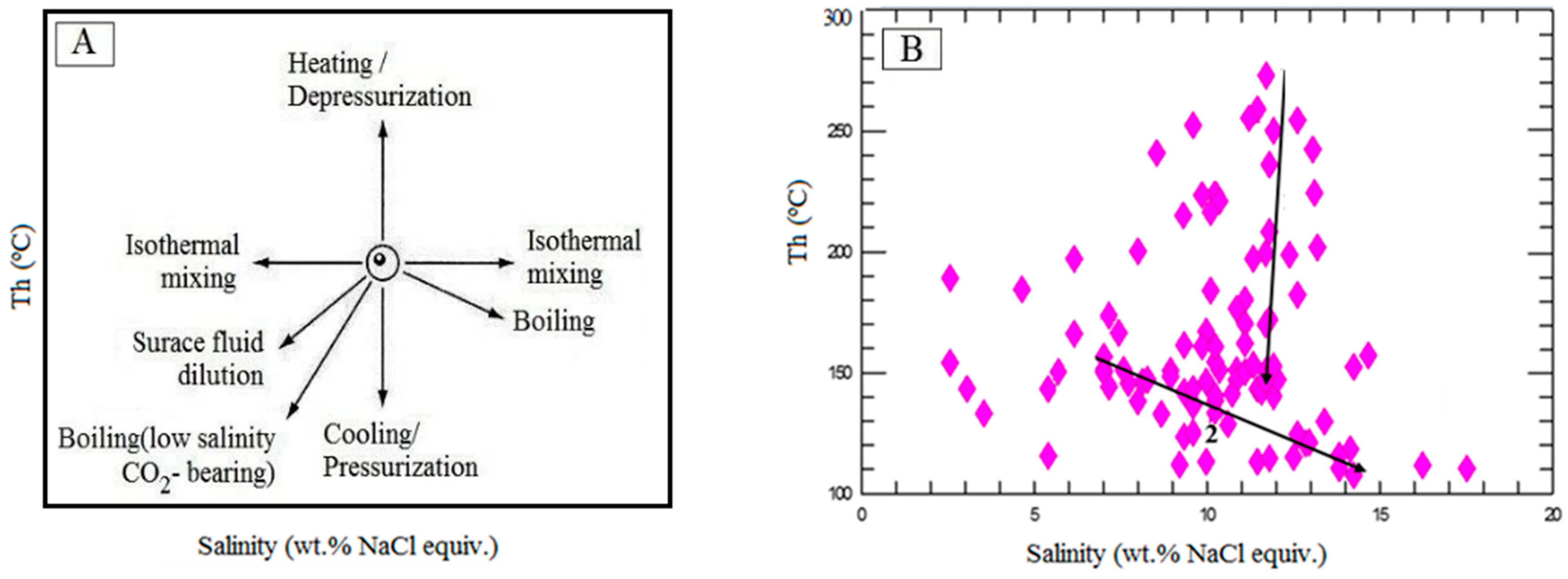
6.1. Boiling Evidence
6.2. Source of Ore-Forming Fluids and Materials
6.3. Evolution of the Ore-Forming Fluids
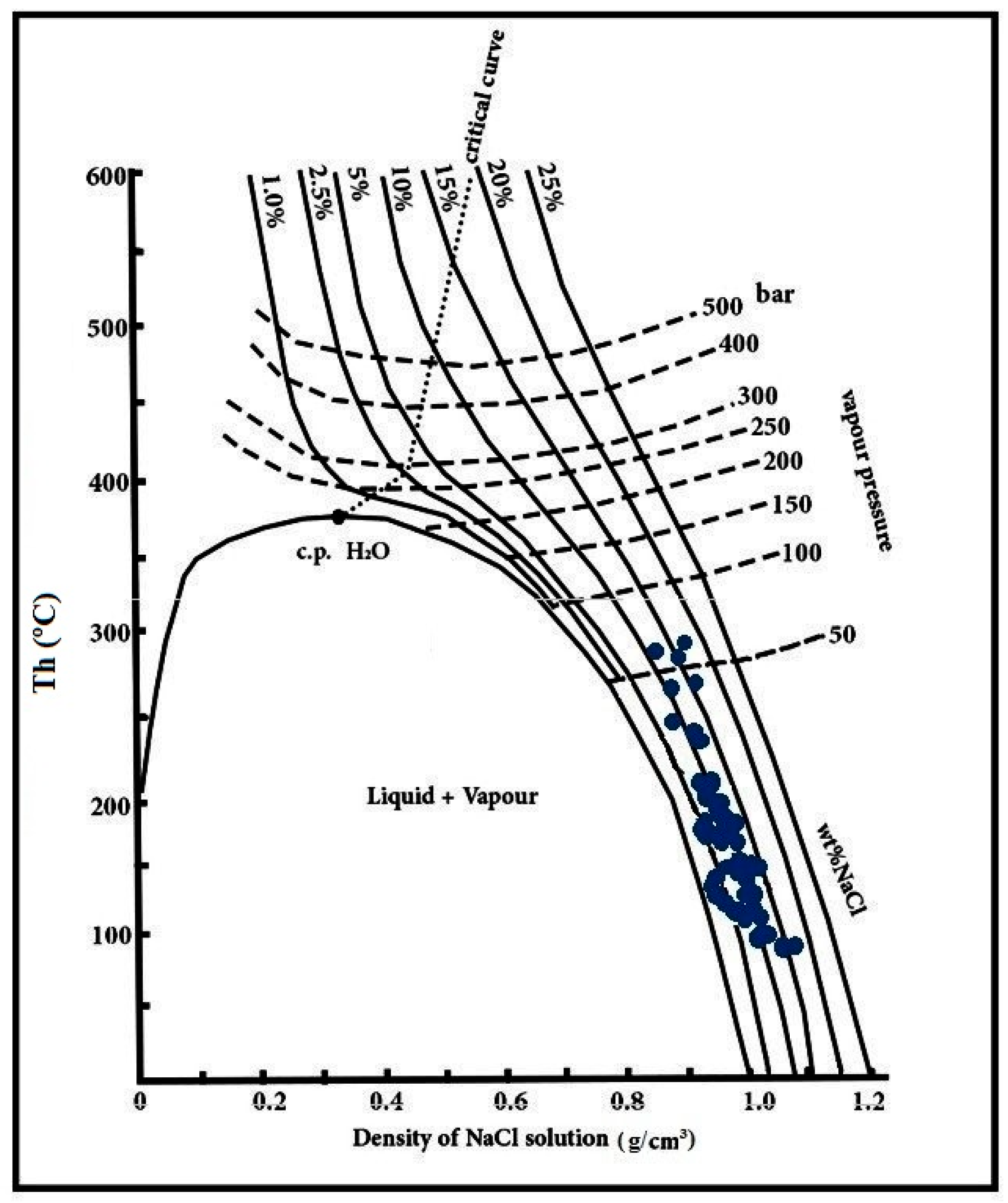
6.4. Trapping Pressure
6.5. Sulfidation State
6.6. Ore Genesis
7. Conclusions
- (1)
- The Eocene volcanic units (green tuff and andesite tuff units) host the deposit. Based on geochemical and petrological studies, the above units have a calc-alkaline composition and a shear and vein-veinlet structure.
- (2)
- Mineralization at the deposit is in the form of sulfides and sulfosalts, including galena, Fe-poor sphalerite, pyrite, chalcopyrite, and tetrahedrite-tennantite.
- (3)
- The alteration types observed at the deposit include carbonate-sericite, argillic, silicic-sulfide, and propylitic with a significant association to the mineralized part.
- (4)
- Studies of sulfur isotopes and fluid inclusions suggest that the Mahour deposit could have formed mainly from magmatic fluids.
- (5)
- The presence of fluid inclusions rich in L and V phases and the coexistence of high-salinity along with low-salinity fluids suggest a boiling mechanism in the Mahour deposit.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Persian Gold Company. Annual Report and Accounts; Persian Gold Company: Tehran, Iran, 2007; 40p. [Google Scholar]
- Persian Gold Company. Interim Results for the Six-Month Exploration in Iran; Persian Gold Company: Tehran, Iran, 2008; 8p. [Google Scholar]
- Richards, J.P.; Sholeh, A. The Tethyan Tectonic History and Cu-Au Metallogeny of Iran. In 2016 Tectonics and Metallogeny of the Tethyan Orogenic Belt; Richards, J.P., Ed., SEG Special Publication: Houston, TX, USA, 2016; Volume 19, pp. 193–212. [Google Scholar]
- Hezarkhani, A.; Williams-Jones, A.E. Controls of alteration and mineralization in the Sungun porphyry copper deposit, Iran: Evidence from fluid inclusions and stable isotopes. Econ. Geol. 1998, 93, 651–670. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Daneshfar, B. Strategic prospectivity review of mineral potential: Middle East region. Rep. Met. Min. Agency Jpn. 2001. 68p. [Google Scholar]
- Zarasvandi, A.; Liaghat, S.; Zentilli, K. Geology of the Darreh-Zerreshk and Ali-Abad porphyry copper deposits, central Iran. Intl. Geol. Rev. 2005, 47, 620–646. [Google Scholar] [CrossRef]
- Zarasvandi, A.; Liaghat, S.; Zentilli, K. Porphyry copper deposits of the Urumieh-Dokhtar magmatic arc, Iran. In Super Porphyry Copper and Gold Deposits: A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2005; pp. 441–452. [Google Scholar]
- Shafiei, B.; Haschke, M.; Shahabpour, J. Recycling of orogenic arc crust triggers porphyry Cu mineralization in Kerman Cenozoic arc rocks, southeastern Iran. Miner. Depos. 2009, 44, 265–283. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Alirezaei, S.; Pan, Y. Various epithermal precious metal systems in the Urumieh-Dokhtar magmatic assemblage, Iran. In Proceedings of the Goldschmidt Conference, Davos, Switzerland, 21–26 June 2009. [Google Scholar]
- Ebrahimi, S.; Alirezaei, S.; Pan, Y. Geological Setting, Alteration, and Fluid Inclusion Characteristics of Zaglic and Safikhanloo Epithermal Gold Prospects, NW Iran; Granite-Related Ore Deposits; Sial, A.N., Bettencourt, J.S., De Campos, C.P., Ferreira, V.P., Eds.; Special Publications; Geological Society: London, UK, 2011; Volume 350, pp. 133–147. [Google Scholar]
- Jamali, H.; Dilek, Y.; Daliran, F.; Yaghubpur, A.; Mehrabi, B. Metallogeny and tectonic evolution of the Cenozoic Ahar–Arasbaran volcanic belt, northern Iran. Intl. Geol. Rev. 2010, 52, 608–630. [Google Scholar] [CrossRef]
- Ghaderi, M.; Kouhestani, H. Chah Zard deposit: The first report of Ag-Au epithermal mineralization with brecciated host in Iran. In Proceedings of the 7th Annual Meeting of Asia Oceania Geosciences Society (AOGS), Hyderabad, India, 5–9 July 2010. [Google Scholar]
- Kouhestani, H.; Ghaderi, M.; Zaw, K. Chah Zard, a breccia-hosted epithermal silver-gold deposit in the Tethyan belt of Iran. In Proceedings of the 24th Victorian Universities Earth & Environmental Sciences Conference (VUEES), Melbourne, Australia, 5 November 2010. [Google Scholar]
- Ghaderi, M.; Kouhestani, H.; Zaw, K.; Meffre, S. U–Pb geochronology and Pb isotope characteristics of Chah Zard Ag–Au epithermal deposit, west-central Iran. In Proceedings of the 11th International Multidisciplinary Scientific Geo-Conference and EXPO (SGEM), Albena, Bulgaria, 19–25 June 2011; pp. 1039–1046. [Google Scholar]
- Ghaderi, M.; Kouhestani, H.; Zaw, K.; Meffre, S. Geochemistry and geochronology study on Chah Zard deposit: A breccia-hosted Ag-Au epithermal mineralization in Iran. In Proceedings of the 8th Annual Meeting of Asia Oceania Geosciences Society (AOGS), Taipei, Taiwan, 8–12 August 2011. [Google Scholar]
- Daliran, F.; Paar, W.; Neubauer, F.; Rashidi, B. New Discovery of epithermal gold at Chahnali prospect, Bazman volcano, SE-Iran. In Proceedings of the Mineral Deposit Research: Meeting the Global Challenge: Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18–21 August 2005; pp. 917–919. [Google Scholar]
- Stocklin, J. Possible ancient continental margins in Iran. In Geology of Continental Margins; Bark, G.D., Drake, C.L., Eds.; Springer-Verlag: New York, NY, USA, 1974; pp. 873–887. [Google Scholar]
- Berberian, M.; King, G.C.P. Toward a paleogeography and tectonic evolution of Iran. Can. J. Earth Sci. 1981, 18, 210–265. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Whitechurch, H.; Vrielynck, B.; Spakman, W.; Monié, P.; Meyer, B.; Wortel, R. Zagros orogeny: A subduction-dominated process. Geol. Mag. 2011, 148, 692–725. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Badrzadeh, Z.; Castro, A. Petrogenesis and U-Pb SHRIMP dating of Tarom plutons. J. Geosci. 2015, 24, 3–20, (In Persian with English abstract). [Google Scholar]
- Berberian, F.; Berberian, M. Tectono-plutonic episodes in Iran. In Zagros Hindukosh, Himalaya Geodynamic Evolution; American Geophysical Union: Washington, DC, USA, 1981; pp. 5–32. [Google Scholar]
- Omrani, J.; Agard, P.; Whitechurch, H.; Benoit, M.; Prouteau, G.; Jolivet, L. Arc-magmatism and subduction history beneath the Zagros Mountains, Iran: A new report of adakites and geodynamic consequences. Lithos 2008, 106, 380–398. [Google Scholar] [CrossRef]
- Babakhani, A.R.; Charkoski, M. Geological 1:100,000 Map of Kuh-e-Latif; Geological Survey of Iran: Tehran, Iran, 1990. [Google Scholar]
- Yilmaz, H.; Oyman, T.; Sonmez, F.N.; Arehart, G.B.; Billor, Z. Intermediate sulfidation epithermal gold-base metal deposits in Tertiary subaerial volcanic rocks, Sahinli/Tespih Dere (Lapseki/western Turkey). Ore Geol. Rev. 2010, 37, 236–258. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; Society for Sedimentary Geology (SEPM): Tulsa, OK, USA, 1994. [Google Scholar]
- Hall, D.L.; Sterner, S.M.; Bodnar, R.J. Freezing point depression of NaCl–KCl–H2O solutions. Econ. Geol. 1988, 83, 197–202. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. In Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 12, 644p. [Google Scholar]
- Goldstein, R.H. Petrographic analysis of fluid inclusions. In Fluid Inclusions: Analysis and Interpretation; Short Course; Samson, I., Anderson, A., Marshall, D., Eds.; Mineralogical Association of Canada: Vancouver, BC, Canada, 2003; pp. 9–53. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 9th ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Bodnar, R.J.; Burnham, C.W.; Sterner, S.M. Synthetic fluid inclusions in natural quartz. III. Determination of phase equilibrium properties in the system H2O–NaCl to 1000 °C and 1500 bars. Geochim. Cosmochim. Acta 1985, 49, 1861–1873. [Google Scholar] [CrossRef]
- White, N.C.; Hedenquist, J.W. Epithermal gold deposits: Styles, characteristics and exploration. SEG Newslet. 1995, 1–13. [Google Scholar] [CrossRef]
- Simmons, S.F.; White, N.C.; John, D.A. Geological characteristics of epithermal precious and base metal deposits. In Economic Geology 100th Anniversary Volume; Society of Economic Geologists: Denver, CO, USA, 2005; pp. 485–522. [Google Scholar]
- Prokofiev, V.Y.; Garofalo, P.S.; Bortnikov, N.S.; Kovalenker, V.A.; Zorina, L.D.; Grichuk, D.V.; Selektor, S.L. Fluid inclusion constraints on the genesis of gold in the Darasun district (eastern Transbaikalia), Russia. Econ. Geol. 2010, 105, 395–416. [Google Scholar] [CrossRef]
- Moncada, D.; Baker, D.; Bodnar, R.J. Mineralogical, petrographic and fluid inclusion evidence for the link between boiling and epithermal Ag-Au mineralization in the La Luz area, Guanajuato mining district, México. Ore Geol. Rev. 2017, 89, 143–170. [Google Scholar] [CrossRef]
- White, N.C.; Hedenquist, J.W. Epithermal environments and styles of mineralization: Variations and their causes, and guidelines for exploration. J. Geochem. Explor. 1990, 36, 445–474. [Google Scholar] [CrossRef]
- Simmons, S.F.; Christenson, B.W. Origin of calcite in a boiling geothermal system. Am. J. Sci. 1994, 294, 361–400. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.R.; Gonzalez-Urien, E. Exploration for epithermal gold deposits. Rev. Econ. Geol. 2000, 13, 245–277. [Google Scholar]
- Simmons, S.F.; Browne, P.R.L. Hydrothermal minerals and precious metals in the Broadlands-Ohaaki geothermal system: Implications for understanding low-sulfidation epithermal environments. Econ. Geol. 2000, 95, 971–1000. [Google Scholar] [CrossRef]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral textures and fluid inclusion petrography of the epithermal Ag–Au deposit. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Ramboz, C.; Pichavant, M.; Weisbrod, A. Fluid immiscibility in natural processes use and misuse of fluid inclusion data: II. Interpretation of fluid inclusion data in terms of immiscibility. Chem. Geol. 1982, 37, 29–48. [Google Scholar] [CrossRef]
- Ouyang, H.; Wu, X.; Mao, J.W.; Su, H.; Santosh, M.; Zhou, Z.; Li, C. The nature and timing of ore formation in the Budunhua copper deposit, southern Great Xing’an Range: Evidence from geology, fluid inclusions, and U-Pb and Re-Os geochronology. Ore Geol. Rev. 2014, 63, 238–251. [Google Scholar] [CrossRef]
- Rye, R.O.; Ohmoto, H. Sulfur and carbon isotopes and ore genesis: A review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Taylor, H.P. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; John Wiley and Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Shepherd, T.J.; Ranbin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie: Glasgow, UK, 1985; 223p. [Google Scholar]
- Faure, K. δD values of fluid inclusion water in quartz and calcite ejecta from active geothermal systems: Do values reflect those of original hydrothermal water? Econ. Geol. 2003, 98, 657–660. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.X.; Geng, X.X.; Li, C.; Liu, F.; Chai, F.M.; Yang, F.Q. Geology and geochemistry of the Qiaoxiahala Fe–Cu–Au deposit, Junggar region, northwest China. Ore Geol. Rev. 2014, 57, 462–481. [Google Scholar] [CrossRef]
- Jiang, H.; Han, J.; Chen, H.; Zheng, Y.; Zhang, W.; Lu, W.; Deng, G.; Tan, Z. Hydrothermal alteration, fluid inclusions and stable isotope characteristics of the Shaquanzi Fe–Cu deposit, Eastern Tianshan: Implications for deposit type and metallogenesis. Ore Geol. Rev. 2018, 100, 385–400. [Google Scholar] [CrossRef]
- Li, S.N.; Ni, P.; Bao, T.; Li, C.Z.; Xiang, H.L.; Wang, G.G.; Huang, B.; Chi, Z.; Dai, B.Z.; Ding, J.Y. Geology, fluid inclusion, and stable isotope systematics of the Dongyang epithermal gold deposit, Fujian Province, southeast China: Implications for ore genesis and mineral exploration. J. Geochem. Explor. 2018, 195, 16–30. [Google Scholar] [CrossRef]
- Li, S.N.; Ni, P.; Bao, T.; Xiang, H.L.; Chi, Z.; Wang, G.G.; Bao, H.; Ding, J.Y.; Dai, B.Z. Genesis of the Ancun epithermal gold deposit, southeast China: Evidence from fluid inclusion and stable isotope data. J. Geochem. Explor. 2018, 195, 157–177. [Google Scholar] [CrossRef]
- Sasaki, A.; Ishihara, S. Sulfur isotopic composition of the magnetite-series and ilmenite-series granitoids in Japan. Contrib. Mineral. Petrol. 1979, 68, 107–115. [Google Scholar] [CrossRef]
- Taylor, B.E.; Wheeler, M.C.; Nordstrom, D.K. Stable isotope geochemistry of acid mine drainage: Experimental oxidation of pyrite. Geochim. Cosmochim. Acta 1984, 48, 2669–2678. [Google Scholar] [CrossRef]
- Balci, N.; Shanks III, W.C.; Mayer, B.; Mandernack, K.W. Oxygen and sulfur isotope systematics of sulfate produced by bacterial and abiotic oxidation of pyrite. Geochim. Cosmochim. Acta 2007, 71, 3796–3811. [Google Scholar] [CrossRef]
- Camprubí, A.; González-Partida, E.; Torres-Tafolla, E. Fluid inclusion and stable isotope study of the Cobre–Babilonia polymetallic epithermal vein system, Taxco district, Guerrero, Mexico. J. Geochem. Explor. 2006, 89, 33–38. [Google Scholar] [CrossRef]
- Vidal, C.P.; Guido, D.M.; Jovic, S.M.; Bodnar, R.J.; Moncada, D.; Melgarejo, J.C.; Hames, W. The Marianas-San Marcos vein system: Characteristics of a shallow low-sulfidation epithermal Au-Ag deposit in the Cerro Negro district, Deseado Massif, Patagonia, Argentina. Miner. Depos. 2016, 51, 725–748. [Google Scholar] [CrossRef]
- Xie, Y.; Li, L.; Wang, B.; Li, G.; Liud, H.; Li, Y.; Dong, S.; Zhou, J. Genesis of the Zhaxikang epithermal Pb-Zn-Sb deposit in southern Tibet, China: Evidence for a magmatic link. Ore Geol. Rev. 2017, 80, 891–909. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, Y.J.; Qi, J.P.; Chen, J.; Dai, M.C.; Li, J. Geology, fluid inclusion and stable isotope study of the Yueyang Ag-Au-Cu deposit, Zijinshan orefield, Fujian Province, China. Ore Geol. Rev. 2017, 86, 254–270. [Google Scholar] [CrossRef]
- Zhong, J.; Pirajno, F.; Chen, Y.J. Epithermal deposits in South China: Geology, geochemistry, geochronology and tectonic setting. Gondwana Res. 2017, 42, 193–219. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, E.; Bi, Z.; Han, R.; Shao, J.; Liu, B.; Chen, J.; Zeng, N. Geochronology and isotope geochemistry studies of an epithermal gold deposit in the northern Lesser Khingan Range, NE China: The Gaosongshan example. Ore Geol. Rev. 2019, 105, 356–374. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system, Far Southeast-Lepanto porphyry and epithermal Cu–Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Naden, J.; Killias, S.P.; Darbyshire, D.P.F. Active geothermal system with entrained seawater as modern analogs for transitional volcanic-hosted massive sulfide and continental magmato-hydrothermal mineralization: The example of Milos Island, Greece. Geology 2005, 33, 541–544. [Google Scholar] [CrossRef]
- Lattanzi, P. Applications of fluid inclusions in the study and exploration of mineral deposits. Eur. J. Mineral. 1991, 3, 689–697. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–279. [Google Scholar] [CrossRef]
- Glennie, K.W. Cretaceous tectonic evolution of Arabia’s eastern plate margin: A tale of two oceans. In Middle East Models of Jurassic/Cretaceous Carbonate Systems; Alsharan, A.S., Scott, R.W., Eds.; Society for Sedimentary Geology, Special Publication: : Houston, TX, USA, 2000; Volume 69, pp. 9–20. [Google Scholar]
- Babaei, A.; Arvin, M.; Babaie, H.A. An oblique convergence and rotation model for the emplacement of the Baft ophiolitic mélange in Iran. Ofioliti 2001, 26, 401–408. [Google Scholar]
- Mohajjel, M.; Fergusson, C.L.; Sahandi, M.R. Cretaceous-Tertiary convergence and continental collision Sanandaj-Sirjan zone western Iran. J. Asian Earth Sci. 2003, 21, 397–412. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Mouthereau, F. Convergence history across Zagros (Iran): Constraints from collisional and earlier deformation. Intl. J. Earth Sci. 2005, 94, 401–419. [Google Scholar] [CrossRef]
- Richards, J.P.; Wilkinson, D.; Ulrich, T. The Tethyan tectonic history and Cu-Au metallogeny of Iran; SEG Special Publication: Houston, TX, USA, 2006; Volume 19, pp. 1455–1496. [Google Scholar]
- Pirajno, F. Hydrothermal Mineral Deposits; Springer-Verlag: Berlin/Heidelberg, Germany, 1992; 709p. [Google Scholar]
- Camprubí, A.; Albinson, T. Epithermal Deposits in México: Update of Current Knowledge, and an Empirical Reclassification; Special Paper; Geological Society of America: Boulder, CO, USA, 2007; Volume 422, pp. 14–39. [Google Scholar]







| Mineral | Inclusion Type | Tm-Ice (°C) | Th (Total) Range (°C) | Salinity (wt.% NaCl equiv.) |
|---|---|---|---|---|
| Sphalerite (n = 100) | LV | −0.1 to −15 | 107.5–298.1 (160) | 1.5–13.7 (6.97) |
| Sample | Mineral | δ34S Sulfide (‰) |
|---|---|---|
| Mac.B1 | Barite | 12.9 |
| Mac.B2 | Barite | 13.2 |
| Mac.B3 | Barite | 12.1 |
| Mac.B4 | Barite | 12.6 |
| Mac.B5 | Barite | 12.3 |
| Galena | 3.0 | |
| Mac.CGS | Chalcopyrite | 3.4 |
| Sphalerite | 3.4 | |
| Mac.C1 | Chalcopyrite | 1.9 |
| Mac.C2 | Chalcopyrite | 1.9 |
| Mac.G1 | Galena | 2.5 |
| Galena | 3.0 | |
| Mac.GG | Sphalerite | 3.3 |
| Galena | 2.6 | |
| Mac.SG | Sphalerite | 3.0 |
| Mac.Gy1 | Gypsum | 5.6 |
| Mac.Gy2 | Gypsum | 4.3 |
| Galena | 2.8 | |
| Mac.GS | Sphalerite | 3.1 |
| Sphalerite | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradiani, Z.; Ghaderi, M.; Tajeddin, H.-A.; Alfonso, P. Genesis of the Mahour Base Metal Deposit, Iran: Constraints from Fluid Inclusions and Sulfur Isotopes. Minerals 2024, 14, 435. https://doi.org/10.3390/min14040435
Moradiani Z, Ghaderi M, Tajeddin H-A, Alfonso P. Genesis of the Mahour Base Metal Deposit, Iran: Constraints from Fluid Inclusions and Sulfur Isotopes. Minerals. 2024; 14(4):435. https://doi.org/10.3390/min14040435
Chicago/Turabian StyleMoradiani, Zahra, Majid Ghaderi, Hossein-Ali Tajeddin, and Pura Alfonso. 2024. "Genesis of the Mahour Base Metal Deposit, Iran: Constraints from Fluid Inclusions and Sulfur Isotopes" Minerals 14, no. 4: 435. https://doi.org/10.3390/min14040435








