1. Introduction
The world’s production of phosphate rock in 2019 was 240 million tons. Phosphate ore is the only significant global resource of phosphorus, and it is found in marine sedimentary phosphorite deposits located in China, Africa, the Middle East, the United States, and in igneous deposits present in Brazil, Canada, Finland, Russia, and South Africa. Eighty percent of the world’s phosphate supply comes from sedimentary deposits [
1].
In Chile, the production of phosphate rock has been minimal. In 2019, only 3405 t were produced when these valuable resources were available. On the other hand, Chile imported 355,416 t phosphate fertilizers for agriculture in the same year [
2].
The global demand for these raw materials has increased and will double in the coming years. With the increase in demand, ore grades have decreased, and the quality of the product required is higher, making the concentration process more complicated [
3].
Tailings can be transformed into valuable secondary mining sources by combining mineral recovery with environmental management, eliminating the need for further size reduction, as the tailings have already been mined, crushed, and ground, and treatment costs are much lower compared to primary minerals. In Chile, copper tailings have a particle size of
d80 of 90 µm, an appropriate size for flotation [
4,
5,
6].
The iron reserves of Minera del Pacífico Company amount to approximately 7000 million tons with a grade exceeding 30% in total Fe. These reserves are located in the so-called Chilean Iron Belt, consisting of around 80 deposits of iron–apatite oxides. This belt of iron deposits stretches approximately 600 km along the Coastal Range, between the regions of Atacama and Coquimbo [
7]. In this line, the tailings produced by the treatment of iron ores from the Cretaceous Iron Belt in the Mountain Range of northern Chile [
8] are found, which contain iron oxide–apatite (IOA) deposits. The concentration process produces a non-magnetic tailing containing apatite, with grades between 1–3% P
2O
5 [
9].
Flotation is a method based on the difference in physicochemical properties between valuable minerals and gangue [
10]. The wettability of the surface plays a decisive role in its buoyancy. The contact angle between the ore surface and the bubble is often used as an ore surface wettability-relevant indicator [
11].
Flotation has been the most widely used technique when a minimum degree of P
2O
5 (28% to 35%) for fertilizer production is required. Similarly, the most common collectors used in phosphate flotation are fatty acids and their salts [
12,
13]. These collectors are employed to recover apatite in alkaline pH. However, the use of these collectors is only when their beneficiation is by the flotation of non-complex apatite minerals.
Under these conditions, the fatty acids become ionized. The carboxyl group reacts with the calcium ions present on the surface of the apatite and in the solution. This reaction produces non-soluble calcium carboxylate, which has Ca-O bonds. The Ca ions of the apatite surface interact with O atoms of the carbonyl group, forming the calcium carboxylate. This compound would precipitate at the mineral/solution interface, hydrophobizing the surface of the apatite due to the chemical bond that presents characteristics of precipitation of substances with low solubility [
14].
Worldwide, tall oil is the principal source of fatty acids used in flotation plants, although, in recent years, alternative sources have appeared, such as rice, soybean, flaxseed, passion fruit, nut, jojoba, coconut, pequi, and grape [
15,
16,
17].
In recent years, synthetic collectors have been developed as more sophisticated reagents when the fatty acids use to float complex phosphate minerals are not feasible due to their low selectivity and metallurgical recovery. In the case of magmatic apatite minerals, a practice that is becoming common is the mixing of synthetic collectors with fatty acids to increase the efficiency of the fatty acid. The disadvantage these collectors have is the high dose of reagents required for good performance [
18].
Iron oxide–apatite (IOA) deposits are characterized by their magnetite–apatite–actinolite associations. These deposits may contain significant amounts of rare earth elements that can be exploited as by-products. They are high-grade mines in magnetite and variable concentrations of apatite, and these contain rare earth elements [
19], which are processed to obtain iron concentrate. Reprocessing these tailings would have the advantage of partially recycling this material to produce a set of critical elements [
20]. Apatites have different buoyancy in the flotation process, and this depends on the geological origin, whether it is of the igneous, sedimentary, or sedimentary metamorphic type.
The circular economy and the utilization of secondary raw materials have gained increasing interest within the mining industry, and the critical raw material extraction from tailings can reduce waste materials and add more economic value to raw ore. Refs. [
21,
22] studied the flotation of apatite from iron tailings from IOA-type deposits. The processing of these tailings is a great challenge since the characteristics of the feed material can limit the possibility of an acceptable high grade in the final product and good recovery. Tailings with different phosphate contents from the magnetic separation process of magnetite have been studied for apatite recovery by flotation. With the results of the tailing beneficiation at the laboratory level and in the pilot plant using tall oil as a collector and sodium silicate as a dispersant in the flotation, an apatite concentrate is obtained with acceptable P
2O
5 grades for the manufacture of fertilizer. Additionally, the apatite concentrate also contains rare earth element concentrates.
Considering that phosphorus recovery could be carried out from tailings [
23,
24] to ensure the supply of phosphorus for fertilizers, the purposes of this work were as follows: (a) to characterize and evaluate the performance of a new synthetic collector, Atrac-2600 (anion collector sold by AkzoNobel-Brazil), to float apatite from iron tailings, different from fatty acid collectors, and (b) to design a laboratory-scale flotation circuit to produce apatite concentrate of commercial interest from the iron tailings generated in the Minera del Pacífico Company, located in the Atacama Region-Chile.
2. Materials and Methods
2.1. Preparation of the Sample
Pure samples of natural calcium phosphate crystals were obtained from the Los Molles mine of the Minera del Pacífico Company in Huasco, Atacama Region, Chile. These samples were reduced in size, and the apatite crystals with the lowest impurity contents were selected using an optical microscope. The sample was divided into quarters, with one portion used to make briquettes to determine the contact angle and the other was ground for zeta potential tests and chemical analysis.
The flotation tests were carried out using tailings obtained from the “El Trigo” deposit of the same company. The sample was dispersed and classified on a 210-micrometer opening sieve, where the retained fraction was removed. The fraction that passed was homogenized and divided into quarters. Then, representative samples were obtained for granulometric, chemical, and mineralogical characterization, and flotation tests at the laboratory level.
2.2. Flotation Tests, Zeta Potential, and Contact Angle Measurements with Pure Apatite
In the flotation tests of pure apatite, sodium hydroxide (NaOH) and sulfuric acid (H
2SO
4) were used as pH regulators, sodium silicate (Si-Na) as a dispersant, methyl isobutyl carbinol as a frother agent, and sodium oleate (O-Na) and Atrac-2600 as collectors. In previous research, the author demonstrated that the Atrac-2600 collector has better recovery and selectivity than sodium oleate [
25]
The zeta potential of apatite was measured using the microelectrophoresis technique in the Zeta Meter System 4.0 equipment. To prepare the suspension, 0.01 g of apatite with a particle size smaller than 37 μm was added to 100 mL solution. The suspension was left to settle for 24 h to allow particles smaller than 5 μm to float. Potassium nitrate (KNO3) was used as a supporting electrolyte at a concentration of 1 × 10−3 mol·L−1 and 1 × 10−5 mol L−1. The pH values were adjusted using sodium hydroxide (NaOH) and hydrochloric acid (HCl). Tests were also performed using sodium oleate and Atrac-2600.
The contact angle was measured using the sessile drop method. The equipment used was a Ramé-Hart Model 190 CA goniometer. The apatite sample was immersed in three different liquids: deionized water, a solution prepared with sodium oleate, and another with Atrac-2600, and at different pH values (2, 4, 6, 8, 10, and 12) to measure the contact angle. The purified apatite mineral sample was mounted on an epoxy resin briquette, polished to a mirror surface, and then immersed in the quartz cell containing the test liquids. The cell was mounted on the goniometer slide, and then an air bubble was generated with the goniometer microsyringe and placed on the apatite surface. Finally, the contact angle was measured three times on both sides of the bubble.
2.3. Flotation Tests of Tailings Containing Apatite
The flotation tests were performed in a Denver D12 flotation cell subaeration of 2.5 L. In all flotation tests, the solids concentration was kept constant at 35% with a pH of 10, 1200 rpm, aeration rate of 10 L min−1, and the conditioning and flotation time was 10 min. The mineralized froths were recovered by scraping the froth layer every 15 s and adding water to the test to maintain the suspension level periodically. The first flotation tests were performed to evaluate the type and dose of the collector and frother agent, keeping the dispersant constant based on the grade and recovery of P2O5.
2.4. Kinetic Flotation Tests and Open Cycle Tests
Kinetic flotation tests were performed on the rougher, cleaner, and scavenger stages to determine the optimal flotation time. The experimental procedure applied in the flotation kinetics for each stage was identical. However, no reagents were added during the cleaner and scavenger flotation stages. The mineralized froths were removed at time intervals of 0–1, 1–3, 3–5, 5–7, and 7–10 min. Subsequently, the concentrates and tailings obtained were filtered, dried at 80 °C, weighed to recover the mass, and analyzed to establish the chemical composition in all flotation tests. Open-cycle tests were performed after determining the optimal flotation times to calculate the split factors of the three flotation stages. These tests were performed using the optimal times established in the flotation kinetics.
3. Results
Table 1 presents the chemical composition of the tailing sample to be fed to the flotation tests. The chemical analyses were performed using the colorimetric technique for phosphorus, the potassium dichromate volumetric technique for iron, and atomic absorption spectroscopy for silica, aluminum, calcium, magnesium, and sodium.
Table 2 presents the mineralogical analysis performed on the tailing sample. The different mineralogical species that form the samples can be observed, where 3.4% corresponds to apatite. The other mineralogical species contained in high concentrations are iron oxyhydroxide and aluminum silicates, such as feldspars, amphibole, pyroxene, epidote, and micas, which is consistent with the chemical composition presented in
Table 1, which shows that the most abundant elements are silicon, iron, aluminum, calcium, and magnesium.
Table 3 presents the results obtained in the chemical analysis of the purified apatite sample. A high content of phosphate and lime in the sample can be observed. It was established that it is a chloro-apatite sample based on its composition and from the scanning electron microscopy with an energy-dispersive X-ray spectroscopy (EDS) analysis.
According to the results shown in
Table 1 and
Table 2, there is agreement with respect to the values obtained in the chemical analysis and the mineralogical species present in the tailings sample studied. On the other hand,
Table 3 presents values that show high purity of the apatite sample used in the zeta potential and contact angle tests.
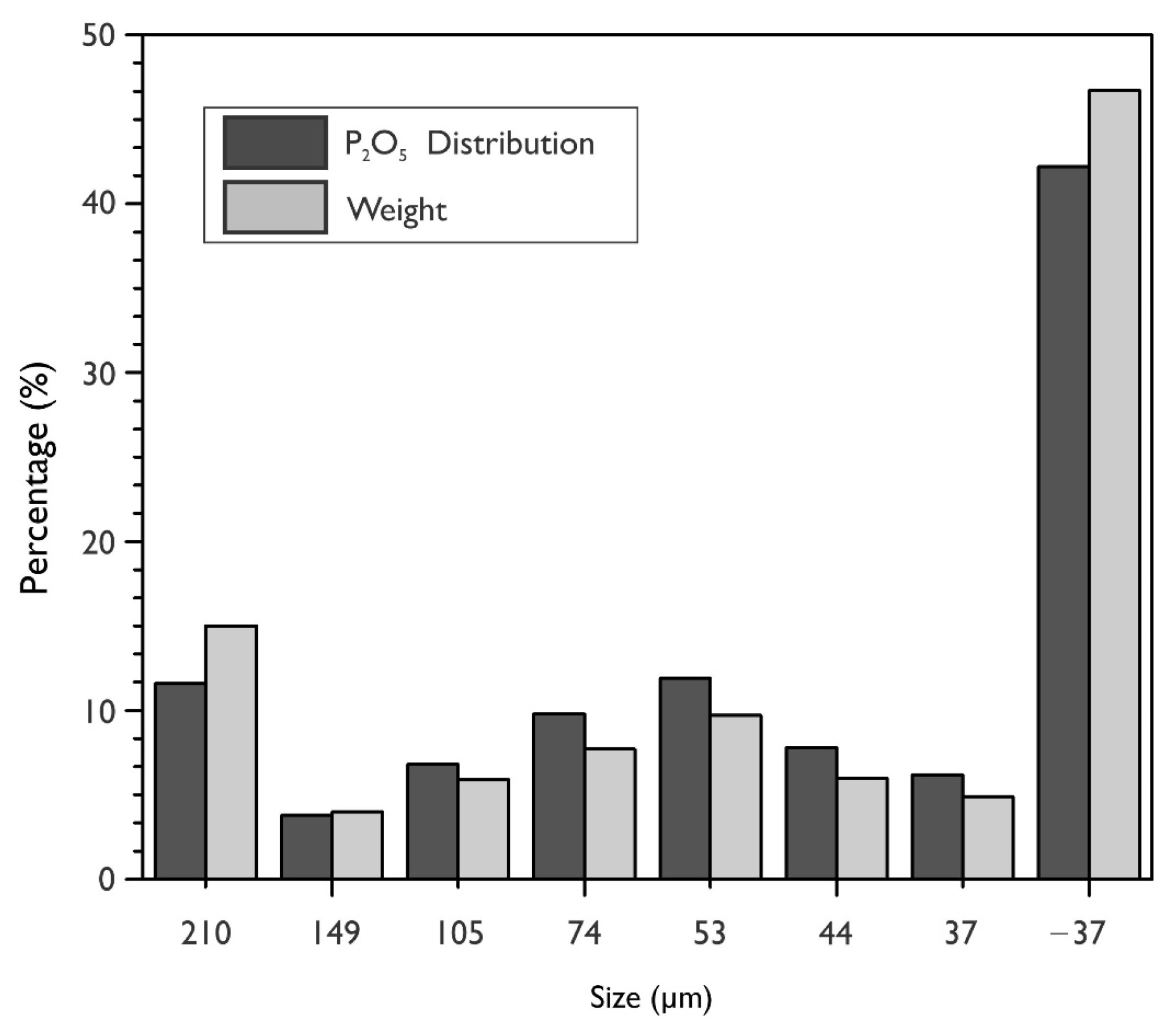
Figure 1 shows the particle size analysis results and the percentage distribution of P
2O
5 for each particle size fraction in the tailing. It is observed that 46.7% of the particles are smaller than 37 microns, and 42.2% of P
2O
5 of the total phosphate is contained under this same particle size. It is worth mentioning that the high percentage of material lower than 53 microns corresponds to 67.3%.
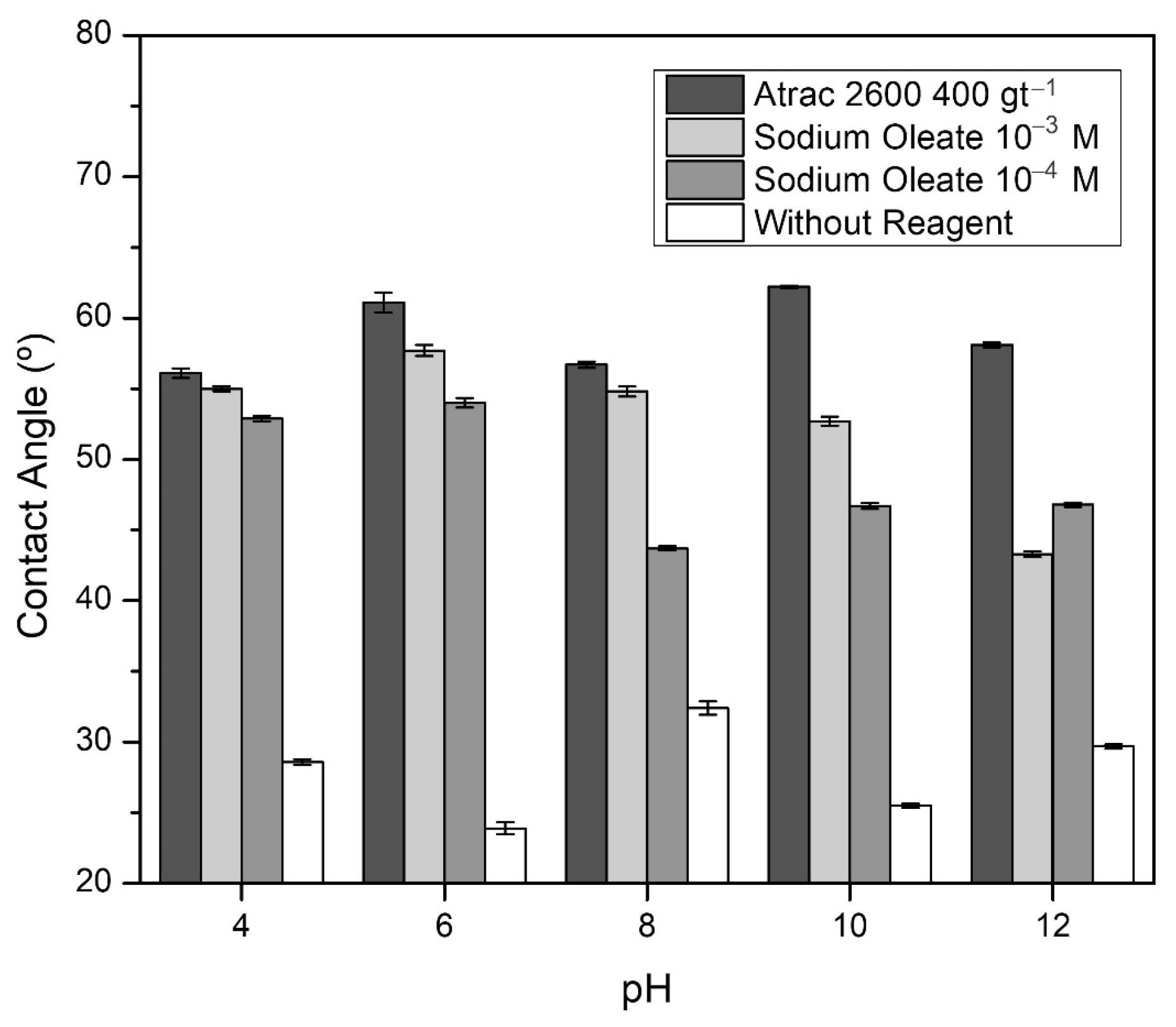
Figure 2 shows that the contact angle without using collectors is low and presents results between 23.9° and 42.4°. However, with sodium oleate in doses of 10
−4 mol·L
−1 and 10
−3 mol·L
−1, the contact angle increases to 56.0° and 57.7°, respectively. It is also observed that the best results were obtained with Atrac-2600 throughout the pH range studied, standing out at pH 10 with a maximum angle of 63.4°. Such results are consistent with previous publications [
26].
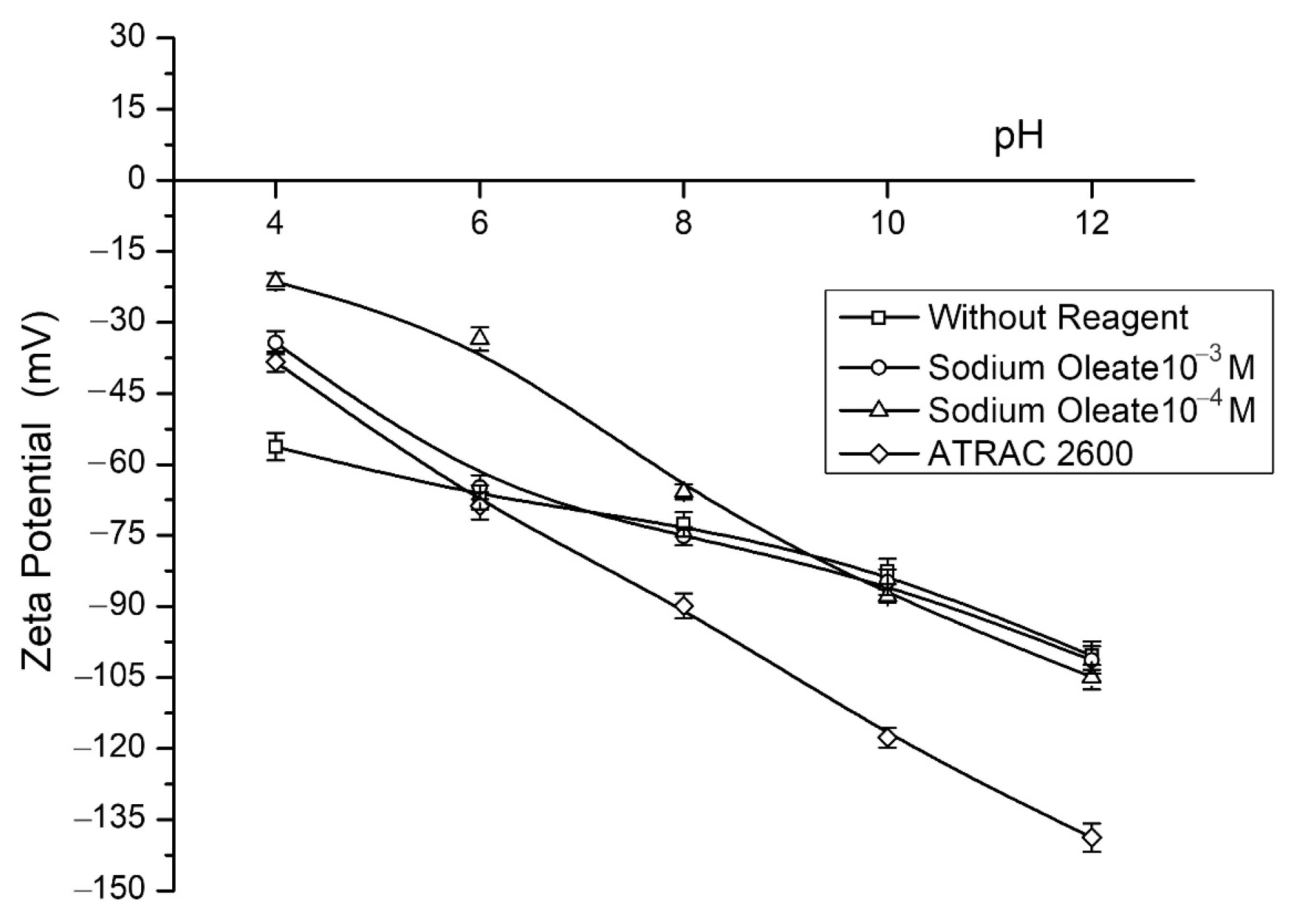
Figure 3 shows the zeta potential results as a pH function without a collector and employing sodium oleate and Atrac-2600. These tests were performed with KNO
3 as the neutral electrolyte and NaOH and H
2SO
4 as the pH modifiers across a pH range of 2 to 12.
The graph illustrates that the apatite particles have a negative surface both with and without the presence of collectors. According to [
27], the electrokinetic properties of apatite depend on the first-order potential-determining ions (Ca
+2,
, and OH
−), while H
+ ions would be the second-order potential-determining ions. The surface charges become slightly negative when applying sodium oleate in concentrations of 10
−4 mol·L
−1 and 10
−3 mol·L
−1. Using Atrac-2600, more negative zeta potentials are obtained concerning the values obtained for apatite without a collector, which would indicate adsorption of the anionic collector on the surface of the mineral despite the high repulsion that would be present at a basic pH. In this case, Atrac-2600 would be specifically adsorbed by chemisorption with apatite, hydrophobizing the surface of the mineral. Diverse researchers [
27,
28] have reported a high variability of the apatite isoelectric point, encountering PIE values between 2 and 8. This difference is due to the origin, composition, and substitutions, among other factors.
Several investigations on reagents used in the flotation of apatite, such as fatty acids, oleic acid, and sodium oleate, have shown through micro-flotation tests, zeta potential measurements, and XPS analysis, that the collector is chemically adsorbed on the surface of the apatite and that it is formed between the hydrogen of the fatty acid and the oxygen of PO
4, at the surface of the apatite [
29,
30]. Ref. [
31] correlated that it was chemisorbed by the CaO bonds’ formation on the apatite surface. Also, the molecules (C
17H
33CONH
2) and micelles (C
17H
33CONH
2) with the oleic acid amide can be chemisorbed on the apatite surface by Ca-O bonds or Ca-N formation. In this study, the flotation of the apatite occurs at an alkaline pH, which suggests possible chemical adsorption of the collector, considering that at this pH, the apatite presents a negative zeta potential, which is increased by the presence of the collector, by the specific adsorption of this reagent on the surface of the mineral.
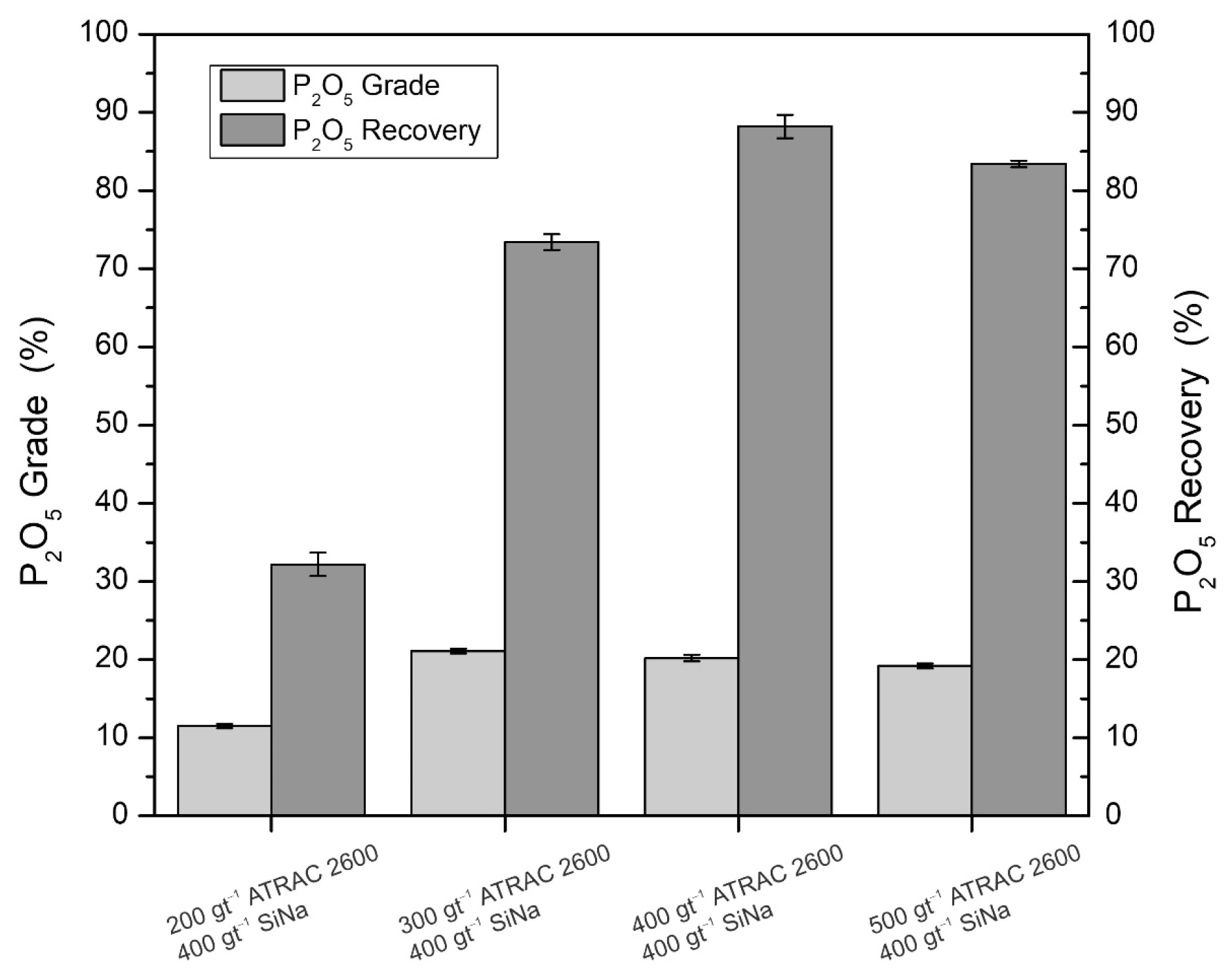
Figure 4 shows the metallurgical recovery and the P
2O
5 grade for different doses of Atrac-2600 (200, 300, 400, and 500 gt
−1) using 400 gt
−1 of sodium silicate. It is observed that the recovery has a behavior directly proportional to the collector dose; namely, as the dose increases, the recovery increases as well. Similar results were stated by [
32], who reported that higher doses of the synthetic collector MD 20,723 increased apatite recovery.
It is observed that the best result is obtained with a dose of 400 gt
−1 of Atrac-2600 and 400 gt
−1 of sodium silicate. Furthermore, a concentrate is obtained with a grade of 20.2% P
2O
5 and a recovery of 88.2%, with a high enrichment ratio of 11.2. This matter indicates a high selectivity concerning SiO
2 and Fe
2O
3, favored by the presence of sodium silicate, whose function is the dispersion of the gangue, the increase of its hydrophilicity, and the inhibition of its flotation [
33,
34].
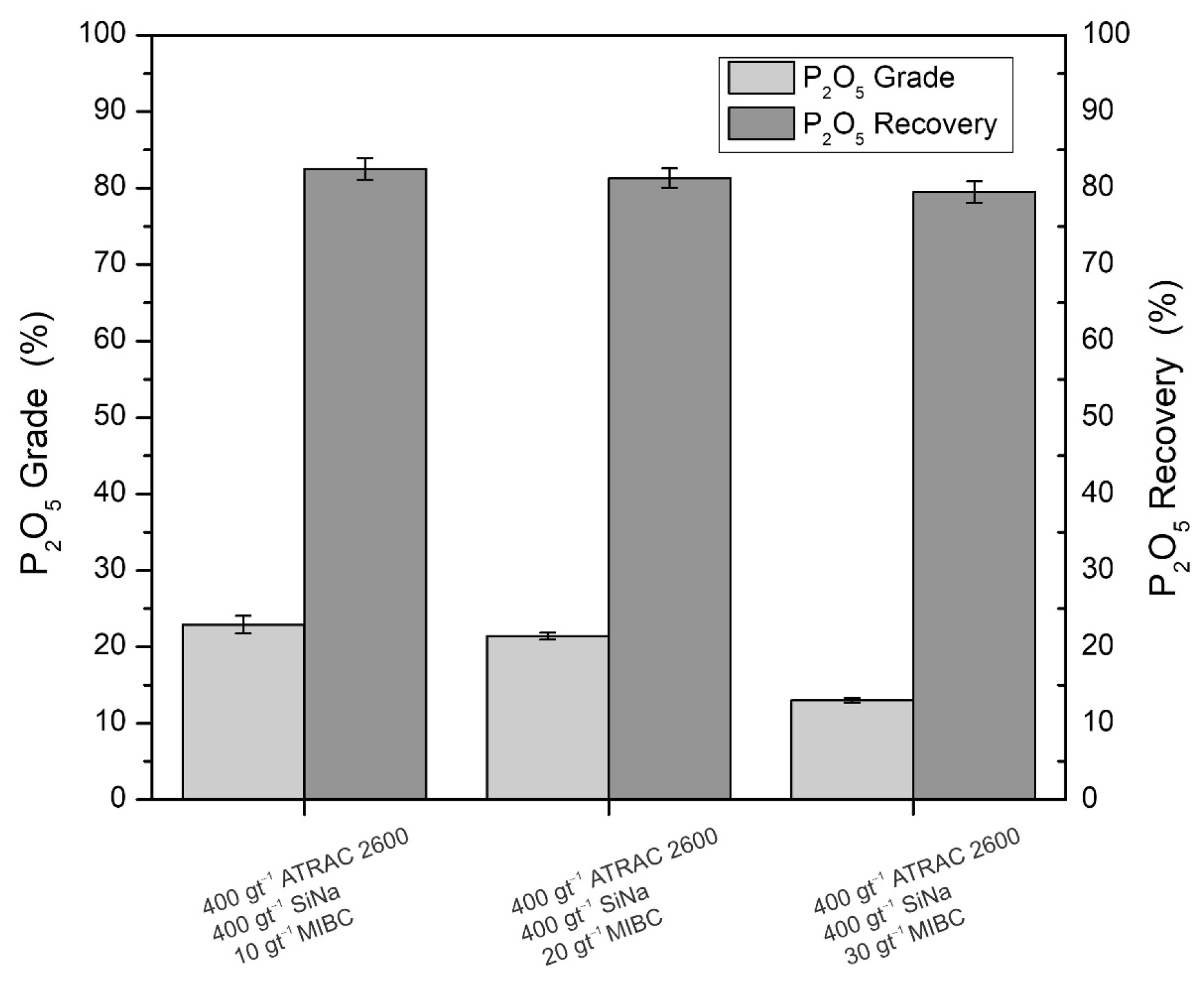
Figure 5 shows the dose effect of the methyl isobutyl carbinol (MIBC) frother agent on the metallurgical recovery and P
2O
5 grade for an Atrac-2600 dose of 400 gt
−1 and 400 gt
−1 of sodium silicate.
The figure shows that the addition of the frother decreases the recovery of P
2O
5, and without the addition of the frother, only adding 400 gt
−1 of Atrac-2600 and 400 gt
−1 of sodium silicate, a recovery of 88.2% is obtained (See
Figure 4). With the addition of 10 gt
−1 of the frother, in addition to 400 gt
−1 of Atrac-2600 and 400 gt
−1 of sodium silicate, a recovery of 82.5% is obtained (See
Figure 5). However, the P
2O
5 grade increases from 20.2% to 22.9% by adding 10 gt
−1 of the frother. Higher doses of the frother reduce the grade and P
2O
5 recovery.
3.1. Flotation Kinetics
The results of the flotation kinetics were adjusted to the model proposed by [
35]:
where R is the recovery (%) of valuable metal in an instant t (min), with
representing the maximum estimated recovery (%) and k is the first-order kinetic.
The kinetic parameters, confidence interval of fit at a 90% significance level (C.I.), standard error of recovery (S.E.), and correlation coefficient (R
2) obtained for each flotation stage presented in
Table 4 were determined using the SOLVER function of the Microsoft Excel spreadsheet program [
36].
The correlation coefficient values indicate that the experimental recoveries fit the Garcia Zúñiga model quite well, i.e., over 98.4% of the recovery variation can be explained by the variation in flotation time [
35].
Table 4 also presents, as expected, that the magnitude of the kinetic constant (k) that represents the flotation speed of the particles in each stage for the cleanest stage (k = 0.30 min
−1) is greater than for the rougher stage (k = 0.28 min
−1), and the magnitude of the kinetic constant (k) for the rougher stage is greater than that for the scavenger stage (0.12 min
−1).
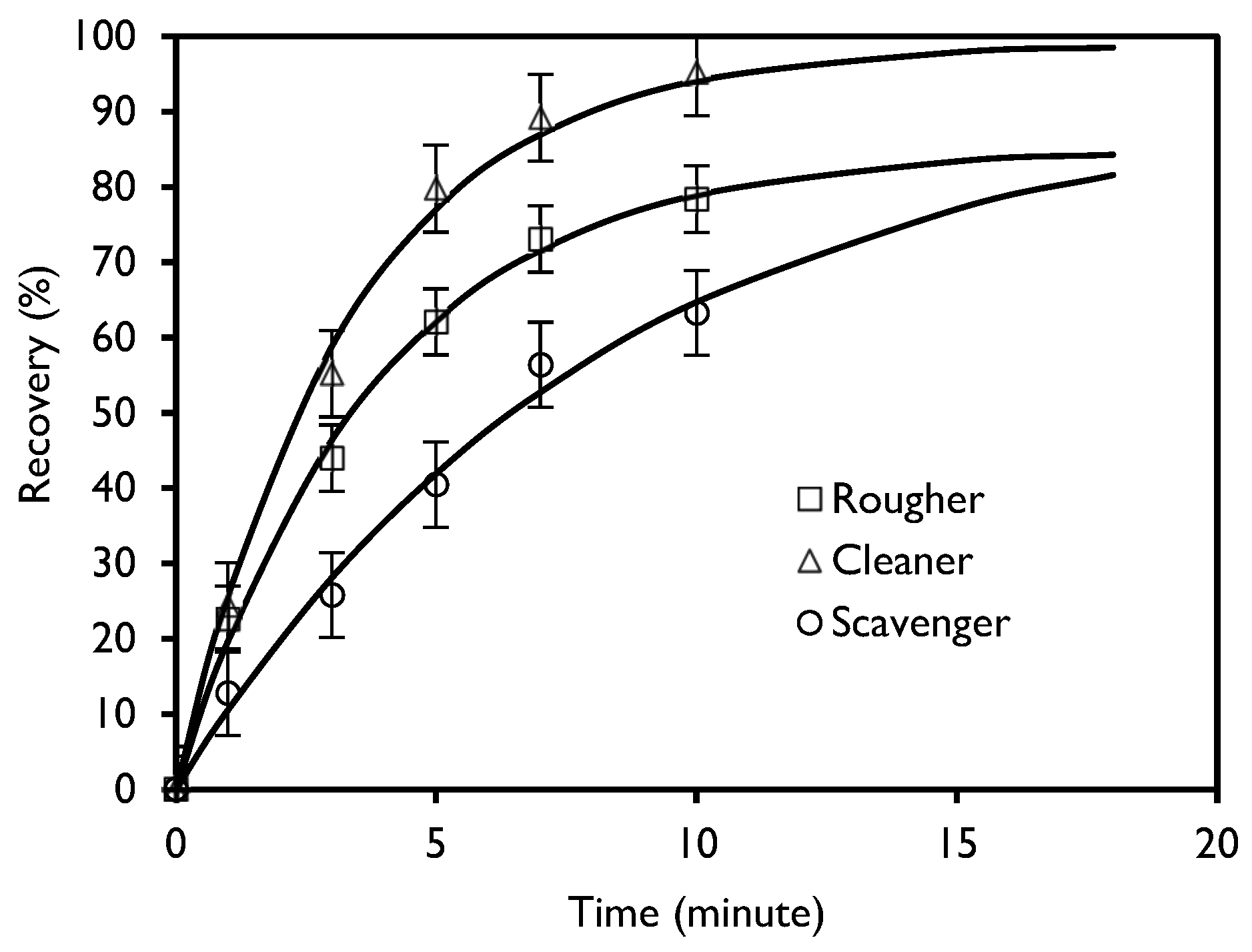
Figure 6 shows the accumulated recoveries as a function of time for the rougher, cleaner, and scavenger kinetics. The symbol represents the accumulated experimental recovery, while the line indicates the accumulated recovery calculated using the García Zuñiga model.
It is observed that after 10 min of flotation, the recovery of apatite is 78%; however, longer times do not increase the recovery and result in a reduction in concentrate grade. The optimal flotation time for the rougher stage is established as 10 min, yielding an instantaneous and cumulative P2O5 grade of 5.7% and 19.5% in the concentrate, respectively. The tailing, with a 0.45% P2O5 grade, will undergo reprocessing in the scavenger stage. The recovery for the scavenger stage was 63.3%, and the optimum time to achieve this recovery according to the Agar criteria was 7.6 min, with P2O5 grades of 2.2% in the concentrate and 0.20% in the scavenger tailing. The instantaneous grade obtained for the cleaner stage was 21.7% of P2O5, and the cumulative grade was 30.2% of P2O5 for 6.8 min.
3.2. Determination of the Split Factor
The split factor concept represents the percentage by weight of each component fed to a flotation stage that appears in the concentrate, i.e., it corresponds to the apatite and weight recoveries for the rougher, scavenger, and cleaner flotation stages. The magnitude of the split factor depends mainly on the flotation time, the physical and chemical properties of the slurry, and the mineral characteristics.
Table 5 presents the split factor calculated with the open cycle tests performed with the optimal flotation time determined by flotation kinetics.
3.3. Simulation of Flotation Circuits
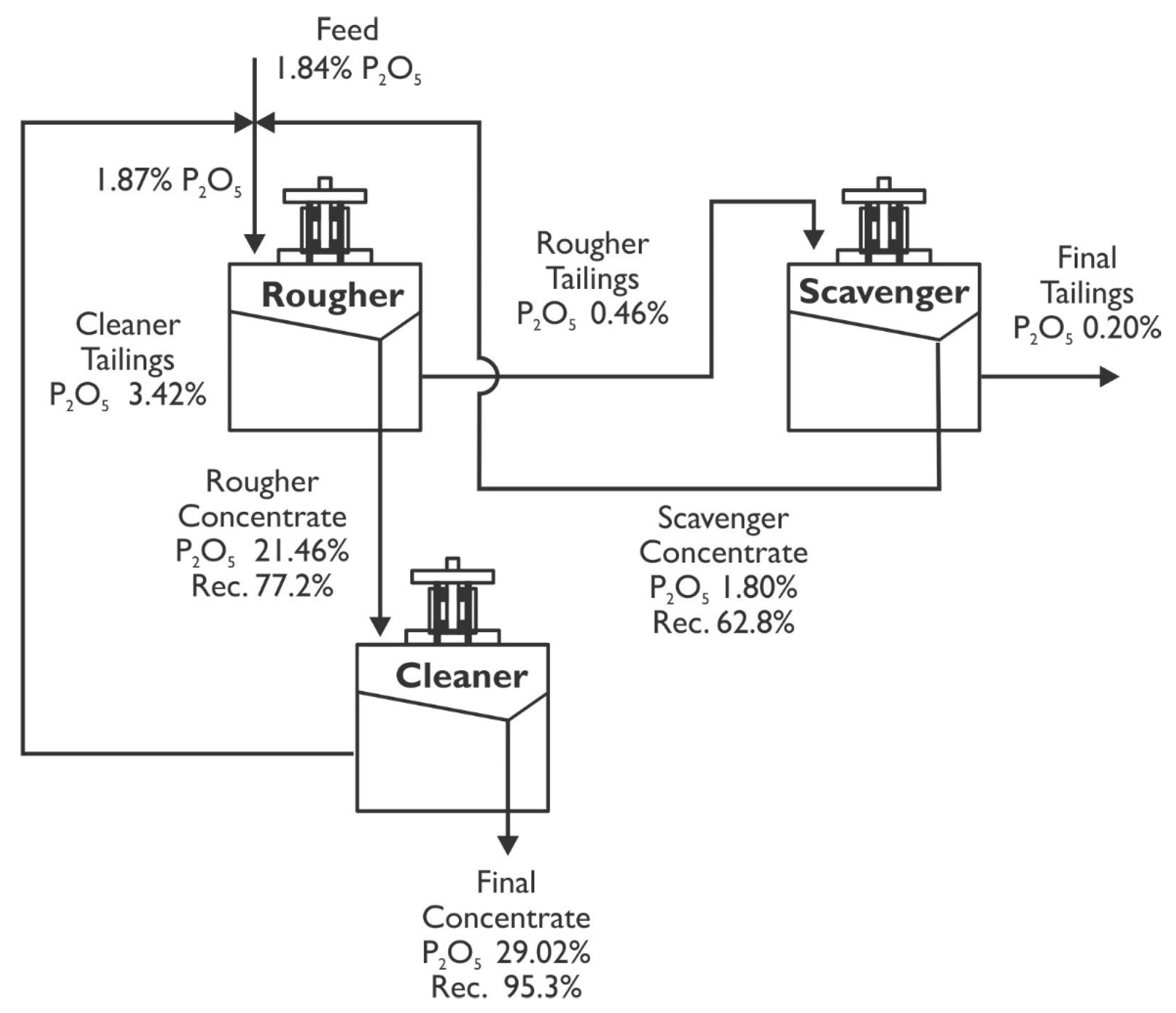
Figure 7 shows the flotation circuit simulation using the split factor method to recover apatite from the magnetic concentration tailings of iron ore. The equations system that describes the mass balance of the flotation circuit, represented in
Figure 7, was solved by the matrix inversion method using the MINVERSA and MMULT functions of Excel.
Table 6 presents the grade and recovery of P
2O
5 for each of the stages along with the global flotation circuit shown in
Figure 7. The feasibility of producing concentrates with a grade of 29.1% and recovering 89.6% of the P
2O
5 contained in the iron tailings by implementing a flotation circuit comprising the rougher–scavenger–cleaner stages is observed in the table. To achieve this grade and recovery of P
2O
5, it is only necessary to condition the iron tailings with 400 gt
−1 of Atrac-2600, 400 gt
−1 of sodium silicate, a 10 min of conditioning time, pH adjustment to 10, and flotation times set at 10, 7.6, and 6.8 min for the rougher–scavenger–cleaner and recleaner stages, respectively.