A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures
Abstract
1. Introduction
2. Materials
3. Experimental Methods
4. Results and Discussion
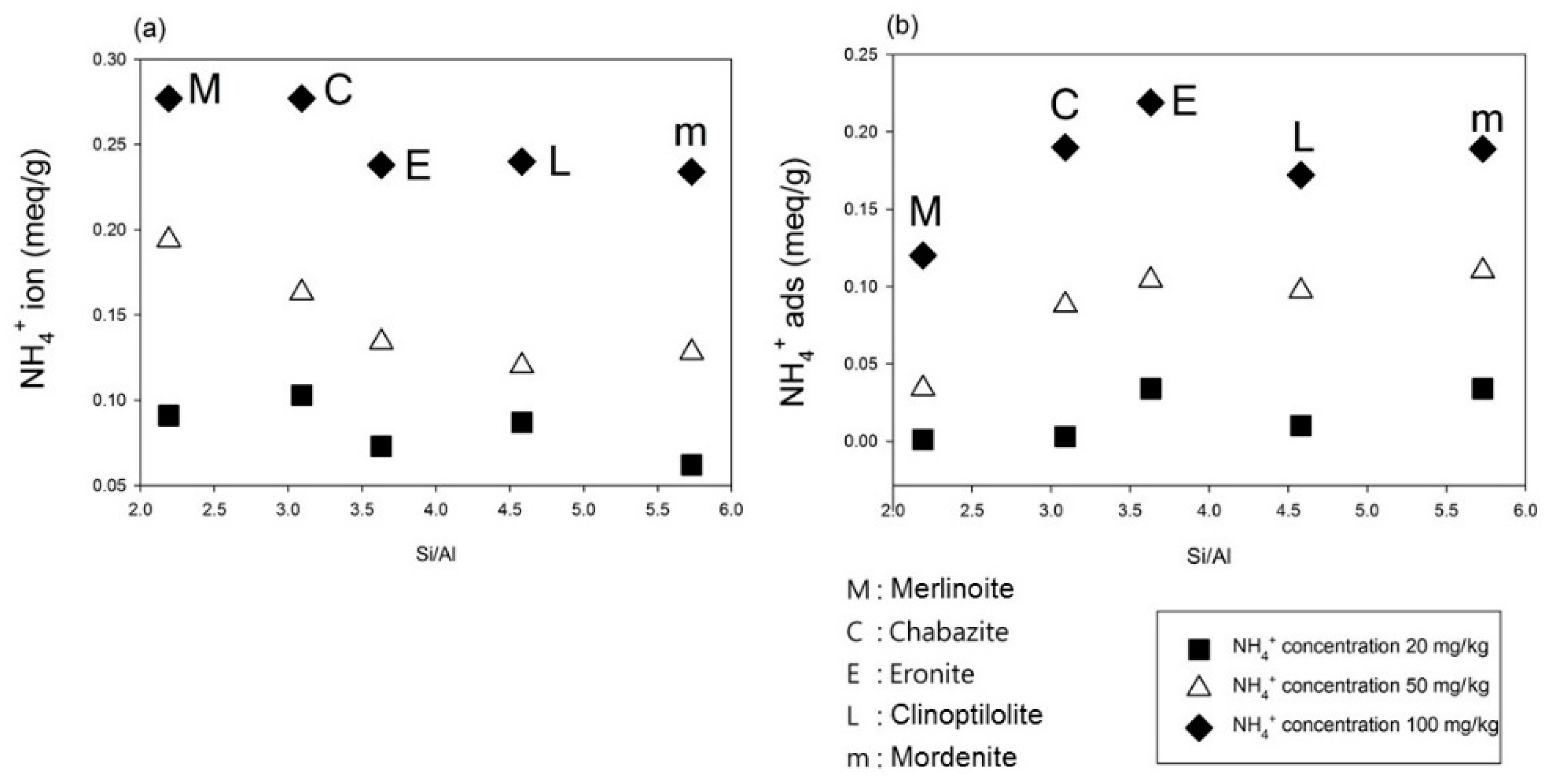
4.1. Percentage of Ion Exchange and Adsorption in Different Concentrations
4.2. The Relationship of Ion Exchange and Adsorption with Zeolite Composition
4.3. Specific Surface Area of Zeolites
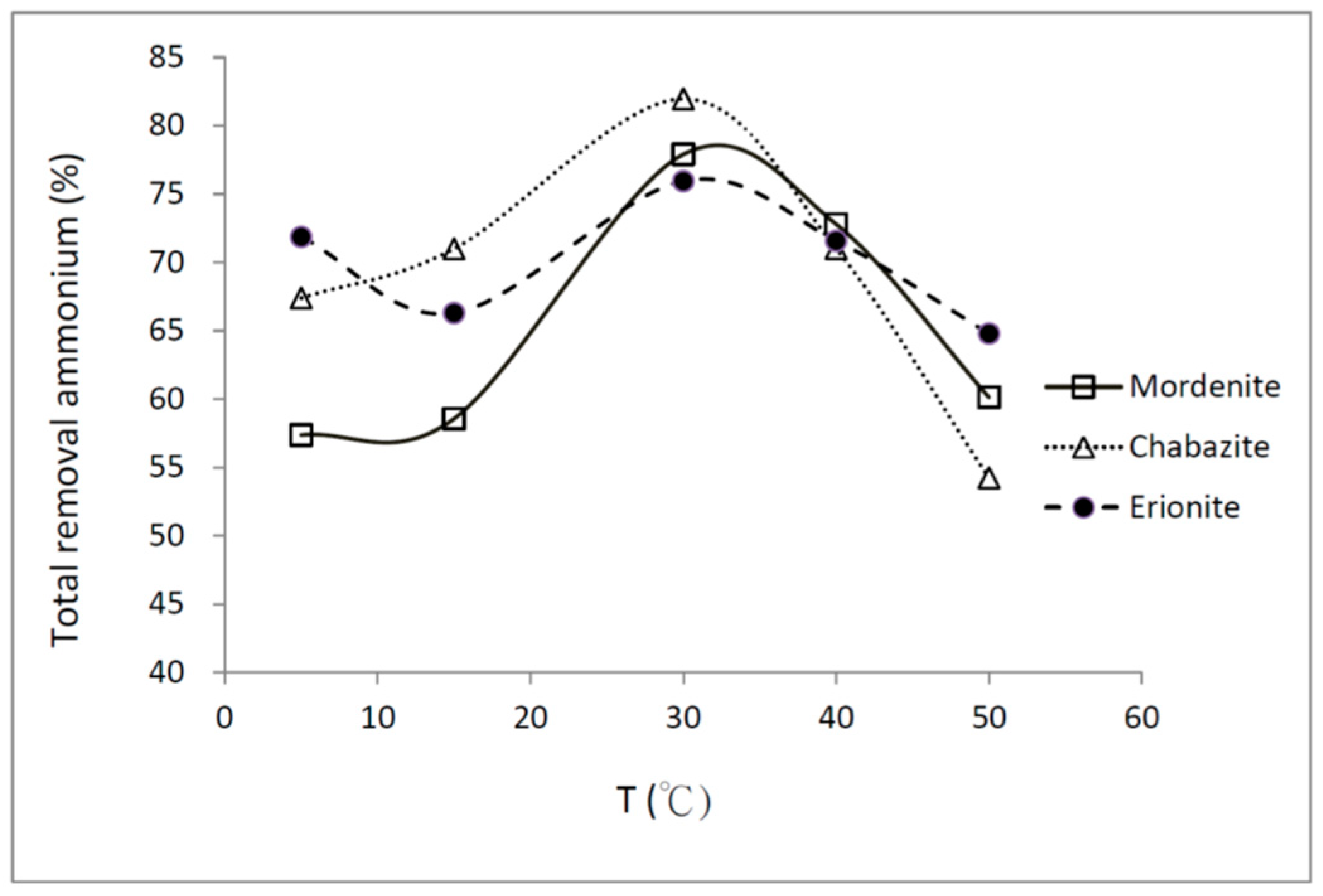
4.4. Temperature Effect
4.4.1. Total Ammonium Removal at Different Temperatures
4.4.2. Zeolite Selectivity at Different Temperatures
4.5. Kinetic Diffusion of Ammonium
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kithome, M.; Paul, J.W.; Lavkulich, L.M.; Bomke, A.A. Kinetics of ammonium adsorption and desorption by natural zeolite clinoptilolite. Soil Sci. Soc. Am. J. 1998, 62, 622–629. [Google Scholar] [CrossRef]
- Wiesmann, U. Biological nitrogen removal from wastewater. Adv. Biochem. Eng. Biotechnol. 1994, 51, 114–153. [Google Scholar]
- Nguyen, M.L.; Tanner, C.C. Ammonium removal from wastewaters using natural New Zealand zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef]
- Townsend, R.P.; Loizidou, M. Ion-exchange properties of natural clinoptilolite, ferrierite and mordenite: 1. Sodium-ammonium equilibria. Zeolites 1984, 4, 191–195. [Google Scholar] [CrossRef]
- Mondale, K.D.; Carland, R.M.; Aplan, F.F. The comparative ion exchange capacities of natural sedimentary and synthetic zeolites. Miner. Eng. 1995, 8, 535–548. [Google Scholar] [CrossRef]
- Green, M.; Mels, A.; Lahav, O.; Tarre, S. Biological ion exchange process for ammonium removal from secondary effluent. Water Sci. Technol. 1996, 34, 449–458. [Google Scholar] [CrossRef]
- Ioannidis, S.; Anderko, A. Equilibrium modeling of combined ion-exchange and molecular adsorption phenomena. Ind. Eng. Chem. Res. 2001, 40, 714–720. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Weatherley, L.R.; Miladinovic, N.D. Comparison of the ion exchange uptake of ammonium ion onto New Zealand clinoptilolite and mordenite. Water Res. 2004, 38, 4305–4312. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Ramos, R.; Monsivais-Rocha, J.E.; Aragon-Piňa, A.; Berber-Mendoza, M.S.; Guerrero-Coronado, R.M.; Alonso-Davila, P. Removal of ammonium from aqueous solution by ion exchange on natural and modified chabazite. J. Environ. Manag. 2010, 91, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; Yan, C.; Al-Ani, Y.; Dawood, A.S.; Ibrahim, A.; Zhou, C.; Wang, H. An investigation into the adsorption removal of ammonium by salt activated Chinese (Hulaodu) natural zeolite: Kinetics, isotherms, and thermodynamics. J. Taiwan Inst. Chem. Eng. 2014, 45, 554–564. [Google Scholar] [CrossRef]
- Englert, A.H.; Rubio, J. Characterization and environmental application of a Chilean natural zeolite. Int. J. Miner. Process. 2005, 75, 21–29. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of ammonium from greywater using natural zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M. Removal of ammonium ions by selected natural and synthetic zeolites. Gospodarka Surowcami Mineralnymi—Miner. Res. Manag. 2010, 26, 133–148. [Google Scholar]
- Alshameri, A.; Ibrahim, A.; Assabri, A.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics (Clinoptilolite and montmorillonite). J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. [Google Scholar] [CrossRef]
- Chen, H.F.; Fang, J.N.; Lo, H.J.; Song, S.R.; Chen, Y.L.; Chung, S.H.; Lee, C.Y.; Li, L.J.; Lin, I.C. The synthesis of merlinoite. West. Pac. Earth Sci. 2002, 2, 371–386. [Google Scholar]
- Chen, H.F.; Lo, H.J.; Song, S.R.; Fang, J.N.; Chen, Y.L.; Li, L.J.; Lin, I.C.; Chung, S.H.; Lee, Y.T. The synthesis of phillipsite. West. Pac. Earth Sci. 2002, 2, 409–420. [Google Scholar]
- Campbell, L.S.; Charnock, J.; Dyer, A.; Hilliber, S.; Chernery, S.; Stoppa, F.; Henderson, C.M.B.; Walcott, R.; Rumsey, M. Determination of zeolite-group mineral compositions by electron probe microanalysis. Mineral. Mag. 2016, 80, 781–807. [Google Scholar] [CrossRef]
- Bish, D.L.; Ming, D.W. Natural zeolites: Occurrence, properties, applications. Rev. Mineral. Geochem. 2001, 45, 654. [Google Scholar]
- Inglezakis, V.J. The concept of “capacity” in zeolite ion-exchange systems. J. Colloid Interface Sci. 2005, 281, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lee, Y. The study of removal ammonium by modified micro-pore zeolite ball. Water Purif. Technol. 2005, 24, 14–17. (In Chinese) [Google Scholar]
- Leyva-Ramos, R.; Aguilar-Armenta, G.; Gonzalez-Gutierrez, L.V.; Guerrero-Coronado, R.M.; Mendoza-Barron, J. Ammonia exchange on clinoptilolite from mineral deposits located in Mexico. J. Chem. Technol. Biotechnol. 2004, 79, 651–657. [Google Scholar] [CrossRef]
- Lebedynets, M.; Sprynsky, M.; Sakhnyuk, I.; Zbytniewski, R.; Golembiewski, R.; Buszewski, B. Adsorption of NH4+ ions onto a natural zeolite: Transcarpathian clinoptilolite. Adsorpt. Sci. Technol. 2004, 22, 731–741. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard. Mater. B 2006, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Han, L.; Ma, H.; Zheng, Y.; Zhang, H.; Liu, D.; Liang, S. Adsorption characteristics of ammonium ion by zeolite 13X. J. Hazard. Mater. 2008, 158, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sarioglu, M. Removal of ammonium from municipal wastewater using natural Turkish (Dogantepe) zeolite. Sep. Purif. Technol. 2005, 41, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.F.; Lin, F.; Pang, W.Q. Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. J. Hazard. Mater. 2007, 142, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, F.G. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Valderrama, C.; Barios, J.I.; Caetano, M.; Farran, A.; Cortina, J.L. Kinetic evaluation of phenol/aniline mixtures adsorption from aqueous solutions onto activated carbon and hyper cross linked polymeric resin (MN200). React. Funct. Polym. 2010, 70, 142–150. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farrana, A.; Luis Cortina, J. Modification of a natural zeolite with Fe(III) for simultaneous phosphate and ammonium removal from aqueous solutions. J. Chem. Technol. Biotechnol. 2016, 91, 1737–1746. [Google Scholar] [CrossRef]



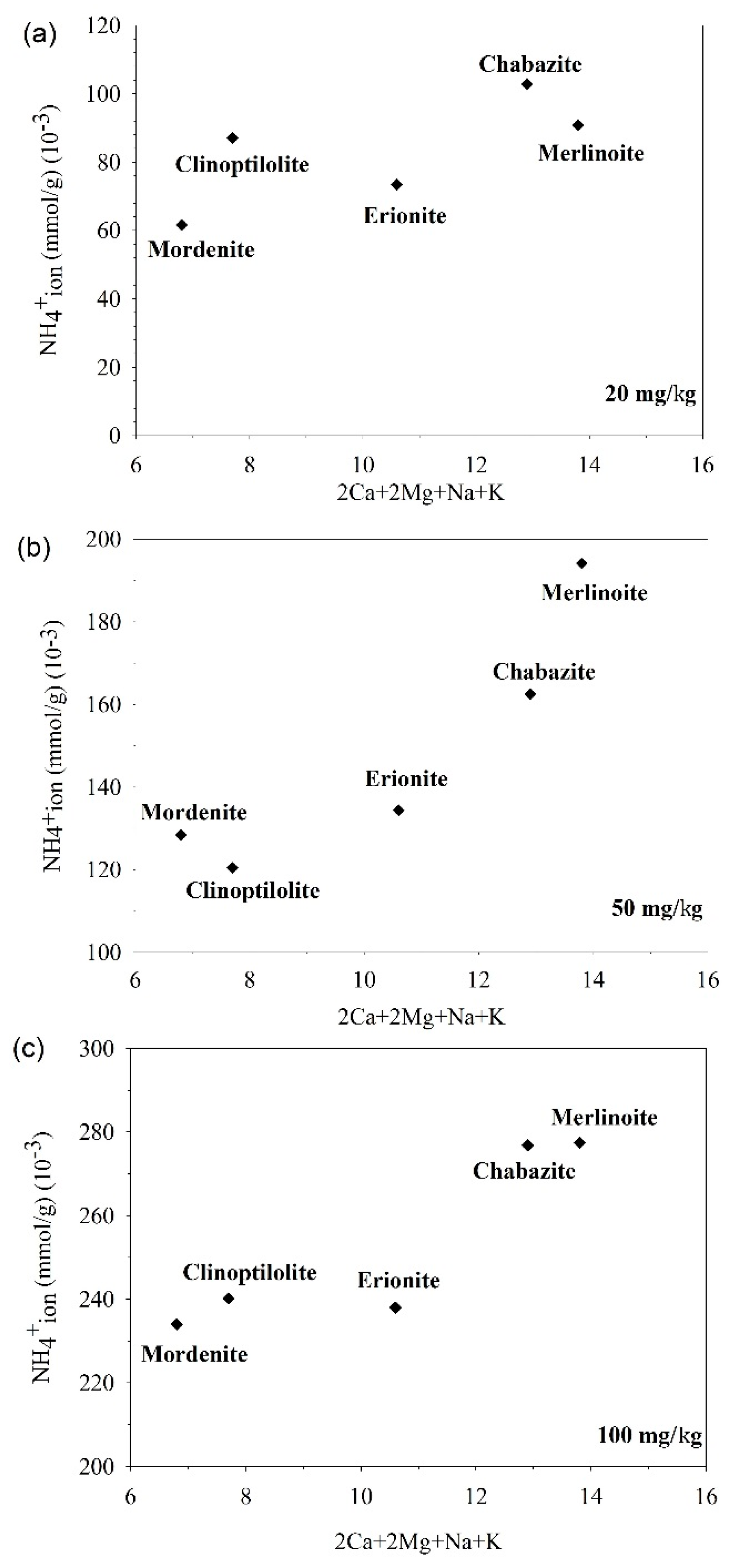


| Zeolite | n | SiO2 | Al2O3 | MgO | CaO | Na2O | K2O | Total (wt %) | Formula | Si/Al | 2Ca + 2Mg + Na + K | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mordenite | 26 | 71.36 | 10.58 | 0.04 | 3.33 | 1.12 | 1.98 | 88.42 | (Ca2.05, Mg0.03, Na1.24, K1.45)Al7.14Si40.93O96 | 5.73 | 6.8 | 85.1468 |
| Chabazite | 5 | 54.51 | 14.98 | 1.27 | 2.23 | 3.41 | 3.42 | 79.95 | (Ca1.58,Mg1.26,Na4.36, K2.89)Al11.65Si36.03O96 | 3.09 | 12.9 | 378.5544 |
| Erionite | 4 | 61.45 | 14.38 | 1.04 | 3.57 | 0.52 | 4.40 | 85.37 | (Ca2.34, Mg0.95, Na0.62, K3.44)Al10.35Si37.58O96 | 3.63 | 10.6 | 395.2574 |
| Clinoptilolite | 14 | 69.14 | 12.82 | 0.05 | 3.70 | 1.58 | 1.94 | 89.38 | (Ca2.27, Mg0.04, Na1.75, K1.42)Al8.63Si39.58O96 | 4.58 | 7.7 | 22.0504 |
| Merlinoite | 13 | 52.15 | 20.19 | 0.04 | 0.42 | 2.61 | 13.07 | 88.53 | (Ca0.29, Mg0.04, Na3.21, K10.59)Al15.07Si33.09O96 | 2.19 | 13.8 | 3.4623 |
| NH4+ Concentration | Zeolites | NH4+ion (meq/g) | Ion Exchange (%) | NH4+ads (meq/g) | Adsorption (%) | NH4+total (meq/g) |
|---|---|---|---|---|---|---|
| Mordenite | 0.062 | 64% | 0.034 | 36% | 0.096 | |
| Chabazite | 0.103 | 97% | 0.003 | 3% | 0.106 | |
| 20 mg/kg | Erionite | 0.073 | 68% | 0.034 | 32% | 0.108 |
| Clinoptilolite | 0.087 | 90% | 0.010 | 10% | 0.097 | |
| Merlinoite | 0.091 | 99% | 0.001 | 1% | 0.092 | |
| Mordenite | 0.128 | 54% | 0.110 | 46% | 0.238 | |
| Chabazite | 0.163 | 65% | 0.088 | 35% | 0.251 | |
| 50 mg/kg | Erionite | 0.134 | 56% | 0.104 | 44% | 0.238 |
| Clinoptilolite | 0.120 | 55% | 0.097 | 45% | 0.217 | |
| Merlinoite | 0.194 | 85% | 0.034 | 15% | 0.228 | |
| Mordenite | 0.234 | 55% | 0.189 | 45% | 0.423 | |
| Chabazite | 0.277 | 59% | 0.190 | 41% | 0.467 | |
| 100 mg/kg | Erionite | 0.238 | 52% | 0.219 | 48% | 0.457 |
| Clinoptilolite | 0.240 | 58% | 0.172 | 42% | 0.412 | |
| Merlinoite | 0.277 | 70% | 0.120 | 30% | 0.398 |
| Mordenite | 1/KMg | 1/Kca | 1/KNa | 1/KK | Release Priority | Zeolite Selectivity |
| 5 °C | 0.554 | 0.082 | 0.169 | 0 | Mg > Na > Ca | Mg < Na < Ca < K |
| 15 °C | 0.768 | 0.055 | 0.112 | 0 | Mg > Na > Ca | Mg < Na < Ca < K |
| 30 °C | 0.961 | 0.096 | 0.343 | 0.040 | Mg > Na > Ca > K | Mg < Na < Ca < K |
| 40 °C | 1.256 | 0.100 | 0.344 | 0.111 | Mg > Na > K > Ca | Mg < Na < K < Ca |
| 50 °C | 0.919 | 0.057 | 0.247 | 0 | Mg > Na > Ca | Mg < Na < Ca < K |
| Chabazite | 1/KMg | 1/Kca | 1/KNa | 1/KK | ||
| 5 °C | 0 | 0.012 | 0.176 | 0 | Na > Ca | Na < Ca < Mg = K |
| 15 °C | 0.008 | 0.005 | 1.951 | 0 | Na > Mg > Ca | Na < Mg < Ca < K |
| 30 °C | 0.007 | 0.004 | 0.310 | 0 | Na > Mg > Ca | Na < Mg < Ca < K |
| 40 °C | 0 | 0 | 0.133 | 0 | Na | Na < Ca = Mg = K |
| 50 °C | 0 | 0 | 0.158 | 0 | Na | Na < Ca = Mg = K |
| Erionite | 1/KMg | 1/Kca | 1/KNa | 1/KK | ||
| 5 °C | 0 | 0.019 | 8.339 | 0 | Na > Ca | Na < Ca < K = Mg |
| 15 °C | 0 | 0.008 | 1.605 | 0 | Na > Ca | Na< Ca < K = Mg |
| 30 °C | 0 | 0.008 | 1.915 | 0.006 | Na > Ca > K | Na < Ca < K = Mg |
| 40 °C | 0 | 0.009 | 4.026 | 0.011 | Na > K > Ca | Na < K < Ca = Mg |
| 50 °C | 0 | 0 | 1.028 | 0 | Na | Na < Ca = K = Mg |
| Parameter | NH4+ Concentration | Mordenite | Chabazite | Erionite | Clinoptilolite | Merlinoite |
|---|---|---|---|---|---|---|
| Radius (μm) | 8.7 | 14.0 | 16.3 | 10.7 | 17.9 | |
| Film diffusion Df (μm2∙s−1) | 20 mg/kg | 0.3 | 2.1 | 4.9 | 0.3 | 1.1 |
| 50 mg/kg | 0.2 | 0.7 | 0.7 | 0.2 | 0.5 | |
| 100 mg/kg | 0.2 | 0.5 | 0.4 | 0.2 | 0.2 | |
| Particle diffusion Dp (μm2∙s−1) | 20 mg/kg | 0.0016 | 0.0059 | 0.0097 | 0.0018 | 0.0139 |
| 50 mg/kg | 0.0013 | 0.0040 | 0.0068 | 0.0019 | 0.0079 | |
| 100 mg/kg | 0.0014 | 0.0052 | 0.0052 | 0.0041 | 0.0053 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-F.; Lin, Y.-J.; Chen, B.-H.; Yoshiyuki, I.; Liou, S.Y.-H.; Huang, R.-T. A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures. Minerals 2018, 8, 499. https://doi.org/10.3390/min8110499
Chen H-F, Lin Y-J, Chen B-H, Yoshiyuki I, Liou SY-H, Huang R-T. A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures. Minerals. 2018; 8(11):499. https://doi.org/10.3390/min8110499
Chicago/Turabian StyleChen, Huei-Fen, Yi-Jun Lin, Bo-Hong Chen, Iizuka Yoshiyuki, Sofia Ya-Hsuan Liou, and Rong-Tan Huang. 2018. "A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures" Minerals 8, no. 11: 499. https://doi.org/10.3390/min8110499
APA StyleChen, H.-F., Lin, Y.-J., Chen, B.-H., Yoshiyuki, I., Liou, S. Y.-H., & Huang, R.-T. (2018). A Further Investigation of NH4+ Removal Mechanisms by Using Natural and Synthetic Zeolites in Different Concentrations and Temperatures. Minerals, 8(11), 499. https://doi.org/10.3390/min8110499




