1. Introduction
The annual sedimentation rate of the world’s reservoirs averages about 1%, but it is much higher in Asia [
1,
2]. Due to climatic, geographic and geologic situations, the reservoir sedimentation problem is very serious in southern Taiwan, R.O.C. All the streams have steep slopes in their catchment areas. The rainfall intensity and runoff flow are strong during the summer typhoon season. Even worse, major irrigation and city water supply reservoirs are located in Pliocene and Pleistocene shale and mudstone areas. The accumulated sediment volume in these reservoirs accounts for about one third or more of the reservoir designed capacity [
3]. To extend the operation life of these reservoirs, desiltation is essential. Reservoir desiltation involves three major processes, namely the removal, deposition and reuse of the silt. The reuse of the silt is the most important one because improper handling of dredged silts will interrupt the removal operation.
The major mineral components of reservoir sediments in southern Taiwan are sand (mostly quartz) and clay (illite and chlorite) [
4]. Since the hardness of quartz is greater than that of the clay minerals, these two types of minerals are concentrated in different size ranges, i.e. sand in the coarse-grained range and clay minerals in the fine-grained range. Using sedimentation technique in mineral processing, it is easy to separate out clay minerals. From the point of view of chemical composition, after the removal of quartz, reservoir silts can be regarded as a natural aluminosilicate mineral source.
Ultramarine with its deep vivid blue is a valuable inorganic pigment. Natural ultramarine is derived from grinding lapis lazuli, which is a rock composed of lazurite with small amounts of calcite, pyroxene, etc. Most of today’s ultramarine is synthetically produced based upon processes invented by Guimet in 1826 and Gmelin in 1828 [
5].
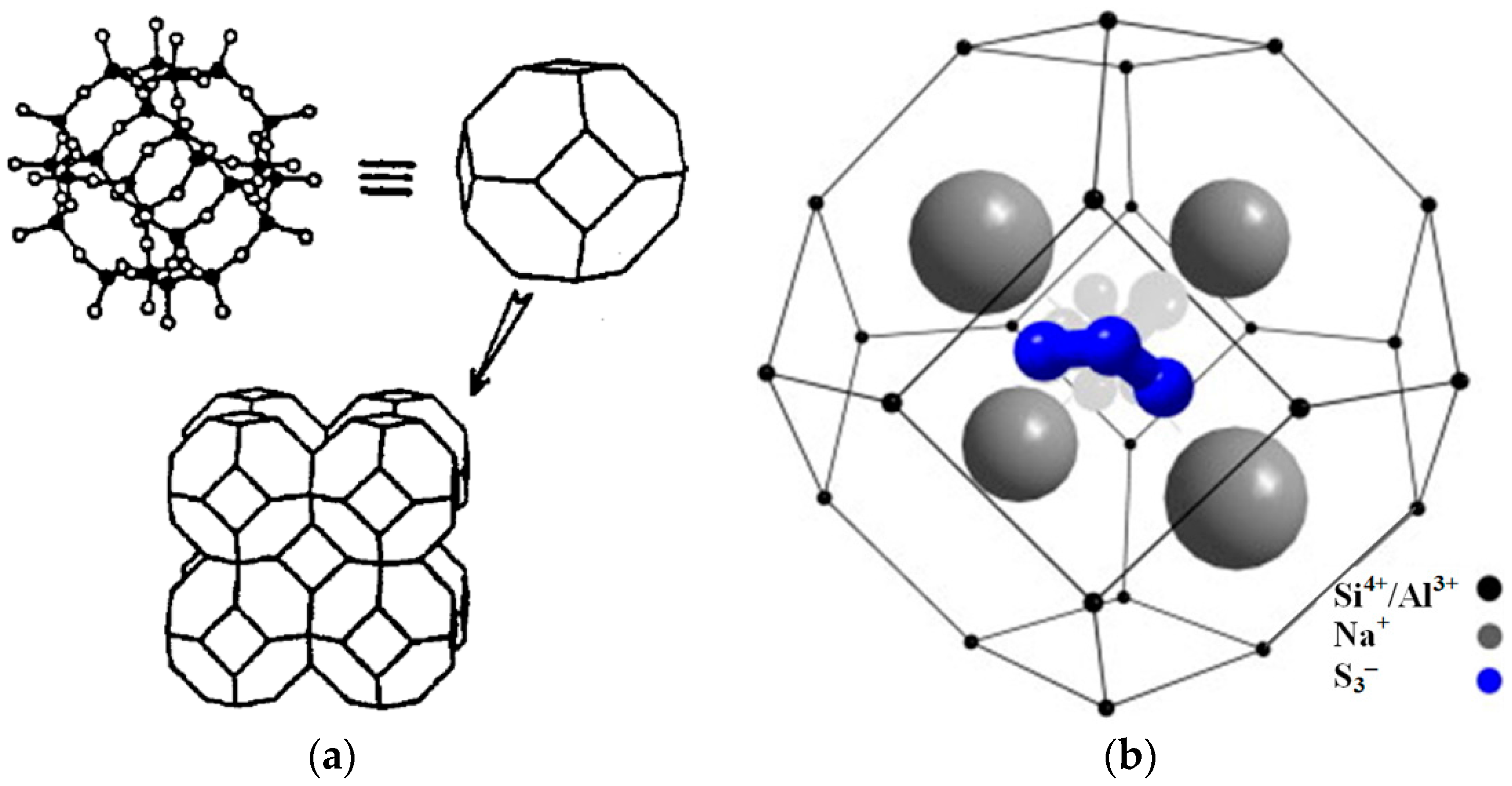
Ultramarine or lazurite has the same crystal structure of sodalite,Na
8[(Al,Si)
6O
24]Cl
2, which is a feldspathoid group mineral with a zeolite structure. In ultramarine, the chloride ions are replaced by the sulfur radical anions S
3− and S
2−. The chemical formula of ultramarine thus is M
8[(Al,Si)
6O
24]X
n, where M is an extra-framework cation, mainly Na
+; X is an extra-framework anion, such as S
2−, S
3−, etc., and n may vary from 2 to 4 [
6,
7,
8,
9]. The anions and cations balance the charge on the ordered aluminosilicate framework. The close-packed cuboctahedra (Al
3Si
3O
12)
3− called β cages organize the sodalite (SOD) structure (
Figure 1a) [
10].The chromophores of ultramarine are polysulfide radical anions encapsulated inside β cages (
Figure 1b) [
7]. Theoretical maximum insertion of chromophores is one S
x− per β cage (x = 2–4). The polysulfide radicals S
2−, S
3− and S
4/S
4− are responsible for the yellow, blue and red colors, respectively [
7,
10,
11,
12,
13,
14,
15]. The cause of color is due to the absorption band at about 400 nm (S
2−), 600 nm (S
3−) and 520 nm (S
4/S
4−), resulted from the transition of electrons among the molecular orbitals of these polysulfide radical anions from ground state to low-lying excited electronic states [
13,
14,
15,
16,
17,
18].
The ratio of Si to Al determines the amount of chromophores encapsulated inside β cages. When fewer Al ions are present in the framework, the required number of sodium ions is reduced for lattice stabilization, and more sodium ions are free to act as counter-ions for the polysulfide. The polysulfide content can be increased and thereby improve color quality [
19,
20]. Within limits set by stability considerations, the proportions of the chemical constituents can vary. Some empirical formulas, likeNa
6.9Al
5.6Si
6.4O
24S
4.2 [
19], are reported.
Traditionally, the raw material used in the synthesis of ultramarine is kaolin clay. The industrial process of ultramarine synthesis involves the dehydration of kaolin at about 550 °C. The dehydrated clay is mixed with sulfur, sodium carbonate and a reducing agent, e.g., coke or active carbon. Heating of the mixture produces a synthesis reaction [
21]. In the reaction, the formation of ultramarine blue proceeds in two stages: first a reduction stage followed by an oxidation stage. In the reduction stage, the mixture is heated to about 750 °C, and held at that temperature for a given period of time. The container for the mixture is covered so that a reducing atmosphere can be maintained within it. When the holding time is reached, the temperature starts to descend, and the reaction enters the oxidation stage. The oxidation stage is achieved by either keeping the mixture at about 450 °C for a fixed time or simply slowing the cooling rate. During this stage, S
2− ions are oxidized to S
3− ions to produce a vivid blue hue [
20,
21].
Besides kaolin, other aluminosilicate sources, including pure chemicals, natural or synthetic minerals and wastes have been used. Chemicals, such as sodium silicate and tetramethylammonium hydroxide [
22]; silicon tetrachloride and aluminum chloride [
8]; silica, diatomaceous earth and sodium aluminate [
23]; natural or synthetic minerals, like zeolite A, X, Y [
24,
25,
26], cancrinite [
27], hydroxycancrinite [
28]; wastes, such as fly ash [
20] and kaolin mine tailings [
29], were reported to be technologically feasible.
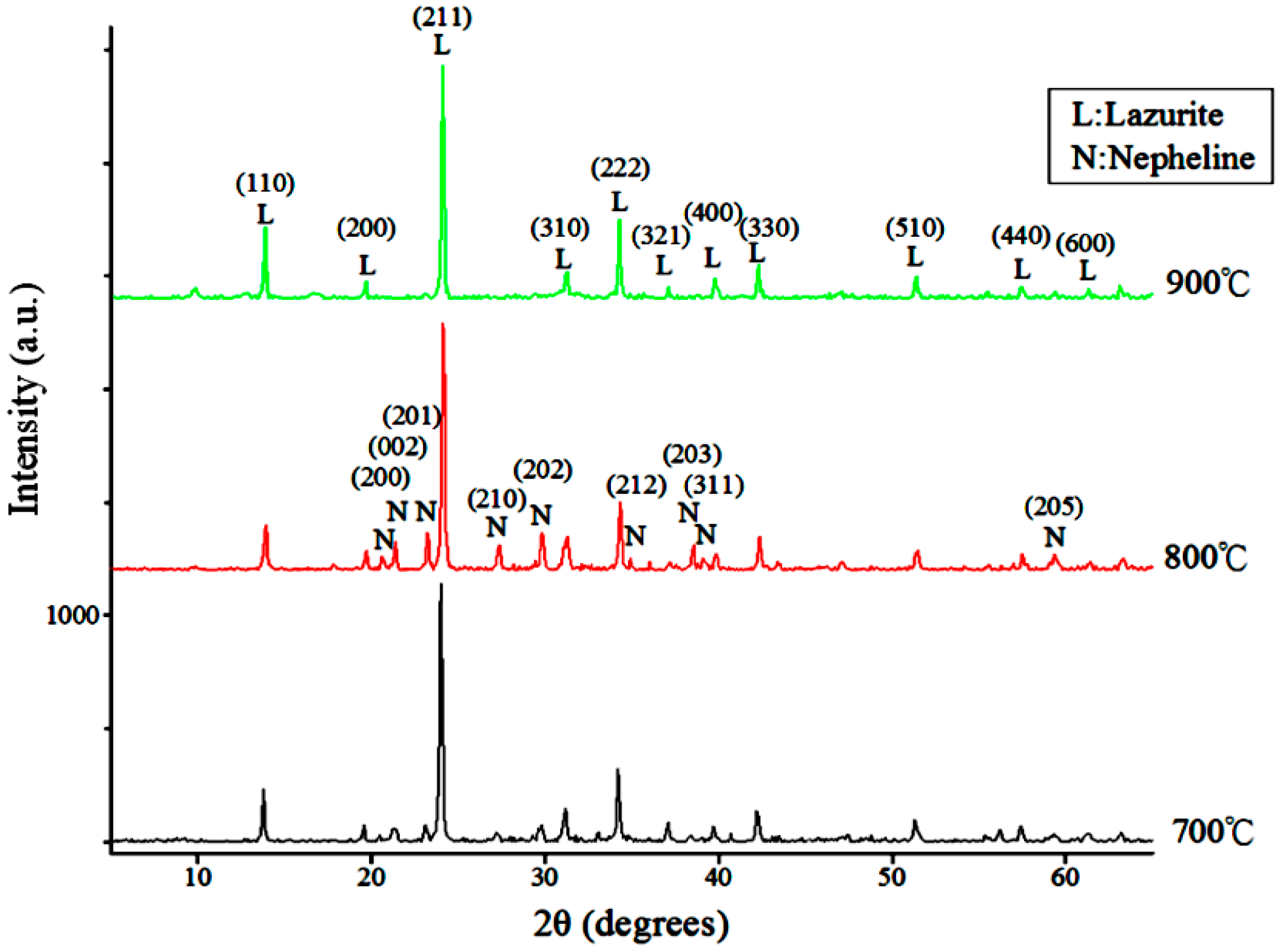
In a previous study, the reservoir silts were first treated hydrothermally to transform them to hydroxycancrinite [
28]; then the hydroxycancrinite was transformed to ultramarine blue via a solid-state reaction process. In this study, reservoir silts were used directly to synthesize ultramarine. The reaction conditions, such as raw materials ratio, calcination temperature and mineral composition of silts on the product phase and color properties, were investigated.
2. Experimental
2.1. Materials
The reservoir silt samples were taken from the city water supply of Nan-Hua Reservoir that is located in Tainan County. The silts were screened through a 200 mesh sieve (75 μm), to remove the non-mineral constituents, such as humus, etc., and then the material was designated as the “raw” silt. The raw silts were further classified via hydraulic sedimentation to separate out the <10 μm, <5 μm, and <2 μm parts.
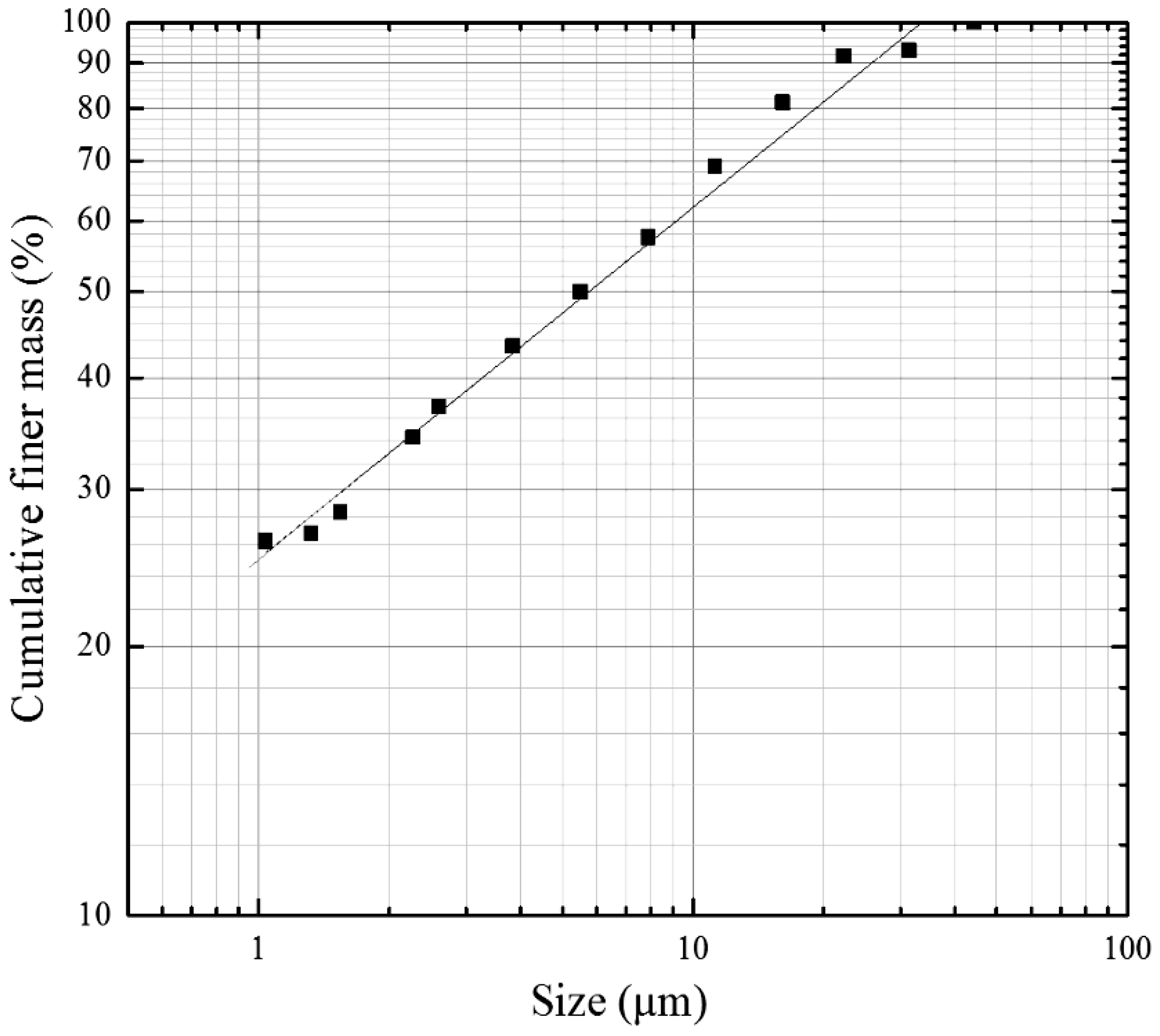
The size distribution of the raw silts is shown in
Figure 2. The distribution fits quite well that of the Gates-Gaudin-Schuhmann distribution function with the distribution modulus of 0.77 and d
50 of 5.7 μm. From the distribution curve, it can be determined that the <10 μm, <5 μm and <2 μm parts account for 62, 47, and 33 wt % of the raw silts, respectively.
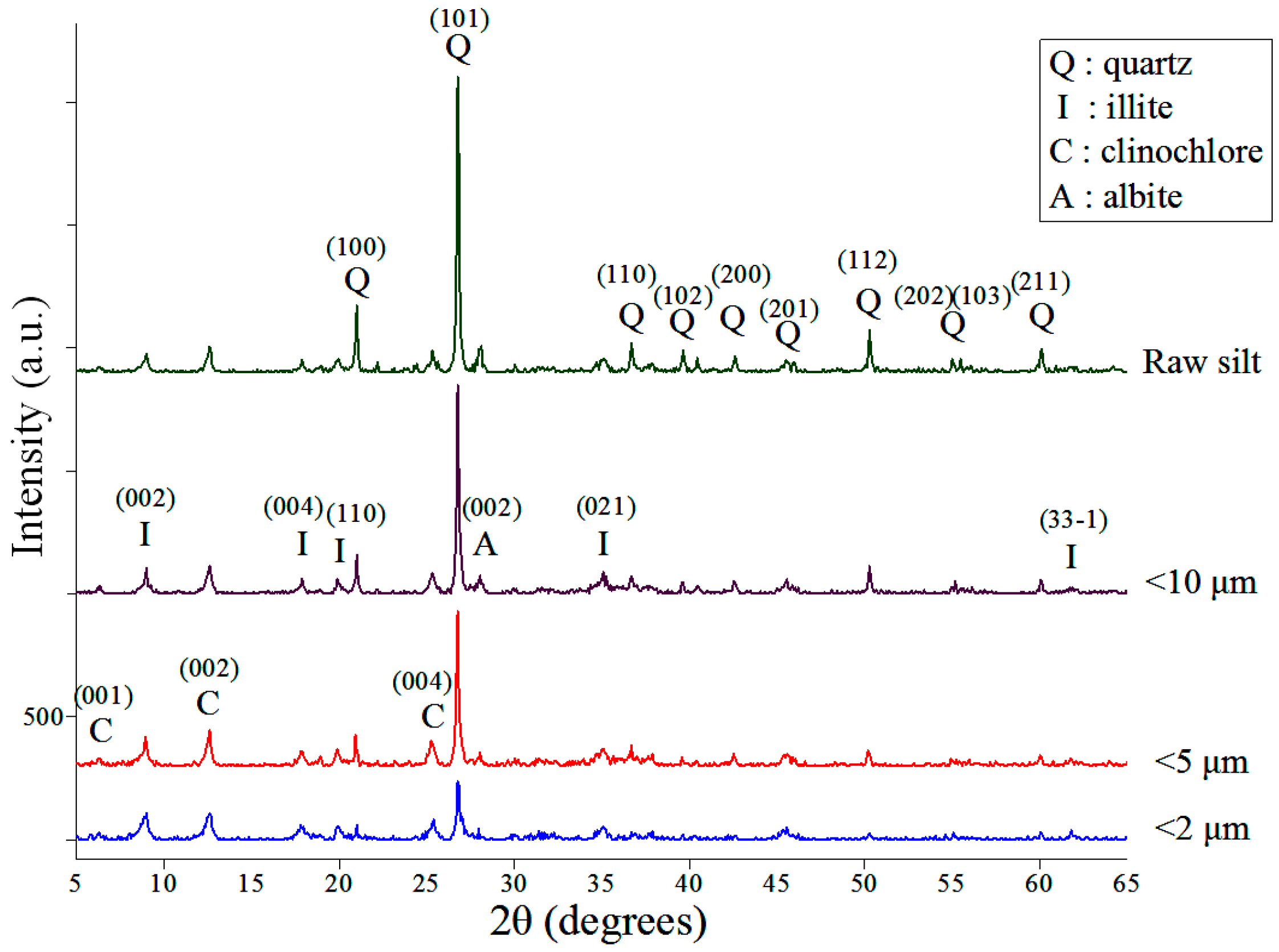
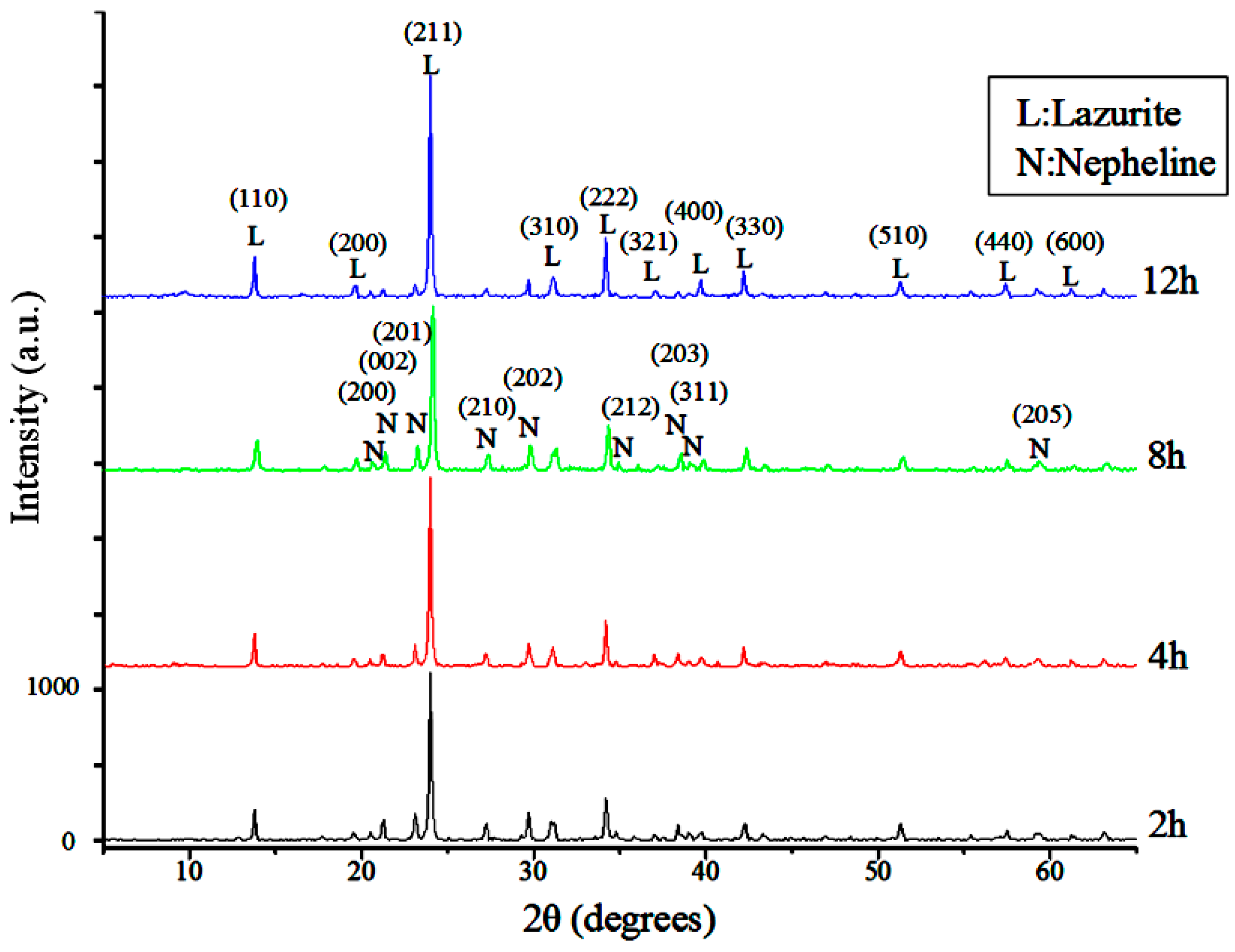
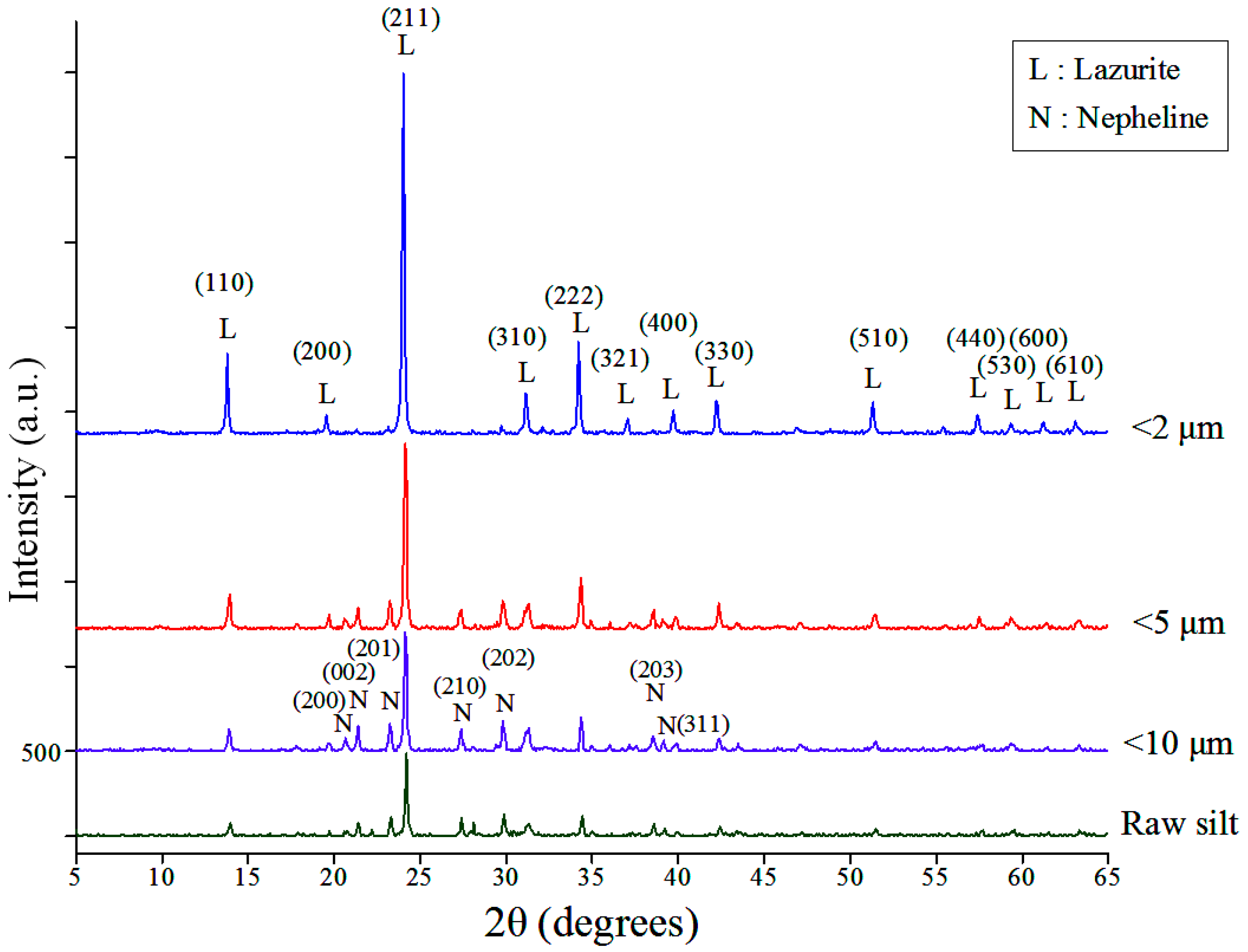
The XRD patterns of the raw and classified silt samples are shown in
Figure 3. The diffraction peaks show that the minerals comprising the silt are quartz, illite, clinochlore and albite. As the size of the silts is decreased, the peaks of quartz and albite decrease rapidly, whereas those of illite and clinochlore are more prominent. The change in mineral composition shows that size classification can separate quartz and albite, which are unusable in the synthesis reaction, from clay minerals effectively.
The chemical compositions of all silt samples determined by XRF are listed in
Table 1. With the removal of quartz from classified silts, the molar ratio of Si/Al decreases from 2.77 to 1.42.
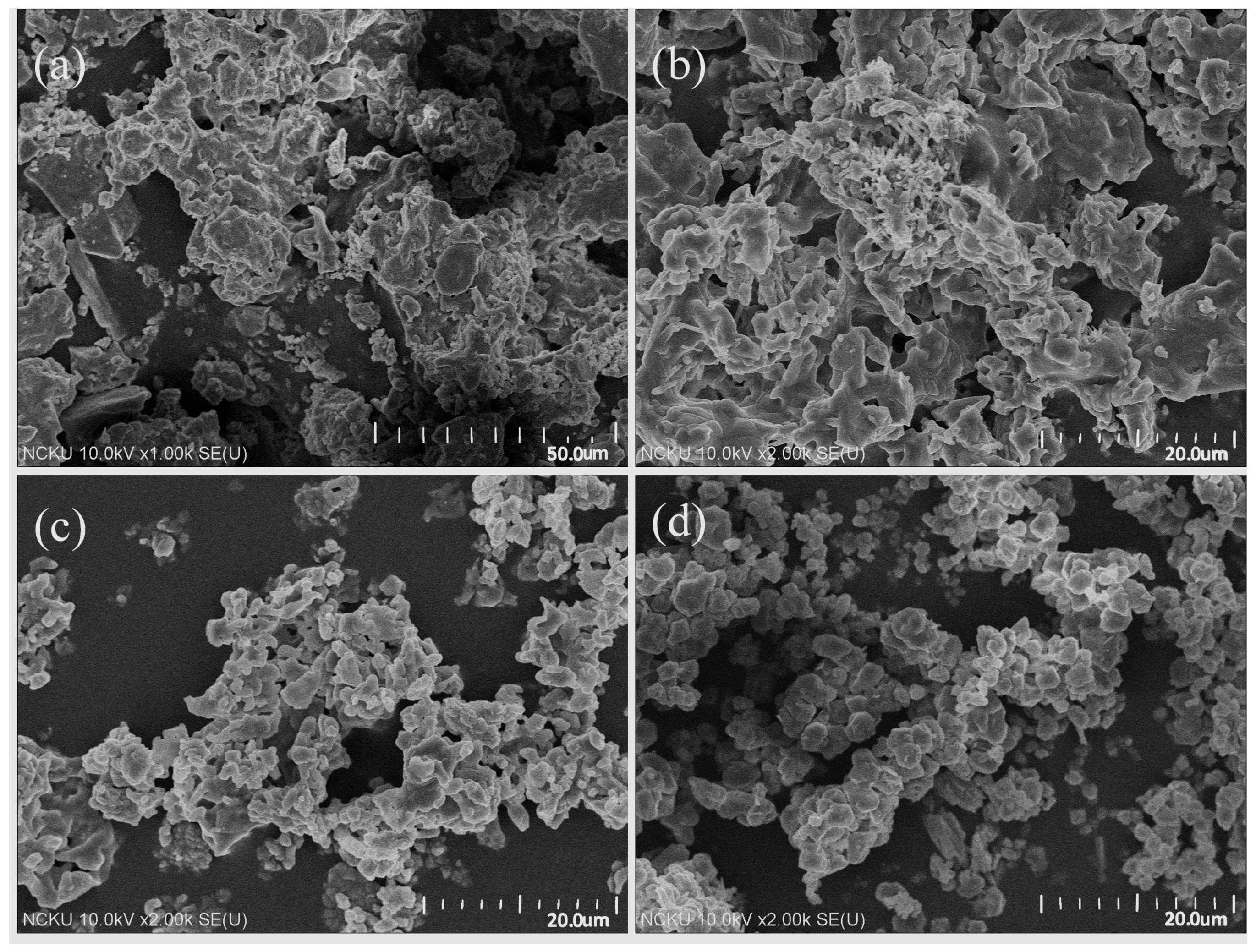
The SEM images of the raw and classified silts are shown in
Figure 4. It is clear that with decreasing particle sizes, the major mineral images gradually vary from massive, which represents quartz, to sheet-like, which represents clay minerals.
2.2. Procedures
The silts were fully mixed with sulfur (purified, Katayama Chemical Co. Ltd., Osaka, Japan) and active carbon (100–400 mesh, Sigma-Aldrich Chemie GmbH, Munich, Germany). An aliquot of sodium carbonate (reagent grade, Fisher Scientific, Leicestershire, UK) solution of assigned concentrations was slowly added to the powder and was hand-kneaded to balls and then dried in air. It was reported [
29,
30] that in the reaction, sulfur and sodium carbonate are reacted as:
From the Na2CO3/S8molar ratio in the above equation, the Na2CO3/S8 weight ratio is approximately equivalent to 1. This ratio was held constant in this study. The dried balls were placed in an alumina crucible covered with a lid and calcined at 700, 800 and 900 °C for 2, 4, 8 and 12 h.
After completion of the reaction, the crucible was allowed to cool down to room temperature in the furnace. The cooling took about 8 h. The reacted balls were mixed with deionized water, stirred thoroughly and filtered several times to remove all dissolved ions. The solids were dried at 120 °C for 24 h and for further analyses.
2.3. Characterization
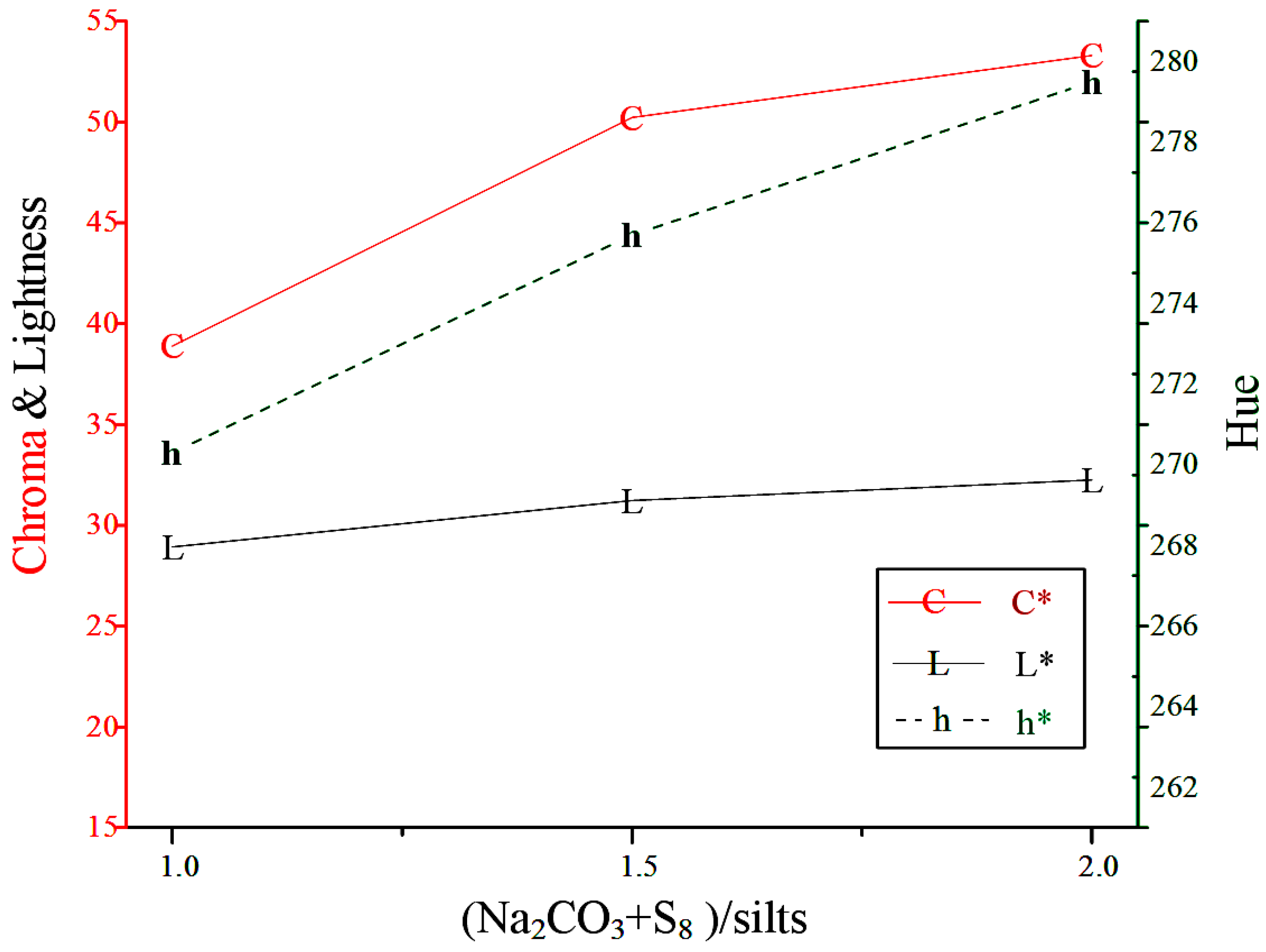
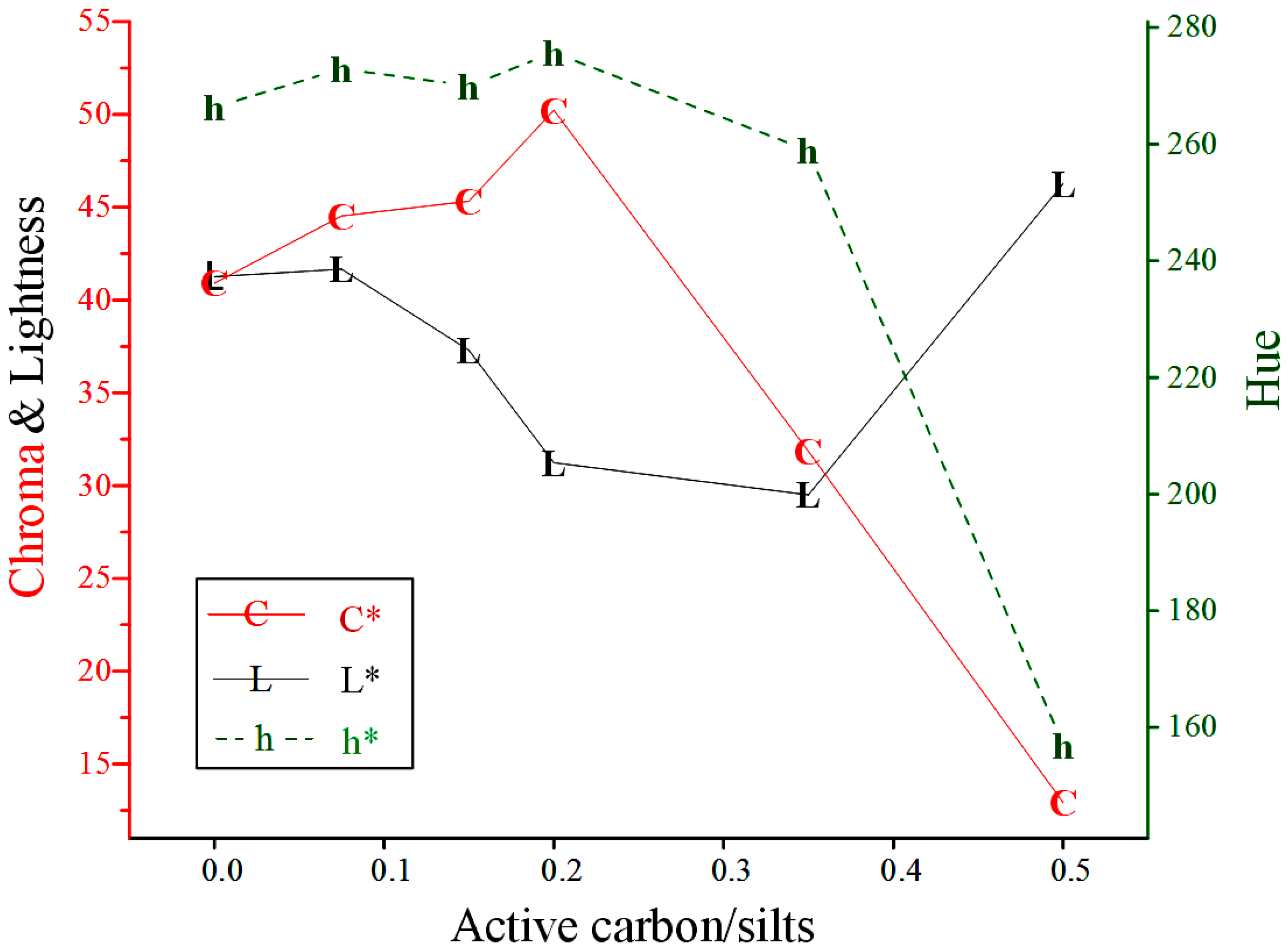
The mineral phases of the sediment and products were characterized using an X-ray diffractometer (DX-2600, Fangyuan Co., Dandong, China) with Cu Kα (λ = 1.5418Å) radiation and a scan rate of 0.04°/s. The chemical compositions were analyzed using an X-ray Fluorescence Analyzer (NEX CG EDXRF elemental analyzer, Rigaku Corp., Tokyo, Japan). The particle size distribution was determined using an Andreasen pipette. The morphology and individual particle sizes were examined using a Hitachi 8000 High-Resolution Scanning Electron Microscope (HRSEM, Hitach Ltd., Ibaraki, Japan). The color characteristics of the synthesized products were measured using a sphere spectrophotometer SP-60 (X-Rite Inc., Grand Rapids, MI, USA) employing the CIELab (Commission Internationale de l'Éclairage) color spaces system. The measurements (Standard Illuminant D65/10°) performed were chromaticity or color saturation (C* = 0 for grey color, C* = 100 for pure chromatic components), lightness (L* = 0 for a black sample, L* = 100 for a white sample) and hue (h* = 0°, 90°, 180°, 270° for red, yellow, green and blue colors, respectively).