Abstract
Cuboid diamonds are particularly common in the placers of the northeastern Siberian platform, but their origin remains unclear. These crystals usually range in color from dark yellow to orange and, more interestingly, are characterized by unusual low aggregated nitrogen impurities (non-aggregated C-center), suggesting a short residence time and/or low temperatures at which they have been stored in the mantle. In order to track possible isotopic signature that could help deciphering cuboid diamond’s crystallization processes, δ13C values, δ15N values, and nitrogen concentrations have been determined in situ in three samples using secondary ion mass spectrometry (SIMS), whereas nitrogen aggregation states have been determined by FTIR spectroscopy. The samples fall out of the δ13C vs. δ15N field of canonical mantle composition. Different scales of carbon and nitrogen fractionation may produce the observed variations. Alternatively, mixing mantle and crustal material would obscure initial co-variations of δ13C values with δ15N or nitrogen content.
1. Introduction
Natural diamonds are the subject of extensive research because they provide unique information on the geochemistry, mineralogy, and P-T-fO2 regimes of the mantle. Most of such studies use inclusions trapped during diamond growth, providing important information about diamond-forming processes. Previous investigations of crystalline inclusions in diamonds from the subcontinental lithospheric mantle have shown that diamonds originated in a range of peridotitic (P-type) and eclogitic (E-type) host-rocks [1]. Diamonds from both parageneses occur with diverse morphological and physical properties, which are widely interpreted as reflecting variations in the conditions of diamond formation, within the Earth’s upper mantle, providing important information about growth conditions and subsequent diamond post-growth histories. Carbon and nitrogen isotopic systems are useful indicators for constraining the crustal and mantle sources and their interactions during subduction [2]. The carbon and nitrogen isotopic compositions of diamonds formed in the mantle have been widely used as a tool for investigating the origin of diamond-forming carbon in the mantle [3] and references therein. The worldwide database shows that the δ13C value of peridotitic diamonds is very narrow, typically around −5‰, whereas eclogitic diamonds can show positive and very negative δ13C values resembling crustal carbonates and crustal organic carbon (from −40‰ to +2‰). Curiously, diamonds from both parageneses can show positive (crust-like) and negative (mantle-like) δ15N values (from −40‰ to +20‰).
The alluvial placers of the northeastern Siberian platform are characterized by specific diamond populations [4]. In particular, diamonds of cubic morphology (cuboids), forming a continuous color series from yellowish-green to yellow and dark orange, are commonly found in such placers. These diamonds have been designated as variety II in the mineralogical classification [5]. Previously described characteristics of the diamonds of variety II, i.e., cuboid morphology, light carbon isotope composition, and low aggregated nitrogen (type Ib—causing specific yellow coloration), suggest an unusual primary source [6,7]. Here, we report variations of δ15N and δ13C in several cuboid diamonds and investigate their nature and origin. This paper is part of a wider study of alluvial diamonds from the northeastern Siberian platform.
2. Materials and Methods
The three cuboid diamonds studied here have been chosen in an alluvial diamond collection originating from the placers of the northeastern Siberian platform. Thirty-six samples from the same collection have been previously analyzed for carbon and nitrogen isotopes, nitrogen content, and aggregation state. The results have been published previously [7]. The millimeter-size samples (2–3 mm) studied here range in color from dark yellow (HL-3) to orange (MTII-27). In order to proceed with in situ geochemical measurements, we polished the samples along either (100) or (110) planes on a conventional diamond scaife (a special metal polishing wheel operating at a rotation speed of 3000 rpm), thereby producing a central plate. Growth patterns of polished plates were observed on a polarized microscope Zeiss Axioscop 40 (Figure 1). All diamonds have concentric cuboid growth zones, which can be distinguished from their orientation and different colors.

Figure 1.
Polished plates of yellow cuboid diamonds from alluvial placers in the northeastern Siberian platform (transmitted light); (a) cubic section; (b,c) dodecahedron polished sections. Zoning growth structures are visible. Points in different growth zones were analyzed.
The infrared spectra of the diamonds were recorded using an FTIR Bruker VERTEX 70 (Billerica, MA, USA) equipped with a HYPERION 2000 microscope. Local spectra were measured in different growth zones at an aperture of 60 μm × 60 μm over a range of 370–7000 cm−1, with 32 scans at a resolution of 2 cm−1. The deconvolution of the experimental spectra between 1100 and 1350 cm−1 made it possible to determine the relative contributions of different N-defects with characteristic spectral lines having known specified shapes.
We used the CAMECA 1280-HR SIMS instrument (CAMECA SAS, Gennevilliers, France) in Deutsches GeoForschungsZentrum in Potsdam (GFZ Potsdam) for determining carbon and nitrogen isotope compositions in small domains in traverses across the diamonds from core to rim. Prior to analyses, the three diamond plates together with reference materials of known compositions were put in indium using a hand operated press. The surface of the 1-inch sample mounts were then cleaned using high-purity ethanol before it was argon sputter-coated with high-purity gold. Carbon and nitrogen data were collected in June 2015 and March 2016, respectively. The analytical details were presented in [8].
Our analyses employed a 133Cs+ primary beam with a total impact energy of 20 keV. The analyses used a 250 pA probe current for nitrogen and a 2.5 nA current for carbon. The beam was focused to a ~4–8 µm diameter with a Gaussian distribution at the sample surface. Secondary ions were extracted using a −10 kV potential applied to the sample holder. Charge compensation involved circa 250 pA low energy electron cloud provided by a normal incidence electron flood gun. The instrument was operated with a circa 80 µm field-of-view, with a 50 eV wide energy window. In the case of carbon isotope measurements, we used a static multi-collection mode operating at a mass resolution of M/dM ≈ 3200, which fully resolves the 13C mass station from the nearby 12C1H molecular isobar. For determining nitrogen concentrations and nitrogen isotope ratios our mass spectrometer was operated in mono-collection mode at a mass resolution of M/dM ≈ 6900 using the peak-stepping sequence 12C2, 12C14N and 12C15N signals.
Each carbon isotope analysis was preceded by a 60 s pre-sputter using a 25 µm raster, so as to remove the 35 nm thick gold coat and also to implant Cs+ in order to establish equilibrium sputtering conditions. An analysis used a 15 µm raster, together with the tool’s dynamic transfer capability, thereby generating a square, flat-bottom sputter crater. Prior to initiating data collection we conducted automatic beam centering routines in both X and Y for the field aperture. A single carbon analysis involved 20 cycles of 4 s integration each and the data were filtered at the 3sd level. Thus, a single analysis required around 3 min of analysis time, including the pre-sputtering. All data were collected in fully automated data acquisition mode.
Prior to initiating nitrogen data collection on a selected target, the diamond region was sputter cleaned for 80 s using a 3 nA Cs+ primary beam rastered over a 20 µm × 20 µm area. Prior to initiating data collection the primary beam current was reduced to 250 pA and the rastered area was reduced to 10 µm × 10 µm, which was compensated using the dynamic transfer function tool. Automatic centering routines were then conducted for X and Y for the field aperture. Our data acquisition involved the peak-stepping sequence: 12C2 (2 s integration time per cycle), 12C14N (4 s) and 12C15N (15 s). A single nitrogen isotope analysis consisted of 50 cycles of this peak-stepping sequence, leading to a total analysis time of ~20 min per acquisition.
Machine calibration used two diamond reference materials contained in the same indium-based sample mount. The samples were cut out from isotopically homogeneous “steady state” parts of up-octahedral sectors in synthetic crystals 140/4 and 150/3 [9]. The synthetic crystals had been characterized for their δ13CVPDB values and nitrogen concentrations refer to “SynAT” reference material [10] at the Edinburgh ion-microprobe facility. Minor pieces of the specific chips used in this study were additionally checked for their δ13С, δ15N, and nitrogen content using static vacuum gas source mass-spectrometry by Dr. A. Verchovsky at Open University (Milton Keynes, UK). The established characteristics are as follows: Crystal 140/4 with δ13CVPDB = −26.5‰, 460 µg/g nitrogen and δ15NAIR = 0‰; Crystal 150/3 with δ13CVPDB = −24.5‰, 215 µg/g nitrogen and δ15NAIR = −5‰.
Our raw isotope data were tested for the presence of a time-dependent linear drift, which was corrected for when needed. Such drifts, which we attribute to changes in sensitivity of the amplifiers in our detector system, were 0.3‰ per hour or less. After correcting for such drift the repeatability of our 12C15N/12C14N determinations on the two reference materials was in the range of 1.1‰–1.5‰ (1sd), which is our best estimate for the random component that might be present in our nitrogen data. Hence, we can conclude that the overall reliability of our δ15N values is better than ±1.5‰ (1sd). Using the same approach for 13C/12C determinations, the overall reliability of our δ13C values is better than ±0.24‰.
3. Results
3.1. Nitrogen Content and Aggregation State
According to FTIR data, the three cuboid diamonds are of mixed type IaA/Ib caused by the presence of an A-center (pair of N atoms [11]) and C-center (single N atom [12]) with additional lines of unknown centers described as X (a sharp peak at 1332 cm−1) and Y (a superposition with a maximum at 1140–1150 cm−1) [13,14]. The nitrogen content (N = NA + NC) in studied diamonds shows little variations from 24 to 133 ppm and is commonly higher in the central zones (Table 1). The nitrogen aggregation (relative abundance of A-centers) in diamonds ISTD-15 and MTII-27 is higher in central zones and nearly constant in diamond HL-3. The nitrogen content determined by SIMS in these diamonds varies in the range 28–217 ppm (Table 1). The higher nitrogen concentrations as determined by SIMS relative to those measured from FTIR are in the central zones. We suppose this discrepancy may be explained by the presence of additional nitrogen beyond that contained in the IR-active A- and C-centers. Additionally, SIMS measurements provide results for the surface area, but FTIR measurements provide results for the entire width of the plate, where input of the other zones is inevitable [15]. Notably, all the areas with low nitrogen content show precise agreement between FTIR and SIMS results.

Table 1.
Isotopic compositions of carbon (δ13C) and nitrogen (δ15N) and nitrogen concentrations (N) in cuboid diamonds from alluvial placers in the northeastern Siberian platform. Also indicated are the nitrogen concentrations as measured by SIMS and FTIR.
3.2. Carbon Isotope Composition
Carbon isotope compositions were analyzed in four to five growth zones of the cuboid diamonds (see Figure 1 and Table 1). δ13C values of the diamonds vary substantially from sample to sample but show very small intra-crystal variations (from −3.8‰ to −4.1‰ in ISTD-15, from −8.9‰ to −9.3‰ in HL-3, and from −15.1‰ to −16.1‰ in MTII-27). These values fall within the previously described carbon isotope range of cuboid diamonds of this variety from this locality [7], which are commonly off-set from the main mantle mode of δ13C at −5.5‰ towards lighter carbon isotope compositions (Figure 2). Nonetheless, the studied cuboid diamonds plot in the field of most known diamonds originate from the worldwide dataset by Stachel and Harris, 1997 [16], and are outlined by Cartigny et al., 2001 [17] (Figure 2). Despite observed wide variations, we did not find any straightforward correlation between carbon isotope compositions and the nitrogen concentration of studied diamonds.
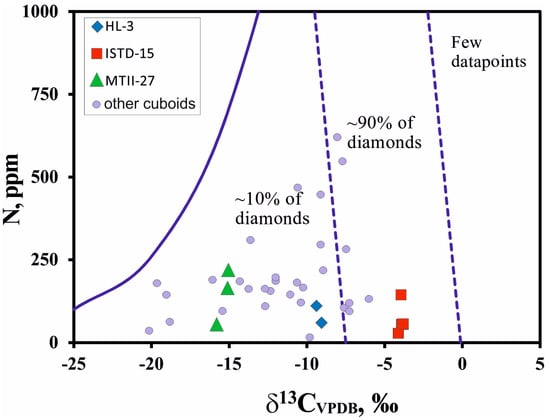
Figure 2.
Carbon isotope values (δ13CVPDB) and nitrogen abundance (N, ppm) in yellow cuboid diamonds from alluvial placers in the northeastern Siberian platform compared to worldwide data summarized by Cartigny et al., 2001 [17].
3.3. Nitrogen Isotope Composition
The nitrogen isotope composition of our cuboid diamonds show a broad range in δ15N values (−5‰ to −22‰ in ISTD-15, −12‰ to −20‰ in HL-3, and −10‰ to −14‰ in MTII-27). We found no correlation between δ15N values and nitrogen concentrations of the diamonds (Table 1). The measured δ15N values are lighter than the average modern mantle value [18]. Compared to diamonds worldwide, the observed range of δ15N in our diamonds is more typical of diamonds from a peridotitic lithology (Figure 3). Diamond ISTD-15 shows wide δ15N variations within the field of peridotitic diamonds against a background of minute variations of carbon isotope composition (Figure 3). Diamonds HL-3 and MTII-27 have a lighter carbon isotope composition, which is rare in peridotitic diamonds, whereas the range of δ15N is close to that seen in ISTD-15, with a pronounced shift to lighter compositions (Figure 3).
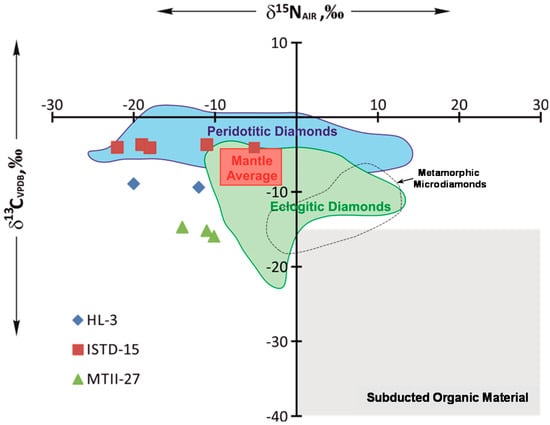
Figure 3.
Carbon (δ13CVPDB) and nitrogen (δ15NAIR) isotope compositions of yellow cuboid diamonds from alluvial placers in the northeastern Siberian platform and comparison with peridotitic and eclogitic diamond data worldwide [3] and metamorphic microdiamonds [31]. Average mantle range and range of subducted organic material for δ13C and δ15N values are also shown [3].
4. Discussion
Diamonds worldwide exhibit a large range of carbon isotope compositions. These variations have been attributed to several factors: (i) isotopic fractionation during fluid/melt migration [17,19] and directly during diamond crystallization, e.g., [20,21,22,23]; (ii) primordial heterogeneities in mantle carbon, e.g., [20,24]; (iii) the addition of isotopically heterogeneous carbon sources incorporated into the mantle by subduction processes [25,26,27]. In the last scenario, the low δ13C values would imply that the carbon originated not as carbonate but as organic carbon. It is supposed that diamonds of eclogitic parageneses were formed from metasedimentary carbon preserved in deeply subducted oceanic crust, whereas diamonds of peridotitic parageneses were formed from a primordial mantle carbon source (δ13C ~ −5‰). It was also suggested that, during the progressive partial melting of subducted material, the relative proportion of the organic and carbonate carbon contribution changes in time/space, thereby generating a wide range of δ13C values [28]. The shift from average mantle δ13C values towards lighter compositions in our studied cuboid diamonds seems typical for diamonds of eclogitic parageneses and their wide range of carbon isotope compositions suggests the formation from distinct mantle and crustal carbon sources [7].
Diamonds with extremely light and heavy nitrogen isotope compositions may reflect sources from an isolated, 15N-depleted primordial mantle reservoir and from an 15N-enriched mantle reservoir that contains recycled crustal components, respectively [29,30,31]. Significant general variations in the nitrogen isotope composition of peridotitic diamonds (Figure 3) may be related to primordial mantle heterogeneity. Very low δ15N values of rare lithospheric and sublithospheric diamonds suggest that the Archaean mantle was more isotopically depleted in 15N than is the case today [18]. However, some evidence for lowering δ15N values down to −40‰ were recently suggested as a result of nitrogen isotope fractionation during diamond crystallization [32]. Importantly, metasedimentary nitrogen is characterized by δ15N values that are invariably positive for throughout the Archean to recent geological periods [33] (Figure 3). It is recognized that eclogitic diamonds with low δ13C values and positive δ15N signatures have been formed from crustal material [30,31]. Alternatively, Cartigny et al., 2001 [17], suggested that the very negative δ13C values associated with positive δ15N values can result from an open-system fractionation of metasomatic fluids.
Our study reveals that δ15N and δ13C variations in two studied cuboid diamonds locate close to known the peridotitic (ISTD-15) and eclogitic (MTII-27) diamond isotopic fields (Figure 3). The mixing between mantle and crustal source cannot properly explain the origin of cuboid diamonds with observed low δ13C values and negative δ15N values. The shift from average mantle δ13C values towards lighter compositions observed for HL-1 and MTII-27 diamonds are typical for a series of diamonds from eclogitic parageneses. On the contrary, variation in nitrogen isotope composition towards light values is attributed to peridotite paragenesis (Figure 3) and results from isotopically light Archean mantle [18]. Using stable isotope data obtained on diamonds from worldwide sources, Hogberg et al., 2016 [23], argued that carbon and nitrogen in diamonds are decoupled. They suggested that even a minor heterogeneity in the primary composition of diamond forming carbon (e.g., due to addition of minor subducted carbon) will completely obscure any possible correlations between δ13C values and either δ15N or nitrogen content. Modeling for mixing between mantle-related and subduction-related carbon also illustrates the impossibility of its matching with associated nitrogen content in diamonds [34]. Thus, C and N isotope data do not provide direct evidence of a relationship between subducted carbon and the formation of cuboid diamonds from alluvial placers of the northeastern Siberian platform. However, the lack of C–N co-variations in isotopically light diamonds can suggest a mixing of mantle and subducted crustal material. It also cannot rule out a possibility of significant nitrogen isotope fractionation against a background of moderate δ13C changes during fluid crystallization [8].
The formation of cuboid diamonds was suggested to proceed through different growth modes: (i) a “fibrous” (columnar) growth in the <111> direction, and (ii) “cuboids” growth of the crystals, which do not show a fibrous internal structure [35]. In both cases, the cuboid habit of diamonds may be a primary feature, reflecting rough growth under high degrees of carbon supersaturation, which operates as the driving force for diamond growth [36]. Most cuboid diamonds worldwide are characterized by a restricted range of carbon and nitrogen isotope compositions, nitrogen concentrations, and aggregation states, suggesting their formation from a common primary source [37,38]. The δ13C and δ15N values in cuboid diamonds generally resemble the average mantle range with a low nitrogen aggregation state (99% of them are IaA diamonds) attributed to short residence times, which indicates a close relationship of their formation to kimberlite magmatism [39,40]. The presence of non-aggregated substitutional single nitrogen defects in our studied cuboid diamonds (Type Ib-IaA) testifies to an even shorter mantle residence time. These features point to the formation of cuboid diamonds during metasomatic events prior to their ascent to the surface in kimberlite magmas. Alternatively, the low aggregation state of nitrogen defects in the cuboid diamonds may be related to their storage in the mantle at relatively low temperature and conditions where an active transformation of nitrogen defects is inhibited [41]. Thus, the observed isotopic and N composition variations in the studied diamonds may also record chemical and isotopic heterogeneities of the host rocks and thermal gradients up to several hundred degrees inside of subducted oceanic plates [42].
5. Conclusions
A population of yellow- to dark orange-colored cuboid diamonds from alluvial placers of the northeastern Siberian platform shows a specific set of morphological and chemical features, suggesting they were derived from a rare primary source. The overall presence of non-aggregated C centers in these diamonds requires their short mantle residence time or low temperatures, where C- to A-centers conversion becomes exceedingly slow. In this study, we have determined multiple δ13C–δ15N values and nitrogen concentrations in three cuboid diamonds using SIMS and FTIR. We found a wide range of δ15N intracrystal variations with no significant fluctuations in the carbon isotope values of the diamonds. The isotope values of the cuboid diamonds falls out of the δ13C vs. δ15N field of average mantle compositions, which is also typical for most cuboid and fibrous diamonds worldwide. This might be evidence of mantle mixing with crustal material that would have obscured any initial δ13C-δ15N co-variations. Chemical heterogeneities of host rocks and different scales of carbon and nitrogen isotopic fractionation may also affect co-variations of δ13C values with δ15N or nitrogen content.
Acknowledgments
This work was supported by state assignment project (0330-2016-0007). SIMS measurements in Potsdam were supported by Russian Scientific Foundation Grant No. 14-17-00054. We thank F. Couffignal for his excellent support during SIMS data acquisition.
Author Contributions
D.Z. studied samples by optical microscopy, polished the plates, and analyzed them via FTIR; V.R. analyzed C and N isotope composition in samples and discussed the results; M.W. contributed analysis tools and discussed the results; D.Z. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sobolev, N.V. Deep-Seated Inclusions in Kimberlites and the Problem of the Composition of the Upper Mantle; Translated from the Russian Edition; AGU: Washington, DC, USA, 1974; 279p. [Google Scholar]
- Wallace, P.J. Volatiles in subduction zone magmas: Concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geotherm. Res. 2005, 140, 217–240. [Google Scholar] [CrossRef]
- Cartigny, P.; Palot, M.; Thomassot, E.; Harris, J.W. Diamond formation: A stable isotope perspective. Annu. Rev. Earth Planet. Sci. 2014, 42, 699–732. [Google Scholar] [CrossRef]
- Afanasiev, V.P.; Zinchuk, N.N.; Koptil, V.I. Polygenesis of diamonds in the context of placer sources in the Northeastern Siberian Platform. Dokl. Earth Sci. 1998, 361, 366–369. [Google Scholar]
- Orlov, Y.L. Mineralogy of Diamond; Wiley Sons, Inc.: New York, NY, USA, 1977; 234p. [Google Scholar]
- Titkov, S.V.; Shiryaev, A.A.; Zudina, N.N.; Zudin, N.G.; Solodova, Y.P. Defects in cubic diamonds from placers in the northeastern Siberian platform: Results of IR microspectrometry. Russ. Geol. Geophys. 2015, 56, 354–362. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Kalinina, V.V.; Reutsky, V.N.; Yuryeva, O.P.; Rakhmanova, M.I. Regular cuboid diamonds from placers on the northeastern Siberian platform. Lithos 2016, 265, 125–137. [Google Scholar] [CrossRef]
- Reutsky, V.N.; Kowalski, P.M.; Palyanov, Y.N.; Wiedenbeck, M. Experimental and theoretical evidence for surface-induced carbon and nitrogen fractionation during diamond crystallization at high temperatures and high pressures. Crystals 2017, 7, 190. [Google Scholar] [CrossRef]
- Reutsky, V.N.; Harte, B.; Borzdov, Y.M.; Palyanov, Y.N. Monitoring diamond crystal growth, acombined experimental and SIMS study. Eur. J. Miner. 2008, 20, 365–374. [Google Scholar] [CrossRef]
- Harte, B.; Fitzsimons, I.C.W.; Harris, J.W.; Otter, M.L. Carbon isotope ratios and nitrogen abundances in relation to cathodoluminescence characteristics for some diamonds from the Kaapvaal Province, S. Africa. Mineral. Mag. 1999, 63, 829–856. [Google Scholar] [CrossRef]
- Davies, G. The A nitrogen aggregate in diamond: Its symmetry and possible structure. J. Phys. C 1976, 9, 537–542. [Google Scholar] [CrossRef]
- Smith, W.; Sorokin, P.; Gelles, I.; Lasher, G. Electron-spin resonance of nitrogen donors in diamond. Phys. Rev. 1959, 115, 1546. [Google Scholar] [CrossRef]
- Lawson, S.L.; Fisher, D.; Hunt, D.C.; Newton, M.E. On the existence of positively charged single-substitutional nitrogen in diamond. J. Phys. 1998, 10, 6171–6180. [Google Scholar] [CrossRef]
- Hainschwang, T.; Fritsch, E.; Notary, F.; Rondeau, B. A new defect center in type Ib diamond inducing one phonon infrared absorption: The Y center. Diam. Relat. Mater. 2012, 21, 120–126. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Pearson, D.G.; Hauri, E.H.; Griffin, B.J. Carbon and nitrogen isotopes systematics within a sector-growth diamond from the Mir kimberlite, Yakutia. Chem. Geol. 2002, 188, 105–123. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. Syngenetic inclusions in diamond from the Birim field (Ghana)—A deep peridotitic profile with a history of depletion and re-enrichment. Contrib. Miner. Petrol. 1997, 127, 336–352. [Google Scholar] [CrossRef]
- Cartigny, P.; Harris, J.W.; Javoy, M. Diamond genesis, mantle fractionations and mantle nitrogen content: A study of δ13C-N concentrations in diamond. Earth Planet. Sci. Lett. 2001, 185, 85–98. [Google Scholar] [CrossRef]
- Cartigny, P.; Marty, B. Nitrogen isotopes and mantle geodynamics: The emergence of life and the atmosphere-crust-mantle connection. Elements 2013, 9, 359–366. [Google Scholar] [CrossRef]
- Galimov, E.M. Isotope fractionation related to kimberlite magmatism and diamond formation. Geochim. Cosmochim. Acta 1991, 55, 1697–1708. [Google Scholar] [CrossRef]
- Deines, P. The carbon isotopic composition of diamonds: Relationship to diamond shape, color, occurrence and vapor composition. Geochim. Cosmochim. Acta 1980, 44, 943–961. [Google Scholar] [CrossRef]
- Thomassot, E.; Cartigny, P.; Harris, J.W.; Viljoen, K.S. Methane-related diamond crystallization in the Earth’s mantle: Stable isotope evidences from a single diamond-bearing xenolith. Earth Planet. Sci. Lett. 2007, 257, 362–371. [Google Scholar] [CrossRef]
- Reutsky, V.N.; Palyanov, Y.N.; Borzdov, Y.M.; Sokol, A.G. Isotope fractionation of carbon during diamond crystallization in model systems. Russ. Geol. Geophys. 2015, 56, 239–244. [Google Scholar] [CrossRef]
- Hogberg, K.; Stachel, T.; Stern, R.A. Carbon and nitrogen isotope systematics in diamond: Different sensitivities to isotopic fractionation or a decoupled origin? Lithos 2016, 265, 16–30. [Google Scholar] [CrossRef]
- Javoy, M.; Pineau, F.; Delorme, H. Carbon and nitrogen isotopes in the mantle. Chem. Geol. 1986, 57, 41–62. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Sobolev, N.V. New portion on very deep subsidence of eclogitizied crustal rocks. Dokl. Akad. Nauk SSSR 1980, 250, 683–685. (In Russian) [Google Scholar]
- Milledge, H.J.; Mendelssohn, M.J.; Seal, M.; Rouse, J.F.; Swart, P.K.; Pillinger, C.T. Carbon isotopic variations in spectral type II diamonds. Nature 1983, 303, 791–792. [Google Scholar] [CrossRef]
- McCandless, T.E.; Gurney, J.J. Diamond eclogites: Comparison with carbonaceous chondrites, carbonaecous shales, and microbial carbon-enriched MORB. Russ. Geol. Geophys. 1997, 38, 394–404. [Google Scholar]
- Bulanova, G.P.; Walter, M.J.; Smith, C.B.; Kohn, S.C.; Armstrong, L.S.; Blundy, J.; Gobbo, L. Mineral inclusions in sublithospheric diamonds from Collier 4 kimberlite pipe, Juina, Brazil: Subducted protoliths, carbonated melts and primary kimberlite magmatism. Contrib. Miner. Petrol. 2010, 160, 489–510. [Google Scholar] [CrossRef]
- Cartigny, P.; Boyd, S.R.; Harris, J.W.; Javoy, M. Nitrogen isotopes in peridotitic diamonds from Fuxian, China: The mantle signature. Terra Nova 1997, 9, 175–179. [Google Scholar] [CrossRef]
- Palot, M.; Cartigny, P.; Harris, J.W.; Kaminsky, F.V.; Stachel, T. Evidence for deep mantle convection and primordial heterogeneity from nitrogen and carbon stable isotopes in diamond. Earth Planet. Sci. Lett. 2012, 357, 179–193. [Google Scholar] [CrossRef]
- Smart, K.A.; Tappe, S.; Stern, R.A.; Webb, S.J.; Ashwal, L.D. Early Archaean tectonics and mantle redox recorded in Witwatersrand diamonds. Nat. Geosci. 2016, 9, 255–259. [Google Scholar] [CrossRef]
- Reutsky, V.N.; Shiryaev, А.А.; Titkov, S.V.; Wiedenbeck, М.; Zudina, N.N. Evidence for large scale fractionation of carbon isotopes and of nitrogen impurity during crystallization of gem quality cubic diamonds from placers of North Yakutia. Geochem. Int. 2017, 55, 988–999. [Google Scholar]
- Cartigny, P. Stable isotopes and origin of diamond. Elements 2005, 1, 79–84. [Google Scholar] [CrossRef]
- Cartigny, P.; Stachel, T.; Harris, J.W.; Javoy, M. Constraining diamond metasomatic growth using C- and N-stable isotopes: Examples from Namibia. Lithos 2004, 77, 359–373. [Google Scholar] [CrossRef]
- Moore, M.; Lang, A.R. On the internal structure of natural diamonds of cubic habit. Philos. Mag. 1972, 26, 1313–1325. [Google Scholar] [CrossRef]
- Sunagawa, I. Growth and morphology of diamond crystals under stable and metastable conditions. J. Cryst. Growth. 1990, 99, 1156–1161. [Google Scholar] [CrossRef]
- Javoy, M.; Pineau, F.; Demaiffe, D. Nitrogen and carbon isotopic composition in the diamonds of Mbuji Mayi (Zaïre). Earth Planet. Sci. Lett. 1984, 68, 399–412. [Google Scholar] [CrossRef]
- Boyd, S.R.; Pineau, F.; Javoy, M. Modelling the growth of natural diamonds. Chem. Geol. 1994, 116, 29–42. [Google Scholar] [CrossRef]
- Boyd, S.R.; Mattey, D.P.; Pillinger, C.T.; Milledge, H.J.; Mendelssohn, M.; Seal, M. Multiple growth events during diamond genesis: An integrated study of carbon and nitrogen isotopes and nitrogen aggregation state in coated stones. Earth Planet. Sci. Lett. 1987, 86, 341–353. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.D.; Rossman, G.R.; Wasserburg, G.J. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Taylor, W.R.; Canil, D.; Milledge, H.J. Kinetics of Ib to IaA nitrogen aggregation in diamond. Geochim. Cosmochim. Acta 1996, 60, 4725–4733. [Google Scholar] [CrossRef]
- Zedgenizov, D.; Rubatto, D.; Shatsky, V.; Ragozin, A.; Kalinina, V. Eclogitic diamonds from variable crustal protoliths in the northeastern Siberian craton: Trace elements and coupled δ13C-δ18O signatures in diamonds and garnet inclusions. Chem. Geol. 2016, 422, 46–59. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).