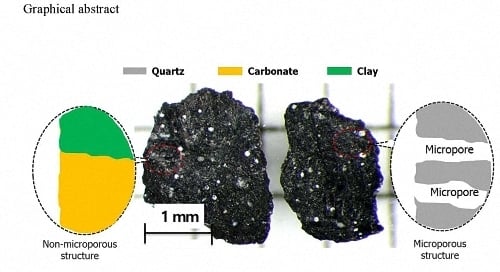
Pore Structure Characterization of Shale Using Gas Physisorption: Effect of Chemical Compositions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shale Sample and Preparation
2.2. X-ray Diffraction (XRD) Analysis
2.3. Low-Pressure N2 and CO2 Isotherm Analysis
3. Results and Discussion
3.1. Mineralogy and Shale Lithofacies
3.2. Pore Characteristics of the Shale with Chemical Composition
3.3. Correlation of the Pore Structure of the Shale with Chemical Composition
4. Conclusions
- The N2 and CO2 gas physisorption technique was found to be suitable for analyzing the pore-related properties of shales (e.g., BET surface area, pore volume, and pore size).
- Chemical composition did not play a critical role in the BJH pore size. However, the chemical composition was critical for the specific/micropore surface area and the total/micropore volume. The silica-rich carbonate shale showed the greatest specific surface area and total pore volume, while the micropore volume and micropore surface area was found to be the greatest for the clay-rich siliceous shale samples.
- Both the micropore surface area and micropore volume were found to be a strong function of the quartz content as compared to the carbonate and clay contents.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuuskraa, V. United States energy information administration. In World Shale Gas Resources: An Initial Assessment of 14 Regions Outside the United States; US Department of Energy: Washington, DC, USA, 2011. [Google Scholar]
- Bustin, A.M.; Bustin, R.M. Importance of rock properties on the producibility of gas shales. Int. Coal Geol. 2012, 103, 132–147. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Wang, H.; Liu, H.; Wei, W.; Fang, J. Shale gas reservoir characterisation: A typical case in the southern sichuan basin of China. Energy 2011, 36, 6609–6616. [Google Scholar] [CrossRef]
- Hu, Q.-H.; Liu, X.-G.; Gao, Z.-Y.; Liu, S.-G.; Zhou, W.; Hu, W.-X. Pore structure and tracer migration behavior of typical American and Chinese shales. Pet. Sci. 2015, 12, 651–663. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, L.; Meegoda, J.N. Pore-scale simulation and sensitivity analysis of apparent gas permeability in shale matrix. Materials 2017, 10, 104. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Solano, N.; Bustin, R.; Bustin, A.; Chalmers, G.; He, L.; Melnichenko, Y.B.; Radliński, A.; Blach, T.P. Pore structure characterization of north american shale gas reservoirs using usans/sans, gas adsorption, and mercury intrusion. Fuel 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the barnett, woodford, haynesville, marcellus, and doig units. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the mississippian barnett shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the sichuan basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Lee, W.; Lee, Y.; Kim, H. Impact of total organic carbon and specific surface area on the adsorption capacity in Horn River shale. J. Pet. Sci. Eng. 2017, 149, 331–339. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-pressure methane sorption isotherms of black shales from the Netherlands. Energy Fuels 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, Z.; Li, X.; Yang, Y.; Zhao, C.; Connell, L.D.; Li, S.; He, J. Experimental study and modelling of methane adsorption and diffusion in shale. Fuel 2014, 117, 509–519. [Google Scholar] [CrossRef]
- Kam, P.; Nadeem, M.; Novlesky, A.; Kumar, A.; Omatsone, E.N. Reservoir characterization and history matching of the Horn River shale: An integrated geoscience and reservoir-simulation approach. J. Can. Pet. Technol. 2015. [Google Scholar] [CrossRef]
- Rietveld, H. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Han, Y.; Kim, H.; Park, J.; Lee, S.-H.; Kim, J.-Y. Influence of Ti doping level on hydrogen adsorption of mesoporous Ti-SBA-15 materials prepared by direct synthesis. Int. J. Hydrogen Energy 2012, 37, 14240–14247. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Han, Y.; Choi, J.; Tong, M.; Kim, H. Synthesis and characterization of high-surface-area millimeter-sized silica beads with hierarchical multi-modal pore structure by the addition of agar. Mater. Charact. 2014, 90, 31–39. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: Berlin, Germany, 2012; Volume 16. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.; Everett, D.; Haynes, J.; Pernicone, N.; Ramsay, J.; Sing, K.; Unger, K. Recommendations for the characterization of porous solids (technical report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Dubinin, M.; Stoeckli, H. Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Int. Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef]
- Wang, G.; Carr, T.R. Marcellus shale lithofacies prediction by multiclass neural network classification in the appalachian basin. Math. Geosci. 2012, 44, 975–1004. [Google Scholar] [CrossRef]
- Ferri, F.; Hickin, A.S.; Reyes, J. Horn river basin–equivalent strata in besa river formation shale, northeastern british columbia (NTS 094K/15). Geosci. Rep. 2012, 1–15. [Google Scholar]
- Abbaszadeh, M.; Nasiri, M.; Riazi, M. Experimental investigation of the impact of rock dissolution on carbonate rock properties in the presence of carbonated water. Environ. Earth Sci. 2016, 75, 1–6. [Google Scholar] [CrossRef]
- Chiou, W.; Faas, R.; Kasprowicz, J.; Li, H.; Lomenick, T.; OBrien, N.; Pamukcu, S.; Smart, P.; Weaver, C.; Yamamoto, T. Microstructure of Fine-Grained Sediments: From Mud to Shale; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Labani, M.M.; Rezaee, R.; Saeedi, A.; Al Hinai, A. Evaluation of pore size spectrum of gas shale reservoirs using low pressure nitrogen adsorption, gas expansion and mercury porosimetry: A case study from the perth and canning basins, western australia. J. Pet. Sci. Eng. 2013, 112, 7–16. [Google Scholar] [CrossRef]






| Sampling Depth (m) | Depth Index (m) | Quartz | Feldspar/Calcite | Dolomite/Plagioclase | Pyrite/Barite | Apatite/Illite | Layer | TOC |
|---|---|---|---|---|---|---|---|---|
| 2325–2330 | 2325 | 33 | 7/6 | 33/0 | 3/0 | 0/18 | Muskwa | 1.4 |
| 2335–2340 | 2335 | 40 | 6/2 | 4/6 | 4/0 | 0/38 | Muskwa | 0.8 |
| 2325–2360 | 2355 | 20 | 11/18 | 14/0 | 2/0 | 0/23 | Upper Otterpark | 1.2 |
| 2375–2380 | 2375 | 18 | 0/54 | 6/6 | 1/0 | 0/10 | Middle Otterpark | 0.5 |
| 2405–2410 | 2405 | 26 | 4/5 | 17/2 | 1/1 | 1/31 | Middle Otterpark | 1.6 |
| 2435–2440 | 2435 | 35 | 6/3 | 22/3 | 10/0 | 0/19 | Lower Otterpark | 1.3 |
| 2445–2450 | 2445 | 58 | 4/2 | 2/4 | 4/1 | 0/28 | Evie | 0.3 |
| 2465–2470 | 2465 | 37 | 2/50 | 2/0 | 1/0 | 0/8 | Evie | 1.7 |
| Depth Index (m) | Shale Lithofacies/Sample Name | Surface Area (m2/g) a | Total Pore Volume (cm3/g) b | BJH Pore Size (nm) c | Micropore Volume (mm3/g) d | Micropore Surface Area (m2/g) d |
|---|---|---|---|---|---|---|
| Shallow Region | ||||||
| 2375 | Silica-rich carbonate shale/sample-1 | 1.61 ± 0.12 | 0.0123 ± 0.0012 | 20.3 ± 1.1 | 0.23 ± 0.08 | 1.51 ± 0.21 |
| 2325 | Carbonate-siliceous shale/sample-3 | 0.82 ± 0.09 | 0.0045 ± 0.0008 | 24.5 ± 1.2 | 0.22 ± 0.04 | 1.98 ± 0.35 |
| 2335 | Clay-rich siliceous shale/sample-5 | 1.00 ± 0.20 | 0.0084 ± 0.0010 | 21.8 ± 0.9 | 0.30 ± 0.09 | 2.17 ± 0.32 |
| 2355 | Mixed shale/sample-7 | 1.25 ± 0.17 | 0.0074 ± 0.0009 | 18.3 ± 2.1 | 0.28 ± 0.11 | 1.79 ± 0.32 |
| Deep Region | ||||||
| 2465 | Silica-rich carbonate shale/sample-2 | 1.79 ± 0.13 | 0.0132 ± 0.0009 | 20.9 ± 1.7 | 0.41 ± 0.02 | 2.57 ± 0.39 |
| 2435 | Carbonate-siliceous shale/sample-4 | 1.28 ± 0.11 | 0.0094 ± 0.0006 | 19.8 ± 2.2 | 0.37 ± 0.05 | 2.56 ± 0.53 |
| 2445 | Clay-rich siliceous shale/sample-6 | 1.13 ± 0.22 | 0.0091 ± 0.0011 | 20.7 ± 1.5 | 0.66 ± 0.11 | 4.38 ± 0.28 |
| 2405 | Mixed shale/sample-8 | 1.65 ± 0.09 | 0.0101 ± 0.0005 | 18.6 ± 0.9 | 0.83 ± 0.09 | 3.88 ± 0.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Kwak, D.; Choi, S.Q.; Shin, C.; Lee, Y.; Kim, H. Pore Structure Characterization of Shale Using Gas Physisorption: Effect of Chemical Compositions. Minerals 2017, 7, 66. https://doi.org/10.3390/min7050066
Han Y, Kwak D, Choi SQ, Shin C, Lee Y, Kim H. Pore Structure Characterization of Shale Using Gas Physisorption: Effect of Chemical Compositions. Minerals. 2017; 7(5):66. https://doi.org/10.3390/min7050066
Chicago/Turabian StyleHan, Yosep, Daewoong Kwak, Siyoung Q. Choi, Changhoon Shin, Youngsoo Lee, and Hyunjung Kim. 2017. "Pore Structure Characterization of Shale Using Gas Physisorption: Effect of Chemical Compositions" Minerals 7, no. 5: 66. https://doi.org/10.3390/min7050066