The Effects of Calcium Ions on the Flotation of Sillimanite Using Dodecylammonium Chloride
Abstract
:1. Introduction
2. Materials and Methods
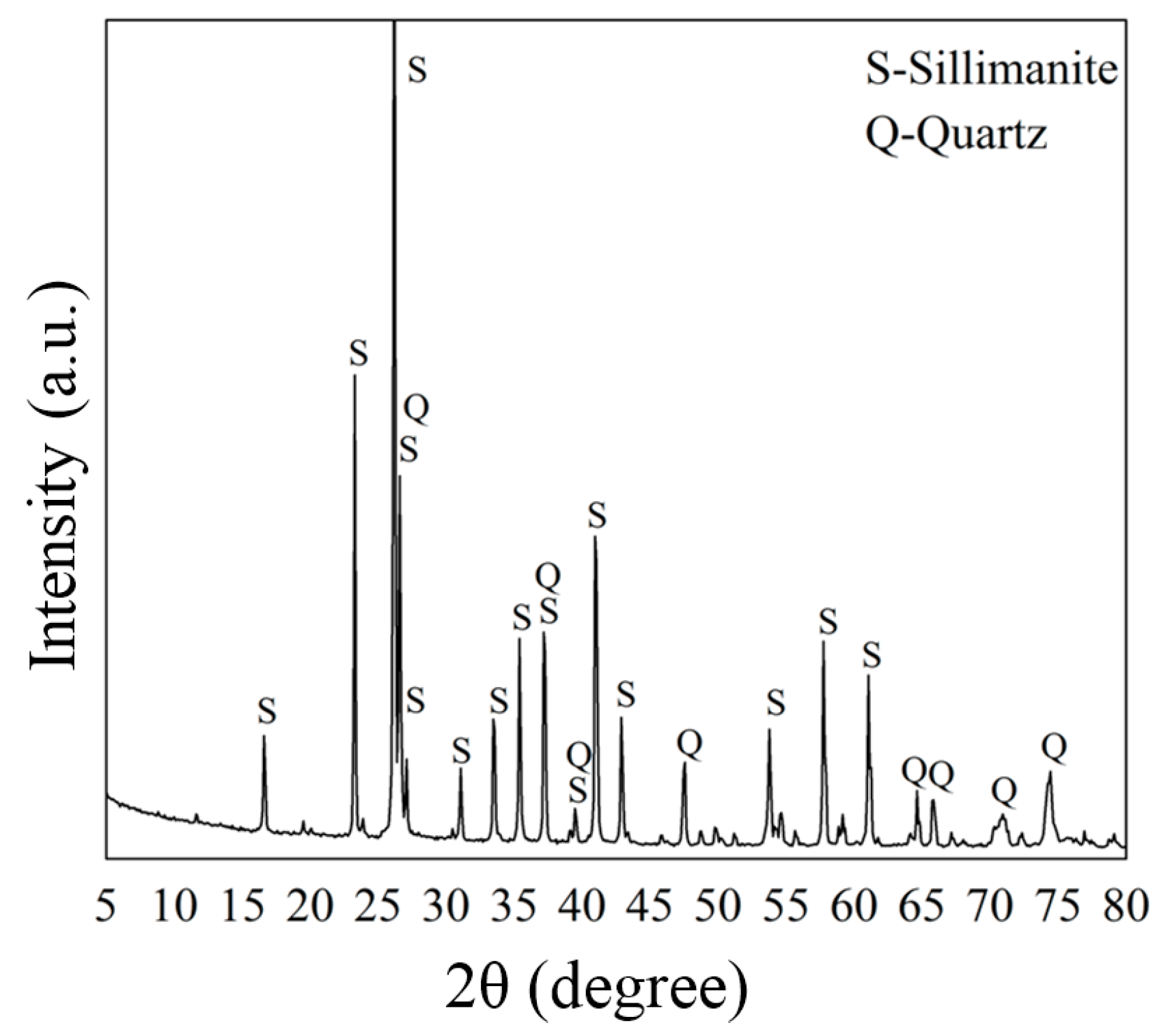
2.1. Materials and Reagents
2.2. Micro-Flotation Experiments
2.3. Zeta Potential Tests
2.4. Computational Methods
3. Results and Discussion
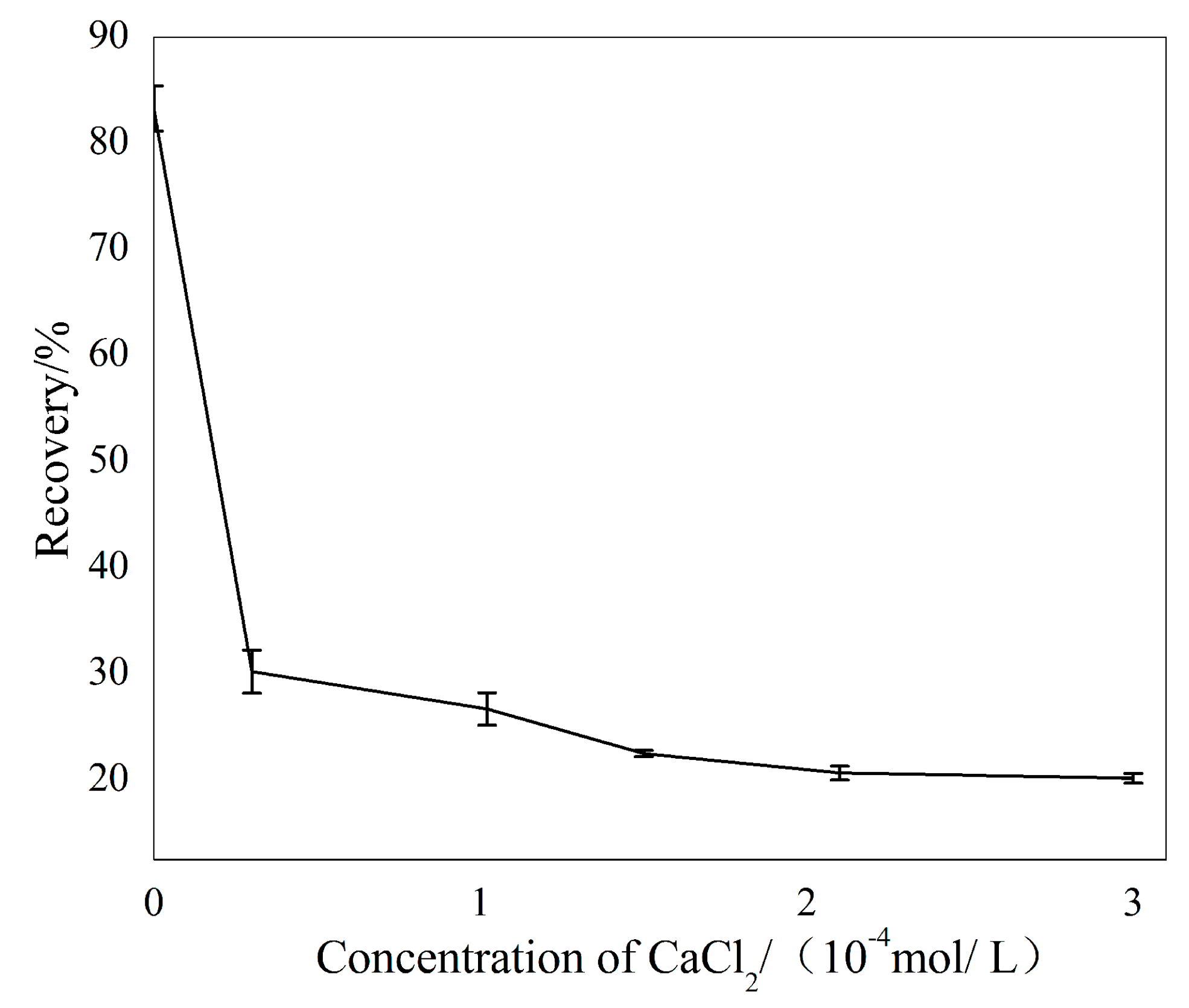
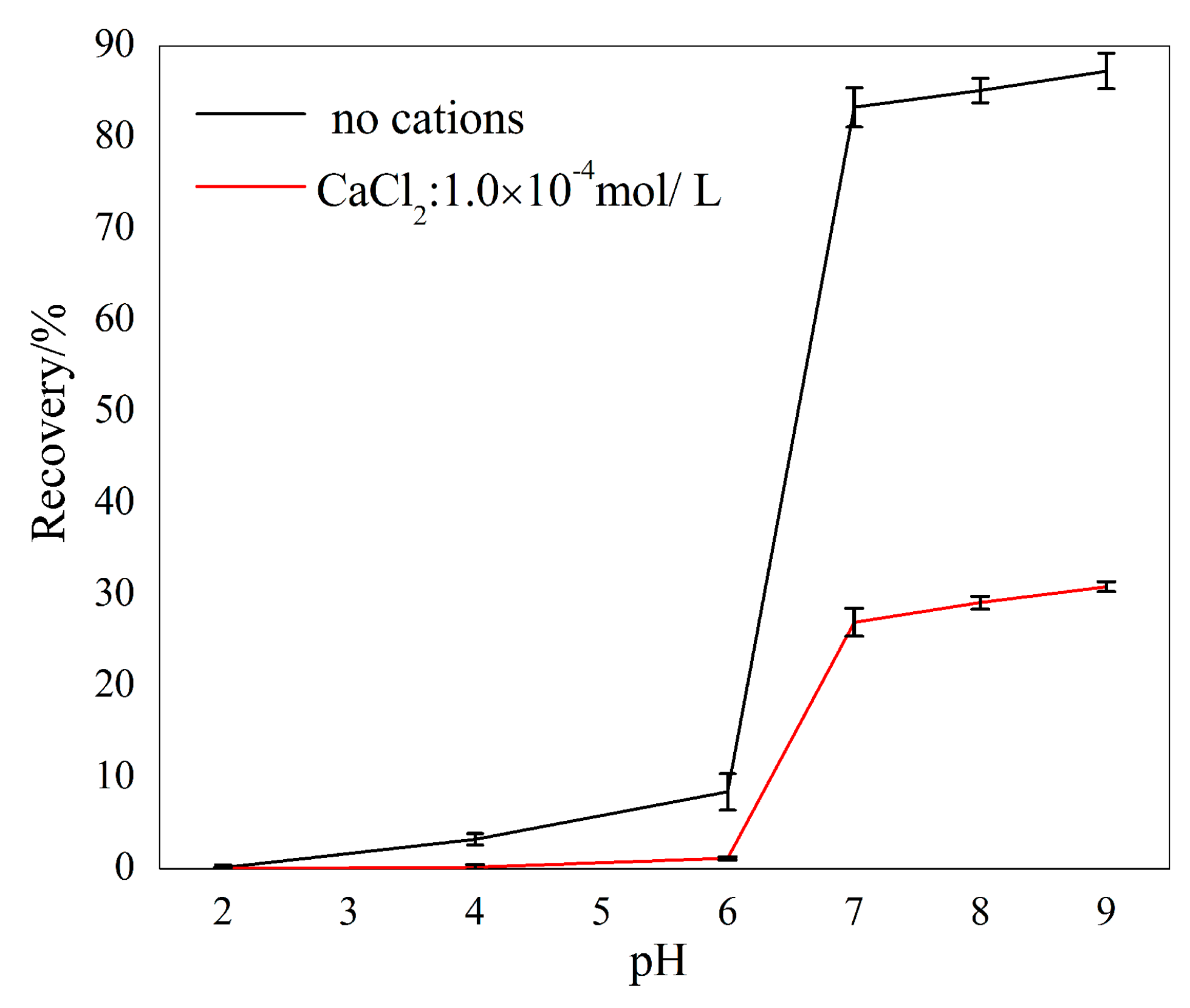
3.1. Micro-Flotation
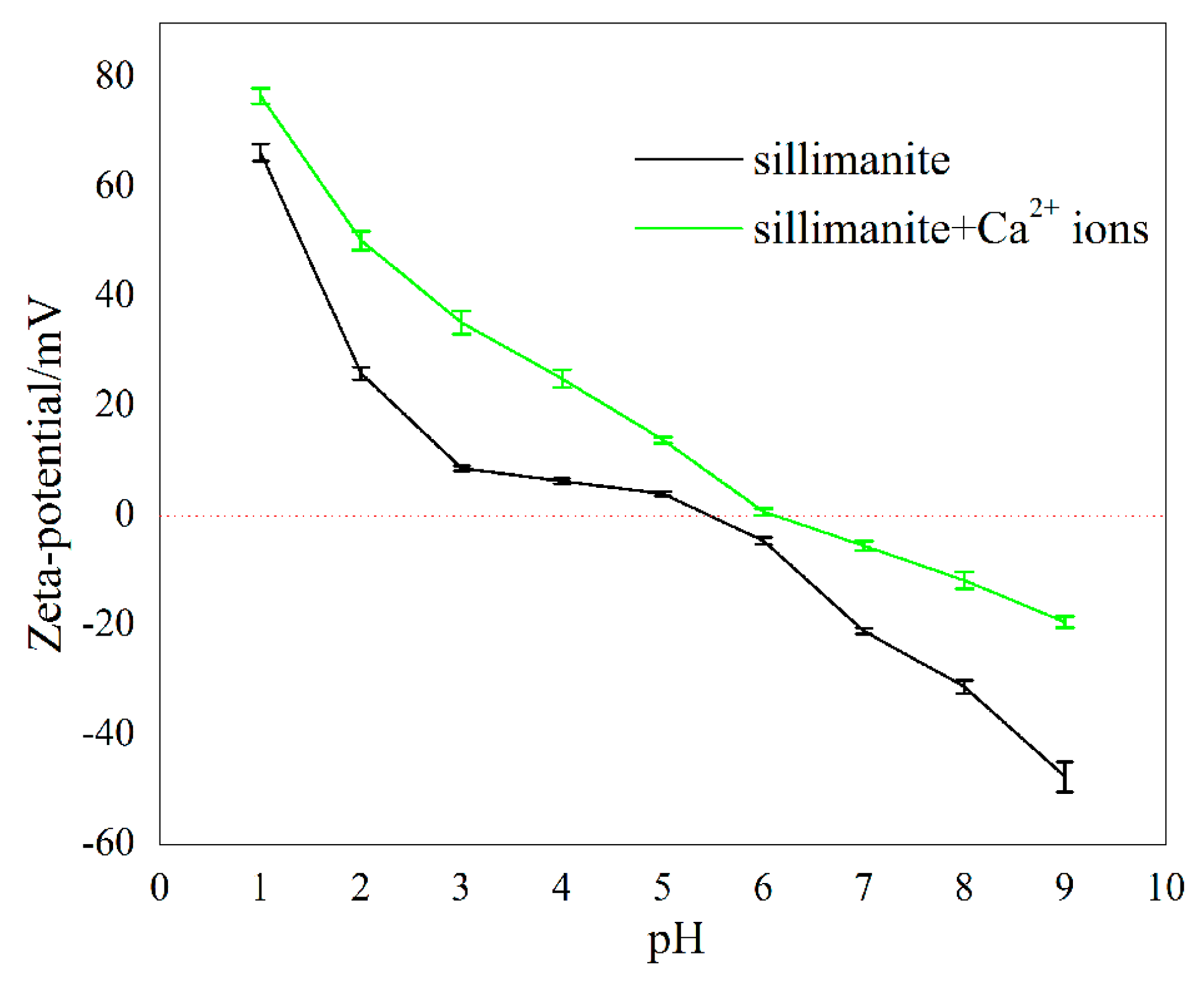
3.2. Zeta Potential Analyses
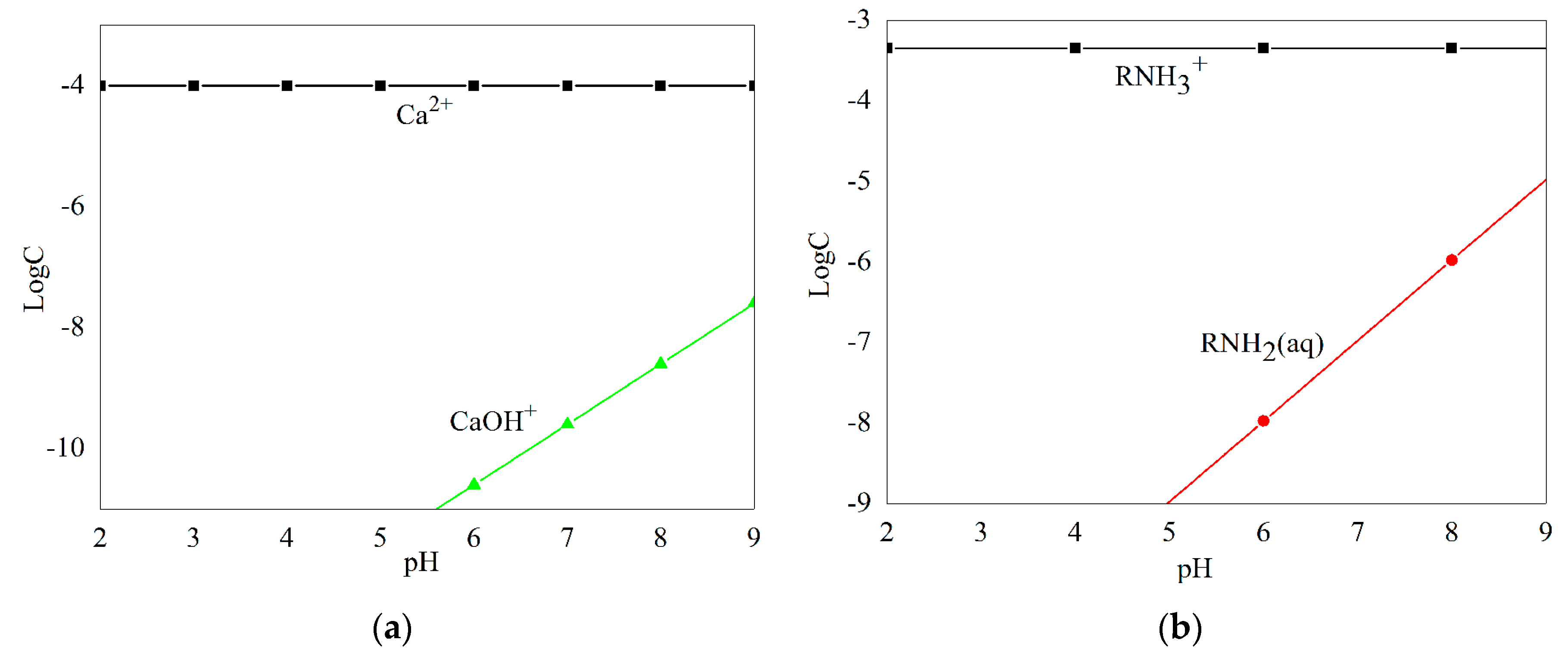
3.3. Solution Chemistry Analyses
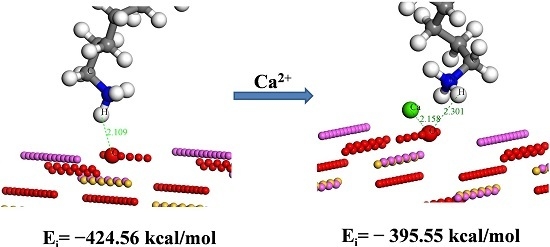
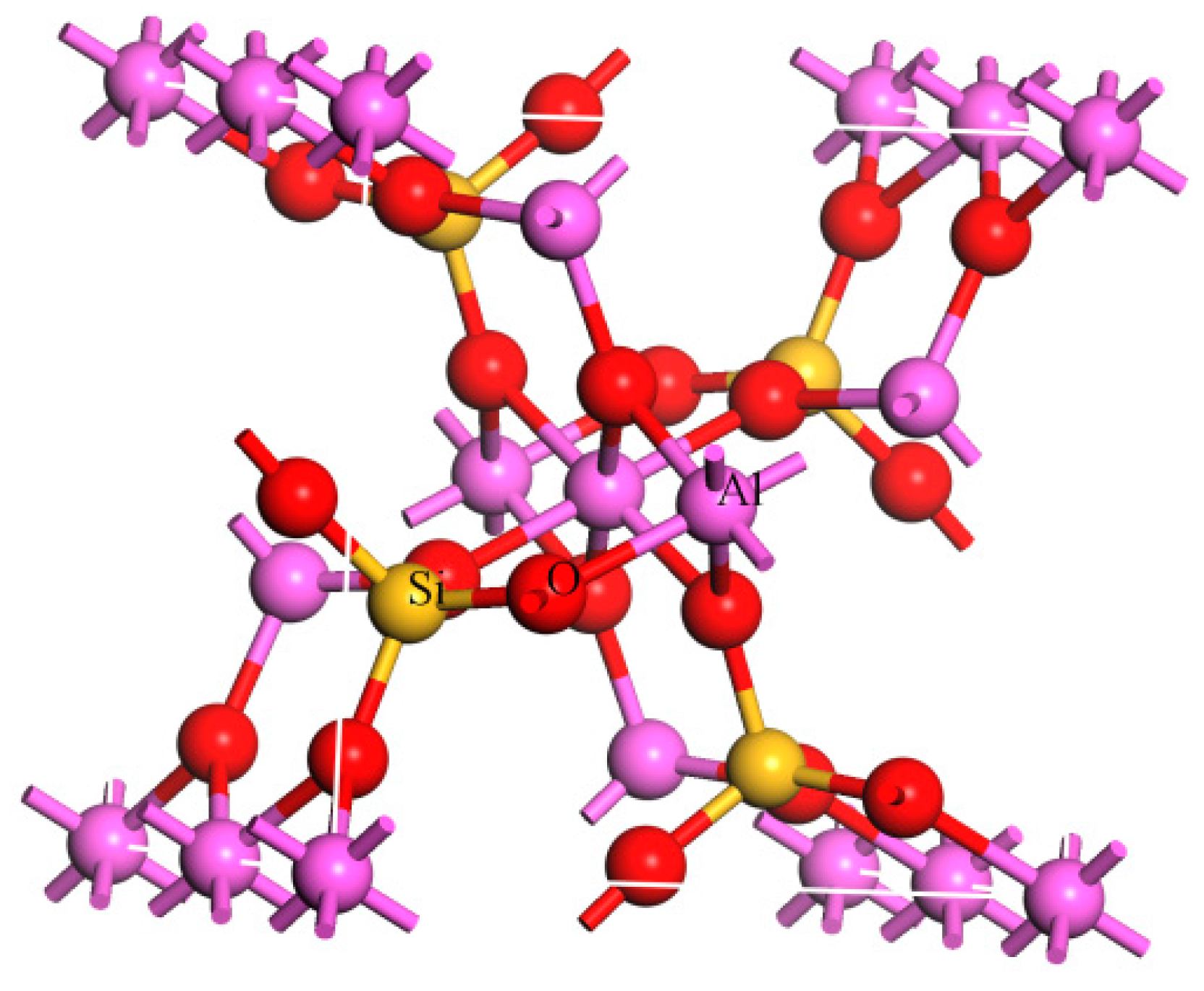
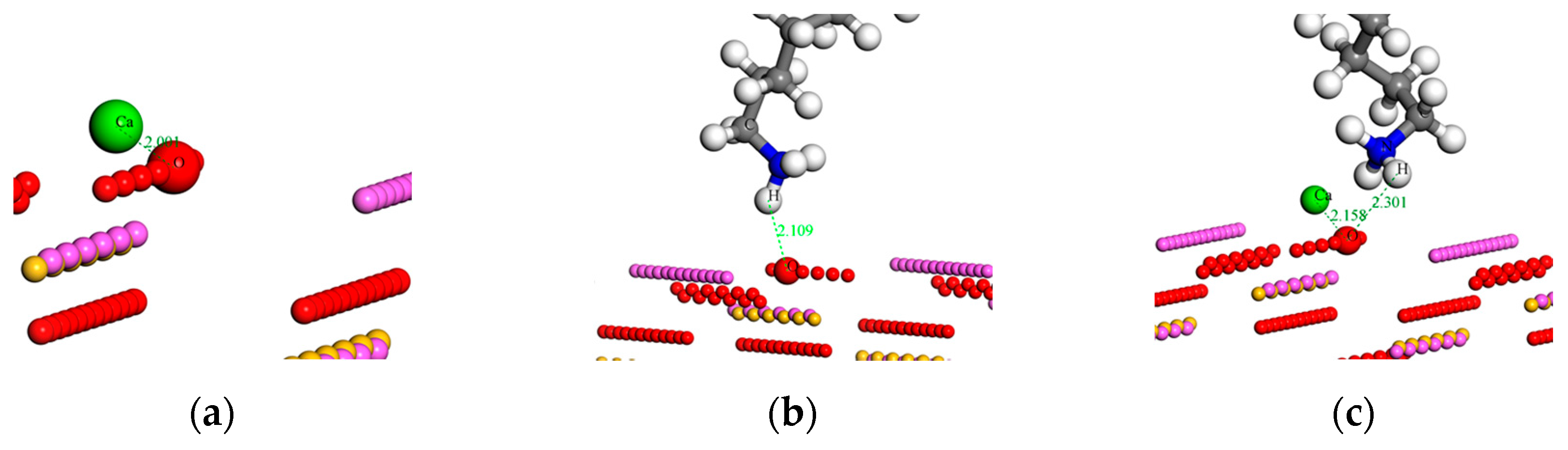
3.4. Molecular Dynamics Calculation Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filippov, L.O.; Duverger, A.; Filippova, I.V.; Kasaini, H.; Thiry, J. Selective flotation of silicates and Ca-bearing minerals: The role of non-ionic reagent on cationic flotation. Min. Eng. 2012, 36, 314–323. [Google Scholar] [CrossRef]
- Murty, C.; Murthy, Ch.V.R.; JaiSankar, N.; Manjeera, P.B.; Kumar, P. Operation and optimization of sillimanite beneficiation from beach sands. Int. J. Intell. Inf. Technol. 2013, 1, 347–355. [Google Scholar]
- Murthy, V.A.N. Beneficiation of sillimanite by flotation. Int. J. Intell. Inf. Technol. 2013, 1, 24–28. [Google Scholar]
- Prabhakar, S.; Bhaskar Raju, G.; Subba Rao, S. Beneficiation of sillimanite by column flotation—A pilot scale study. Int. J. Min. Process. 2006, 81, 159–165. [Google Scholar] [CrossRef]
- Laxmia, T.; Raob, R.B. Upgradation of sillimanite by using advanced air cyclone classifier. Turk. J. Eng. 2013, 3, 46–52. [Google Scholar]
- Jin, J.; Gao, H.; Chen, X.; Peng, Y.; Min, F. The flotation of aluminosilicate polymorphic minerals with anionic and cationic collectors. Min. Eng. 2016, 99, 123–132. [Google Scholar] [CrossRef]
- Prabhakar, S.; Hanumantha Rao, K.; Forsling, W. Dissolution of wollastonite and its flotation and surface interactions with tallow-1,3-diaminopropane (duomeen T). Min. Eng. 2005, 18, 691–700. [Google Scholar] [CrossRef]
- You, C.; Zeng, X. The research of sillimanite flotation collection selection. J. Wuhan Univ. Technol. 1990, 3, 52–58. [Google Scholar]
- Demir, C.; Bentli, I.; Gülgönül, I.; Çelik, M.S. Effects of bivalent salts on the flotation separation of Na-feldspar from K-feldspar. Miner. Eng. 2003, 16, 551–554. [Google Scholar] [CrossRef]
- Fuerstenaua, D.W. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Wang, J.; Xie, Z. Investigation on different behavior and mechanism of Ca (II) and Fe (III) adsorption on spodumene surface. Phys. Probl. Min. Process. 2014, 50, 535–550. [Google Scholar]
- Yu, F.; Wang, Y.; Wang, J.; Xie, Z.; Zhang, L. First-principle investigation on mechanism of Ca ion activating flotation of spodumene. Rare Met. 2014, 33, 358–362. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The effects of Ca (II) and Mg (II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Li, Y.; Lei, D.; Xu, S. Effect of metal ions in pulp on flotation separation of sillimanite from quartz. J. Chin. Ceram. Soc. 2002, 30, 362–364. [Google Scholar]
- Lu, J.; Gao, H.; Jin, J.; Cen, D.; Ren, Z. Effect and mechanism of calcium ion on flotation of andalusite. Chin. J. Nonferr. Met. 2016, 26, 1311–1315. [Google Scholar]
- El-Salmawy, M.S.; Nakahiro, Y.; Wakamatsu, T. The role of alkaline earth cations in flotation separation of quartz from feldspar. Miner. Eng. 1993, 6, 1231–1243. [Google Scholar] [CrossRef]
- Burdukova, E.; Van Leerdam, G.C.; Prins, F.E.; Smeink, R.G.; Bradshaw, D.J.; Laskowski, J.S. Effect of calcium ions on the adsorption of CMC onto the basal planes of New York talc—A ToF-SIMS study. Miner. Eng. 2008, 21, 1020–1025. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Chen, J. DFT simulation of the adsorption of sodium silicate species on kaolinite surfaces. Appl. Surf. Sci. 2016, 370, 403–409. [Google Scholar] [CrossRef]
- Demichelis, R.; Civalleri, B.; D’Arco, P.; Dovesi, R. Performance of 12 DFT functionals in the study of crystal systems: Al2SiO5 orthosilicates and Al hydroxides as a case study. Int. J. Quantum Chem. 2010, 110, 2260–2273. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X. The theoretical study on the efficiencies of 2-[tris(hyroxycarbamoyl) methyl] carboxylic acids as chelating agents in flotation separation of diaspore and aluminosilicates. Sep. Purif. Technol. 2013, 104, 114–120. [Google Scholar] [CrossRef]
- Gocmez, H. The interaction of organic dispersant with alumina: A molecular modelling approach. Ceram. Int. 2006, 32, 521–525. [Google Scholar] [CrossRef]
- Qiang, L.; Li, Z.; Zhao, T.; Zhong, S.; Wang, H.; Cui, X. Atomic-scale interactions of the interface between chitosan and Fe3O4. Colloids Surf. A 2013, 419, 125–132. [Google Scholar] [CrossRef]
- Guide, C.U. Force Field-Based Simulations; Molecular Simulations Inc.: San Diego, CA, USA, 1997. [Google Scholar]
- Ji, Y.; Wang, F.; Duan, L.; Zhang, F.; Gong, X. Effect of temperature on the adsorption of sulfanilamide onto aluminum oxide and its molecular dynamics simulations. Appl. Surf. Sci. 2013, 285, 403–408. [Google Scholar] [CrossRef]
- Bu, H.; Cao, M.; Gao, Y.; Huang, H. Synergic effect of sodium oleate (NaOL) and benzohydroxamic acid (BHA) on flotation of wolframite. Phys. Probl. Min. Process. 2017, 53, 82–93. [Google Scholar]
- Rai, B.; Sathish, P.; Tanwar, J.; Moon, K.S.; Fuerstenau, D.W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Prathab, B.; Subramanian, V.; Aminabhavi, T.M. Molecular dynamics simulations to investigate polymer-polymer and polymer-metal oxide interactions. Polymer 2007, 48, 409–416. [Google Scholar] [CrossRef]
- Mi, H.; Xiao, G.; Chen, X. Theoretical evaluation of corrosion inhibition performance of three antipyrine compounds. Comput. Theor. Chem. 2015, 1072, 7–14. [Google Scholar] [CrossRef]
- Pradip; Rai, B. Molecular modeling and rational design of flotation reagents. Int. J. Miner. Process. 2003, 72, 95–110. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.; Sun, W.; Liu, X. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A 2012, 415, 439–448. [Google Scholar] [CrossRef]
- Pradip; Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of diphosphonic acid based surfactants with calcium minerals. Langmuir 2002, 18, 932–940. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Liu, G. Molecular dynamics simulation of primary ammonium ions with different alkyl chains on the muscovite (001) surface. Int. J. Miner. Process. 2015, 145, 48–56. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, X.; Leng, Y.; Watari, F.; Weng, J.; Feng, B.; Qu, S. Effects of aqueous environment and surface defects on Arg-Gly-Asp peptide adsorption on titanium oxide surfaces investigated by molecular dynamics simulation. J. Biomed. Mater. Res. 2011, 96, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Jin, J.; Gao, H.; Ren, Z.; Chen, Z. The flotation of kyanite and sillimanite with sodium oleate as the collector. Minerals 2016, 6, 90. [Google Scholar] [CrossRef]
- Sun, C.; Yin, W. Flotation Principles of Silicate Minerals; Science Press: Beijing, China, 2001; pp. 216–223. [Google Scholar]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation—A critical review. Miner. Eng. 2014, 66, 13–24. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, B.; Sun, C.; Liu, J.; Sun, H.; Li, Y.; Han, Y. Density functional theory study of α-Bromolauric acid adsorption on the α-quartz (101) surface. Miner. Eng. 2016, 92, 72–77. [Google Scholar] [CrossRef]

| Composition | SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | K2O | ZrO2 | Cr2O3 | V2O5 | MoO3 | Ignition Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 38.09 | 59.85 | 0.08 | 0.27 | 0.13 | 0.02 | 0.59 | 0.07 | 0.14 | 0.08 | 0.68 |
| pH | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Ci (M) | 1.82 × 10−7 | 1.81 × 10−6 | 1.80 × 10−5 | 1.79 × 10−4 | 1.79 × 10−3 | 1.78 × 10−2 | 1.77 × 10−1 | 1.76 |
| Ci/Ci0 | 4.04 × 10−4 | 4.02 × 10−3 | 4.01 × 10−2 | 3.99 × 10−1 | 3.97 | 39.52 | 393.41 | 3916.12 |
| Ci’ (M) | 3.65 × 10−8 | 3.63 × 10−7 | 3.62 × 10−6 | 3.60 × 10−5 | 3.58 × 10−4 | 3.57 × 10−3 | 3.55 × 10−2 | 3.54 × 10−1 |
| Ci’/Ci0 | 8.11 × 10−5 | 8.07 × 10−4 | 8.04 × 10−3 | 8.00 × 10−2 | 7.96 × 10−1 | 7.93 | 78.92 | 785.61 |
| Adsorbate | H2O | Ca2+ | RNH3+ (N) | Ca2+ + RNH3+ (CN) |
|---|---|---|---|---|
| Ei (kcal/mol) | −189.36 | −489.68 | −424.56 | −395.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Ren, Z.; Gao, H.; Lu, J.; Jin, J.; Min, F. The Effects of Calcium Ions on the Flotation of Sillimanite Using Dodecylammonium Chloride. Minerals 2017, 7, 28. https://doi.org/10.3390/min7020028
Chen Z, Ren Z, Gao H, Lu J, Jin J, Min F. The Effects of Calcium Ions on the Flotation of Sillimanite Using Dodecylammonium Chloride. Minerals. 2017; 7(2):28. https://doi.org/10.3390/min7020028
Chicago/Turabian StyleChen, Zhijie, Zijie Ren, Huimin Gao, Jia Lu, Junxun Jin, and Fanfei Min. 2017. "The Effects of Calcium Ions on the Flotation of Sillimanite Using Dodecylammonium Chloride" Minerals 7, no. 2: 28. https://doi.org/10.3390/min7020028