One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials
2.2. Synthesis
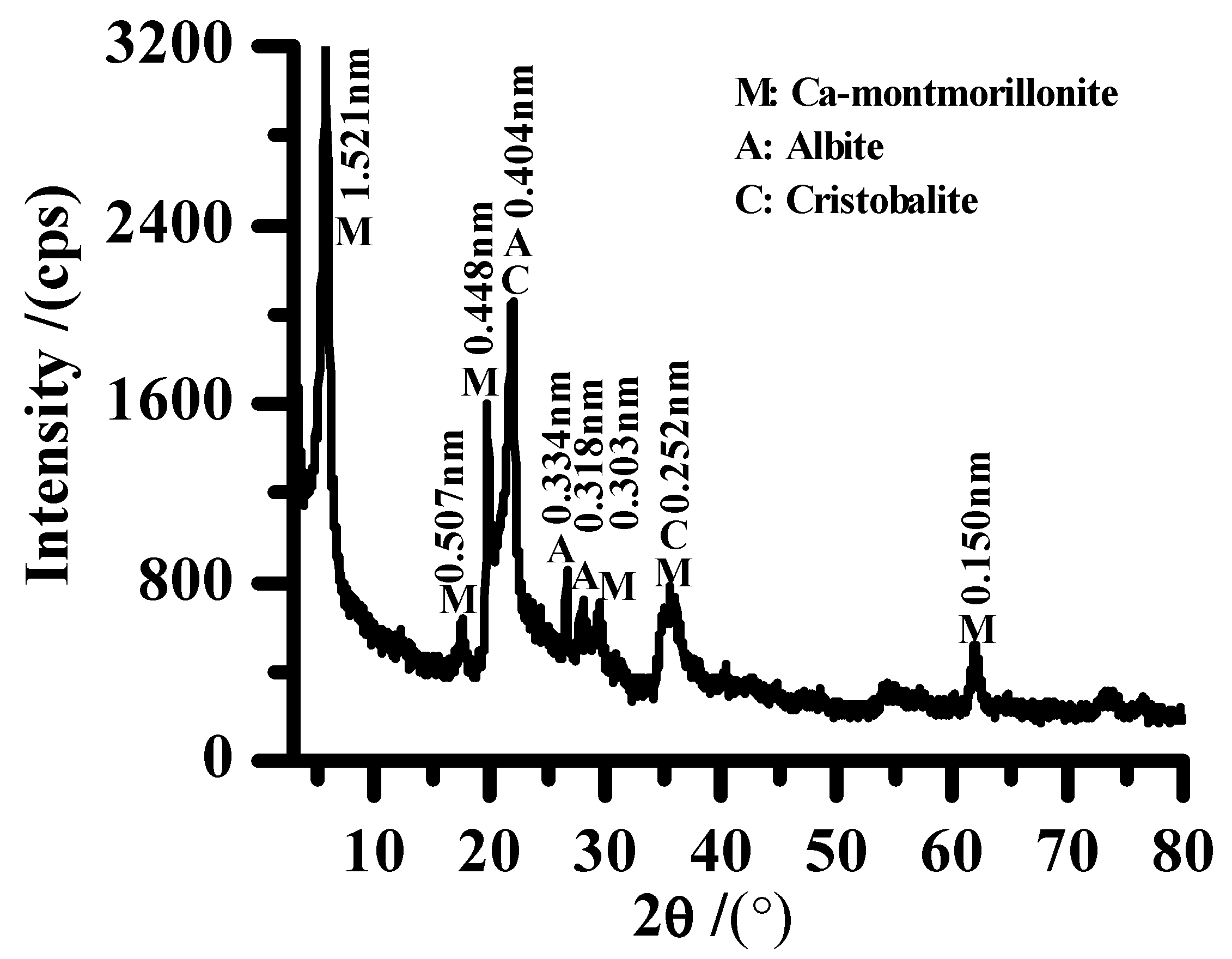
2.3. Characterization
3. Results and Discussion
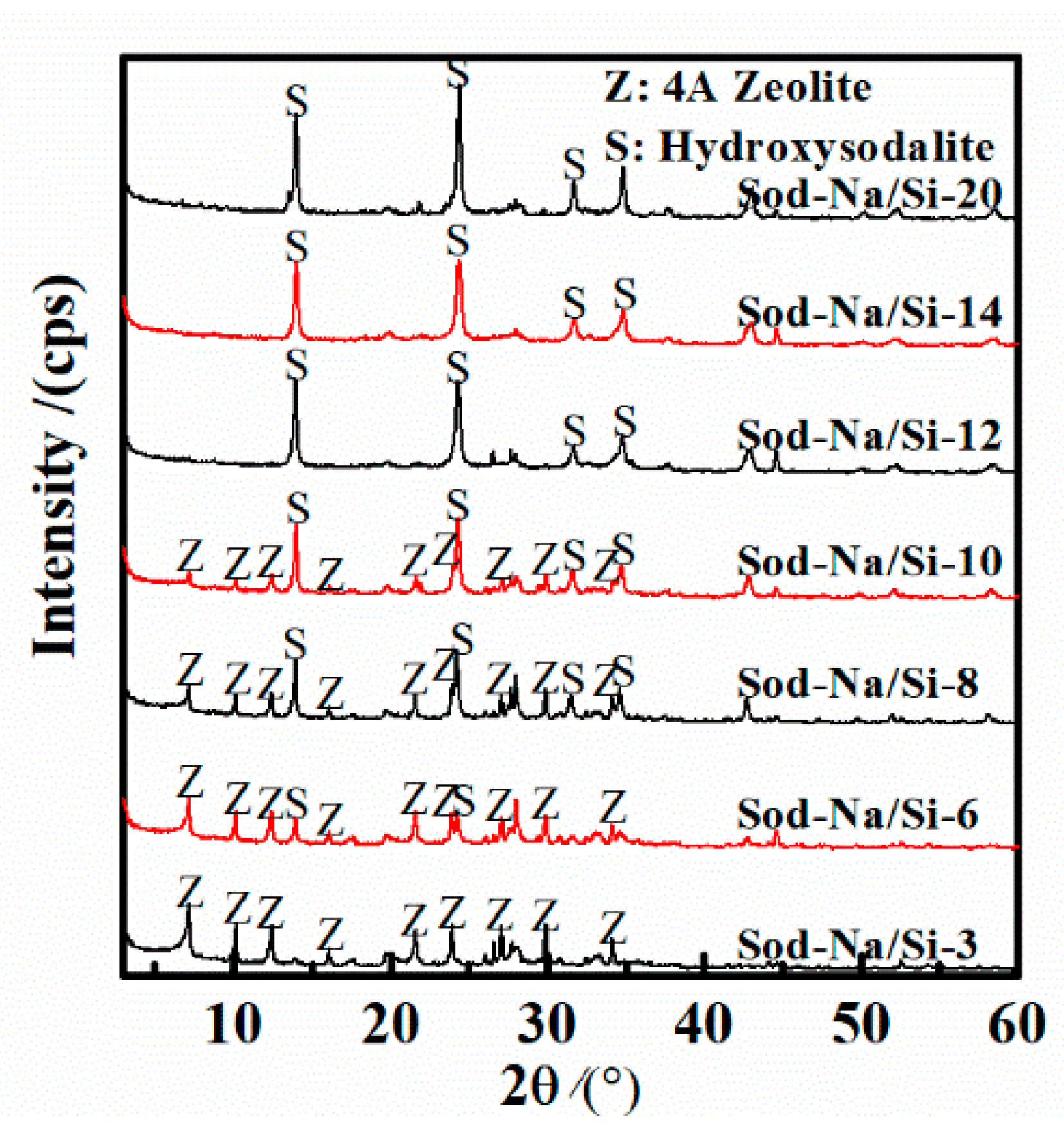
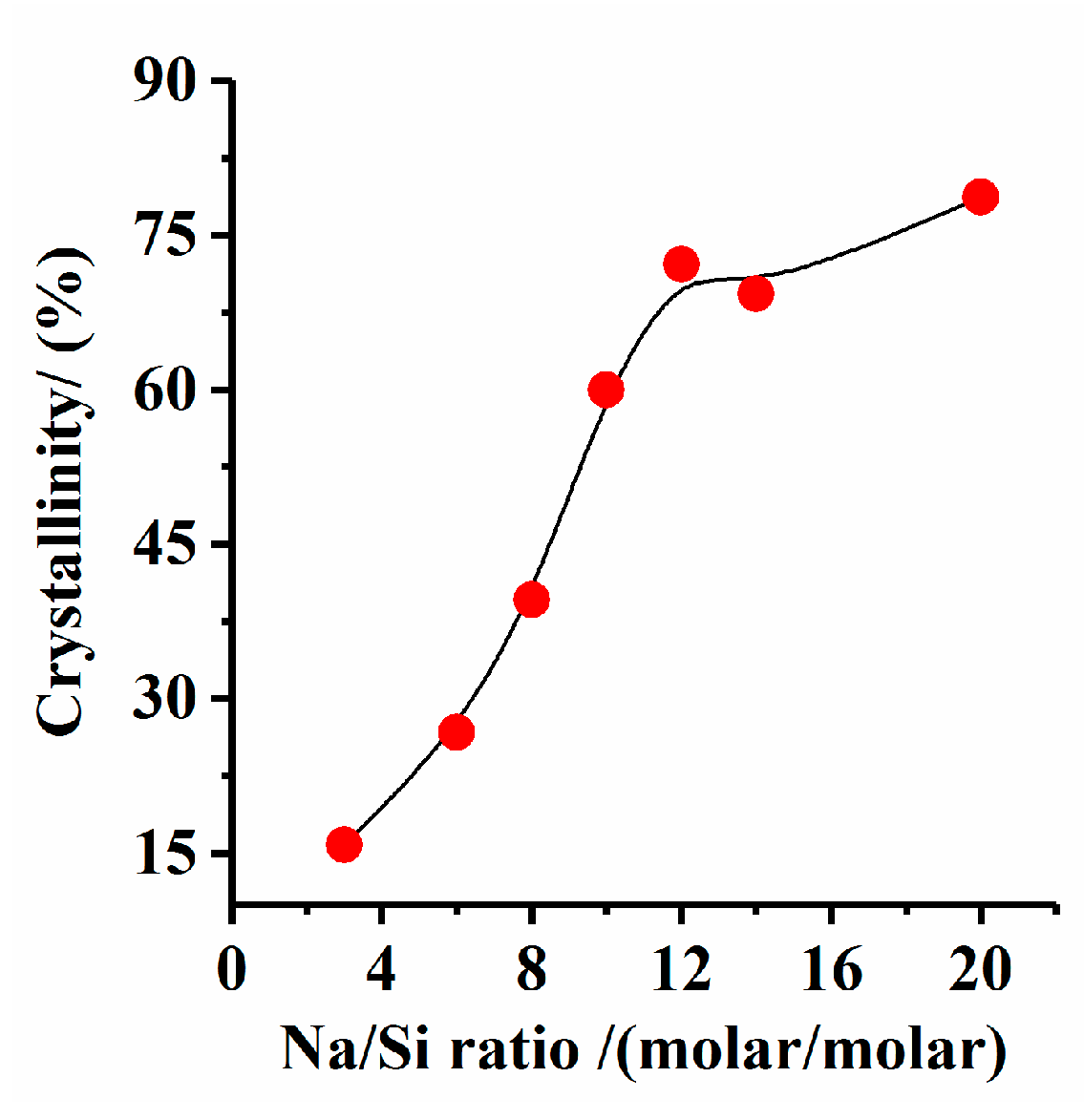
3.1. Effect of Na/Si Molar Ratio
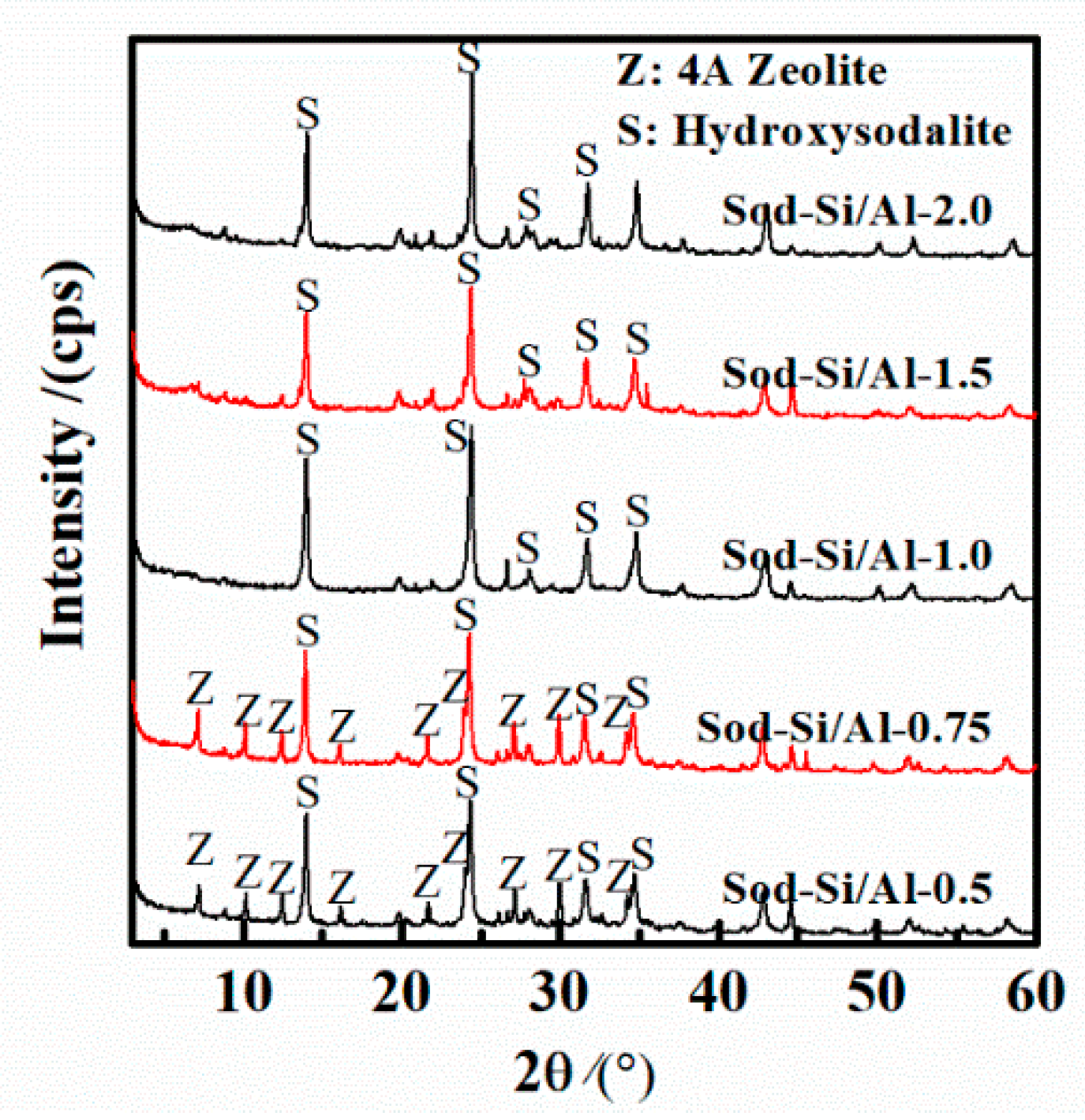
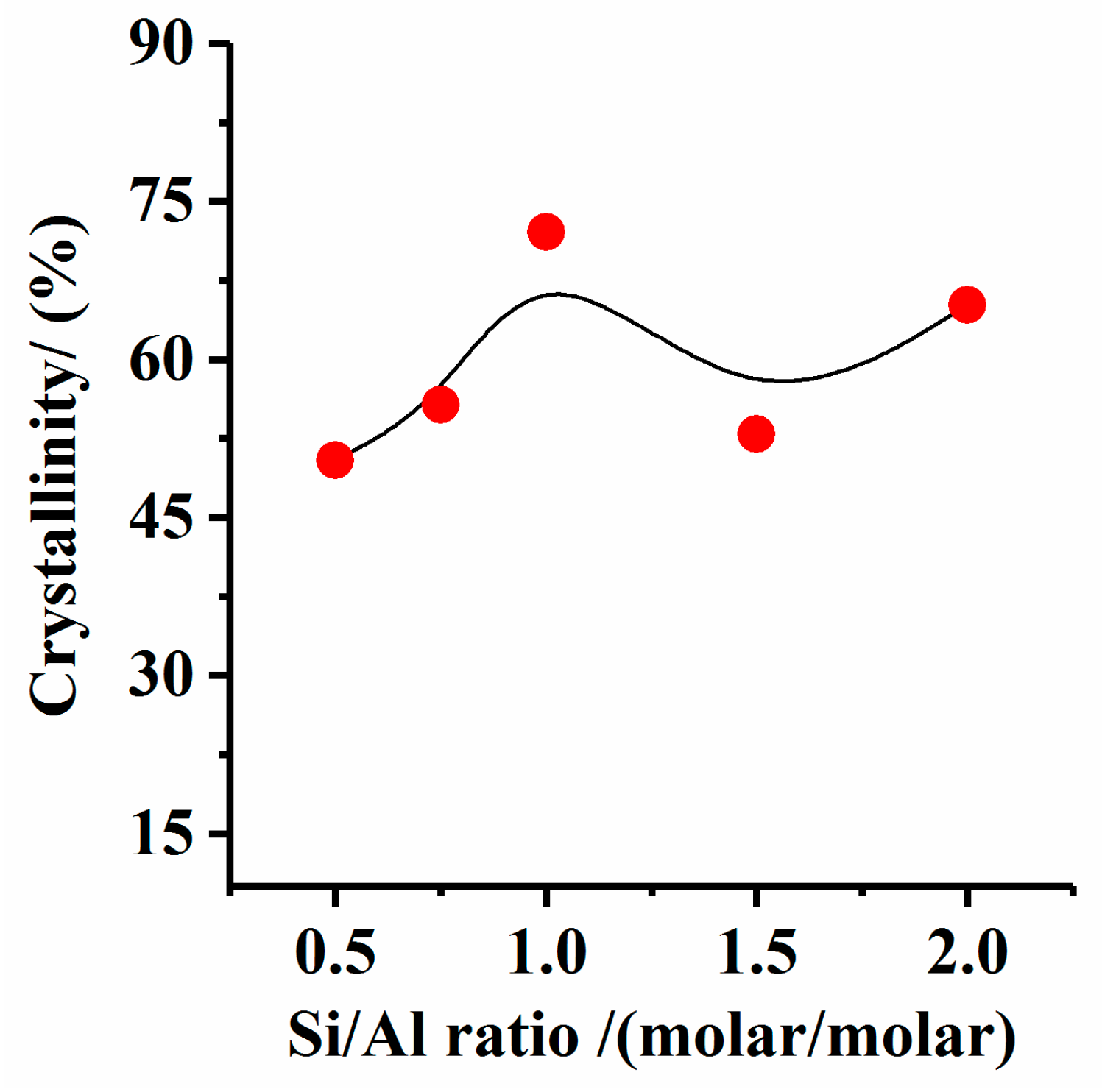
3.2. Effect of Si/Al Molar Ratio
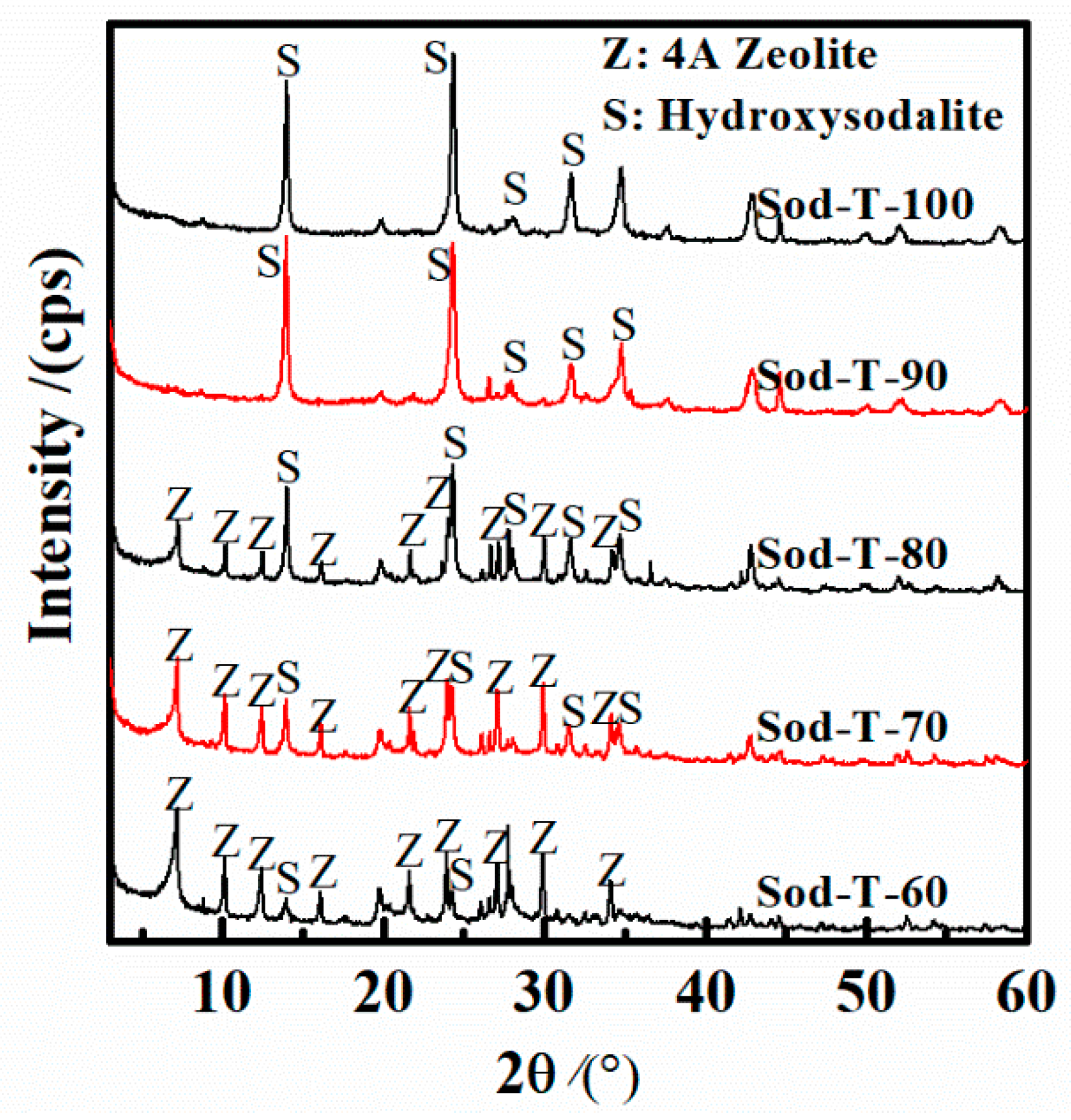
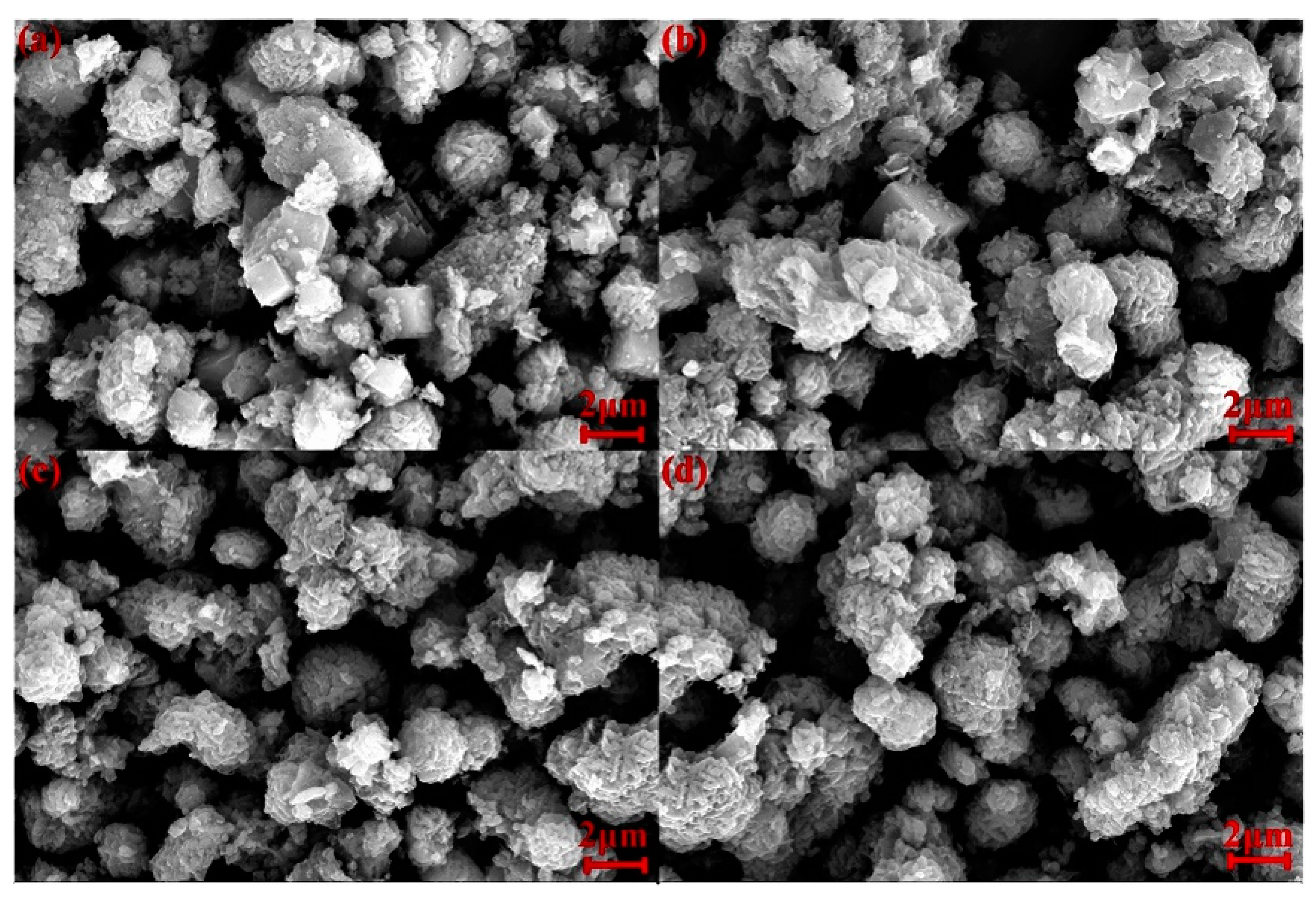
3.3. Effect of Reaction Temperature
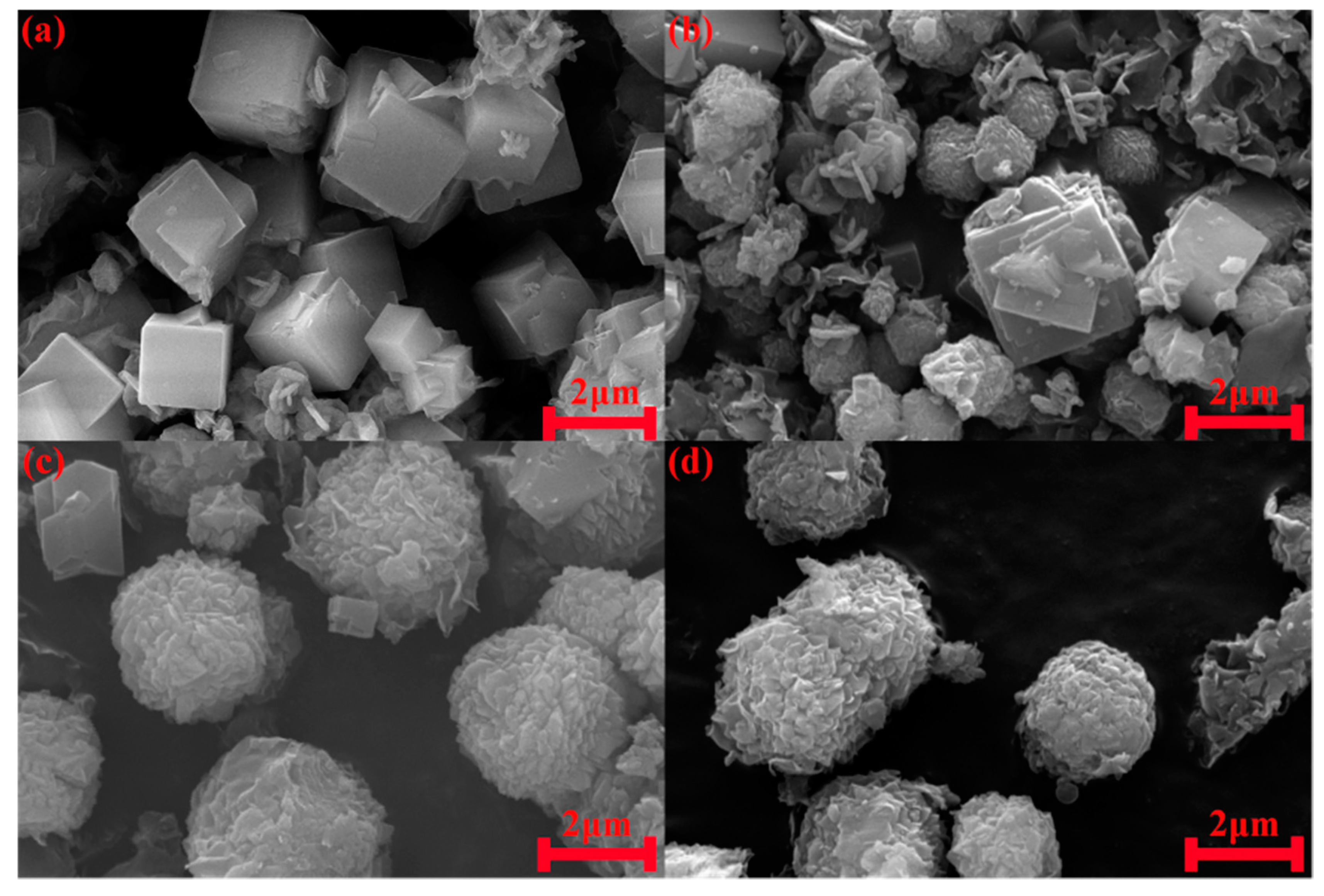
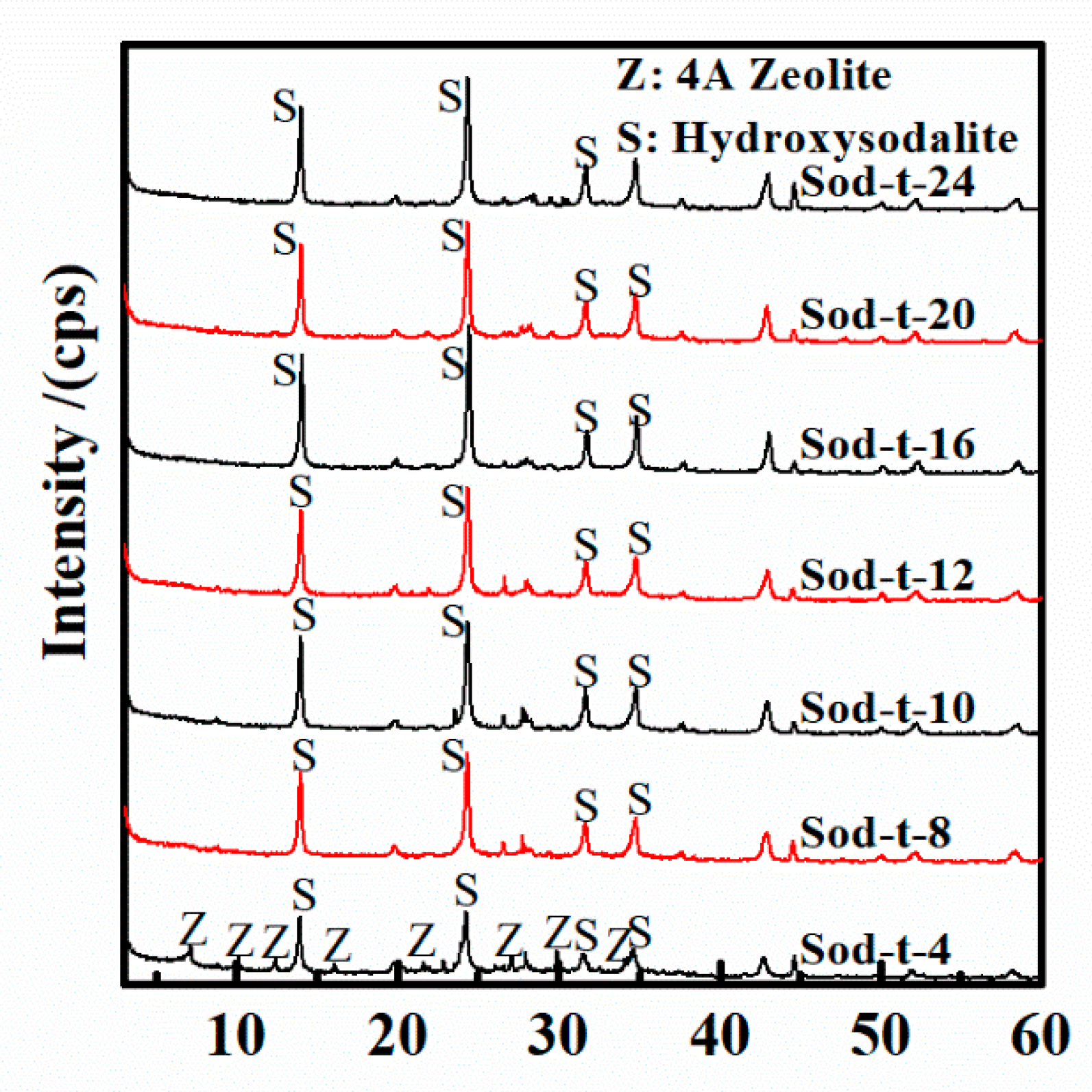
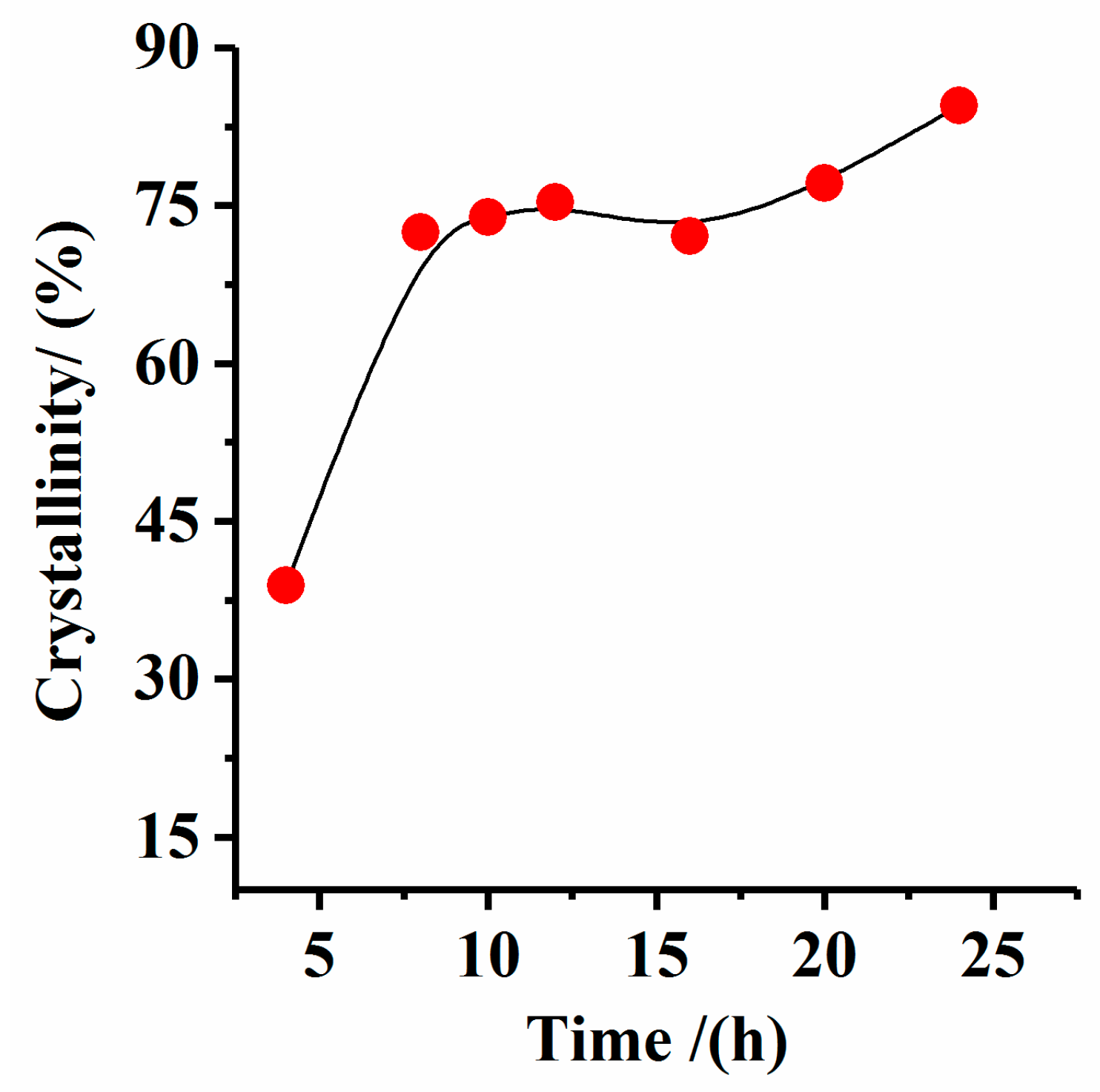
3.4. Effect of Reaction Time
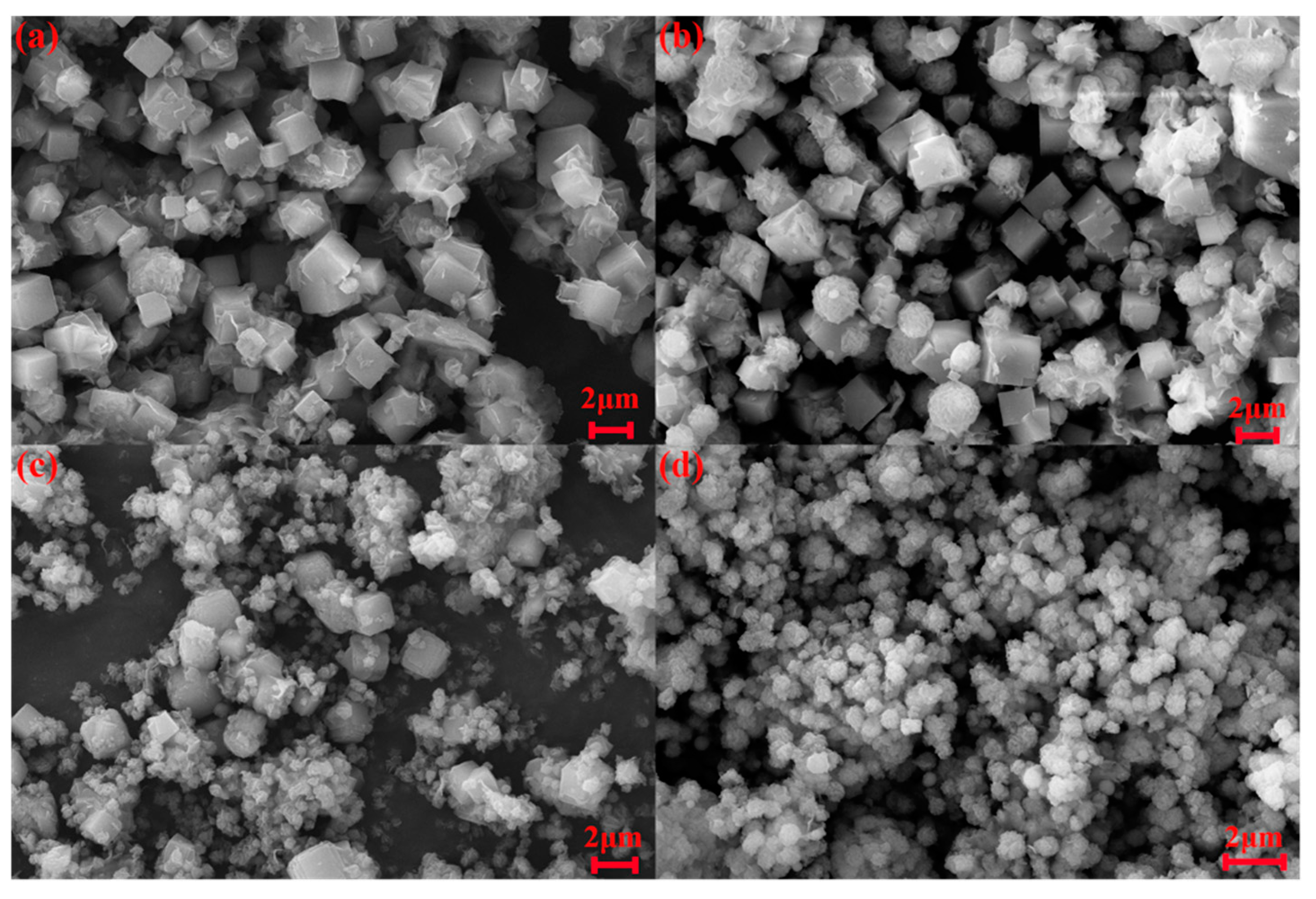
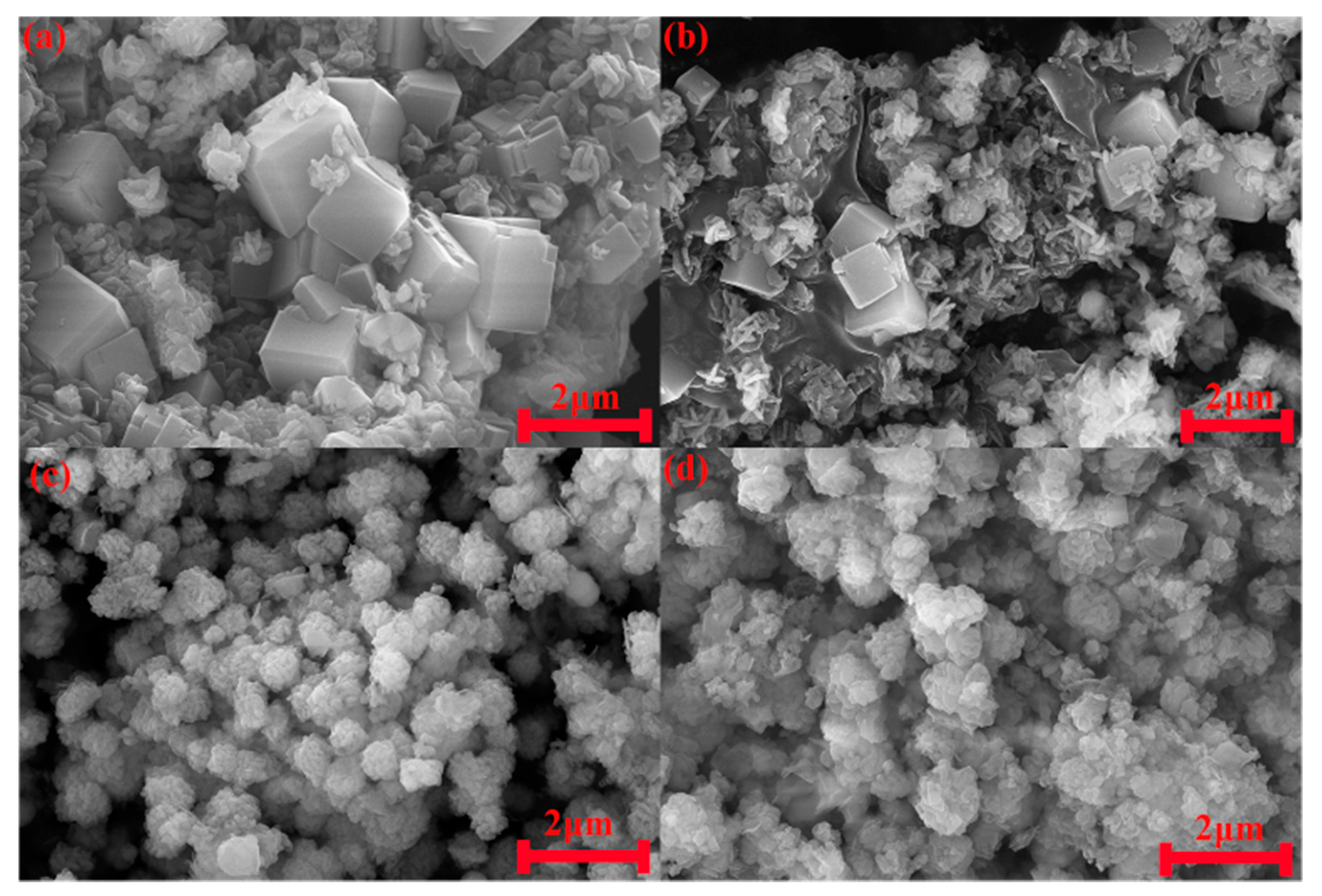
3.5. Characterization of Hydroxysodalite in Optimum Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bibby, D.M.; Dale, M.P. Synthesis of silica-sodalite from non-aqueous systems. Nature 1985, 317, 157. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Yang, X.; Wang, C.; Luo, X. Synthesis of pure sodalite with wool ball morphology from alkali fusion kaolin. Mater. Lett. 2015, 161, 157–159. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.; Wang, H. Synthesis of nanocrystalline sodalite with organic additives. Mater. Lett. 2008, 62, 4028–4030. [Google Scholar] [CrossRef]
- Naskar, M.K.; Kundu, D.; Chatterjee, M. Effect of process parameters on surfactant-based synthesis of hydroxy sodalite particles. Mater. Lett. 2011, 65, 436–438. [Google Scholar] [CrossRef]
- Johnson, G.M.; Mead, P.J.; Weller, M.T. Synthesis of a range of anion-containing gallium and germanium sodalites. Microporous Mesoporous Mater. 2000, 38, 445–460. [Google Scholar] [CrossRef]
- Hu, T.; Qiu, J.; Wang, Y.; Wang, C.; Liu, R.; Meng, C. Synthesis of low Si/Al ratio hydroxysodalite from oil shale ash without pretreatment. J. Chem. Technol. Biotechnol. 2015, 90, 208–212. [Google Scholar] [CrossRef]
- Van den Berg, A.W.C.; Bromley, S.T.; Jansen, J.C. Thermodynamic limits on hydrogen storage in sodalite framework materials: A molecular mechanics investigation. Microporous Mesoporous Mater. 2005, 78, 63–71. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of hydroxy sodalite films as novel water selective membranes. J. Membr. Sci. 2009, 326, 153–160. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of a sodalite membrane reactor in esterification-coupling reaction and separation. Catal. Today 2010, 156, 132–139. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Song, C.; Yang, W.; Liu, J.; Lin, L. Microwave-assisted hydrothermal synthesis of hydroxy-sodalite zeolite membrane. Microporous Mesoporous Mater. 2004, 75, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, M.S.; Mohammadi, T.; Kazemimoghadam, M. Hydrothermal synthesis of hydroxy sodalite zeolite membrane: Separation of H2/CH4. Ceram. Int. 2014, 40, 5889–5896. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Feng, L.; Duanmu, C.; Jin, Y.; Hu, T.; Wu, J. Controllable synthesis of sodalite submicron crystals and microspheres from palygorskite clay using a two-step approach. Powder Tech. 2012, 217, 298–303. [Google Scholar] [CrossRef]
- Passos, F.A.C.M.; Castro, D.C.; Ferreira, K.K.; Simões, K.M.A.; Bertolino, L.C.; Barbato, C.N.; Garrido, F.M.S.; Felix, A.A.S.; Silva, F.A.N.G. Synthesis and characterization of sodalite and cancrinite from Kaolin. In Characterization of Minerals Metals and Materials; Springer: Berlin, Germany, 2017; pp. 279–288. [Google Scholar]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Bouberka, Z.; Kacha, S.; Kameche, M.; Elmaleh, S.; Derriche, Z. Sorption study of an acid dye from an aqueous solutions using modified clays. J. Hazard. Mater. 2005, 119, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Karnland, O.; Olsson, S.; Nilsson, U.; Sellin, P. Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys. Chem. Earth Parts A/B/C 2007, 32, 275–286. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sonderby, C.; Sogaard, E.G. Synthesis and characterization of silicate polymers. J. Sol-Gel Sci. Technol. 2009, 50, 372. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sonderby, C.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079. [Google Scholar] [CrossRef]
- Tang, W.; Han, J.; Zhang, S.; Sun, J.; Li, H.; Gu, X. Synthesis of 4A zeolite containing La from kaolinite and its effect on the flammability of polypropylene. Polym. Compos. 2018, 39, 3461–3471. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, T.; Liu, H.; Wang, C.; Cheng, P.; Chen, D.; Xie, J. An insight into the comprehensive application of opal-palygorskite clay: Synthesis of 4A zeolite and uptake of Hg2+. Appl. Clay Sci. 2018, 165, 103–111. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, H.; Lin, J.; Lin, Z.; Sun, J. Zeolite A synthesized from alkaline assisted pre-activated halloysite for efficient heavy metal removal in polluted river water and industrial wastewater. J. Environ. Sci. 2017, 56, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Itani, L.; Liu, Y.; Zhang, W.; Bozhilov, K.N.; Delmotte, L.; Valtchev, V. Investigation of the physicochemical changes preceding zeolite nucleation in a sodium-rich aluminosilicate gel. J. Am. Chem. Soc. 2009, 131, 10127–10139. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. The mechanism of the sodalite-to-cancrinite phase transformation in synthetic spent Bayer liquor. Microporous Mesoporous Mater. 1999, 31, 287–302. [Google Scholar] [CrossRef]
- Liu, Q.; Navrotsky, A. Synthesis of nitrate sodalite: An in situ scanning calorimetric study. Geochim. Cosmochim. Acta 2007, 71, 2072–2078. [Google Scholar] [CrossRef]
- Fan, W.; Morozumi, K.; Kimura, R.; Yokoi, T.; Okubo, T. Synthesis of nanometer-sized sodalite without adding organic additives. Langmuir 2008, 24, 6952–6958. [Google Scholar] [CrossRef] [PubMed]
- Geon, J.K.; Wha, S.A. Synthesis and characterization of iron-modified ZSM-5. Appl. Catal. 1991, 71, 55–68. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. RSC Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Tounsi, H.; Mseddi, S.; Djemel, S. Preparation and characterization of Na-LTA zeolite from Tunisian sand and aluminum scrap. Phys. Procedia 2009, 2, 1065–1074. [Google Scholar] [CrossRef]
- Mofrad, A.M.; Peixoto, C.; Blumeyer, J.; Liu, J.; Hunt, H.K.; Hammond, K.D. Vibrational spectroscopy of sodalite: Theory and experiments. J. Phys. Chem. C 2018, 122, 24765–24779. [Google Scholar] [CrossRef]
- Khajavi, S.; Kapteijn, F.; Jansen, J.C. Synthesis of thin defect-free hydroxy sodalite membranes: New candidate for activated water permeation. J. Membr. Sci. 2007, 299, 63–72. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Helffrich, G.R.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaAlSiO4-NaCl. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef]
- Gaidoumi, A.E.; Benabdallah, A.C.; Bali, B.E.; Kherbeche, A. Synthesis and characterization of zeolite HS using natural pyrophyllite as new clay source. Arabian J. Sci. Eng. 2018, 43, 191–197. [Google Scholar] [CrossRef]




| Sample | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | TiO2 | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Bentonite | 67.73 | 11.54 | 1.75 | 1.64 | 1.74 | 0.01 | 0.09 | 0.28 | 15.30 |
| NO. | Bentonite (g) | NaOH (g) | NaAlO2 (g) | T (°C) | t (h) | H2O (mL) |
|---|---|---|---|---|---|---|
| Sod-Na/Si-3 | 1 | 0.99 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-6 | 1 | 2.35 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-8 | 1 | 3.25 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-10 | 1 | 4.15 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-12 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-14 | 1 | 5.96 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-20 | 1 | 8.67 | 0.74 | 90 | 12 | 35 |
| Sod-Si/Al-0.5 | 1 | 4.61 | 1.67 | 90 | 12 | 35 |
| Sod-Si/Al-0.75 | 1 | 4.91 | 1.05 | 90 | 12 | 35 |
| Sod-Si/Al-1.0 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-Si/Al-1.5 | 1 | 5.21 | 0.43 | 90 | 12 | 35 |
| Sod-Si/Al-2.0 | 1 | 5.28 | 0.28 | 90 | 12 | 35 |
| Sod-T-60 | 1 | 5.06 | 0.74 | 60 | 12 | 35 |
| Sod-T-70 | 1 | 5.06 | 0.74 | 70 | 12 | 35 |
| Sod-T-80 | 1 | 5.06 | 0.74 | 80 | 12 | 35 |
| Sod-T-90 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-T-100 | 1 | 5.06 | 0.74 | 100 | 12 | 35 |
| Sod-t-4 | 1 | 5.06 | 0.74 | 90 | 4 | 35 |
| Sod-t-8 | 1 | 5.06 | 0.74 | 90 | 8 | 35 |
| Sod-t-10 | 1 | 5.06 | 0.74 | 90 | 10 | 35 |
| Sod-t-12 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-t-16 | 1 | 5.06 | 0.74 | 90 | 16 | 35 |
| Sod-t-20 | 1 | 5.06 | 0.74 | 90 | 20 | 35 |
| Sod-t-24 | 1 | 5.06 | 0.74 | 90 | 24 | 35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Sun, H.; Peng, T.; He, Q. One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures. Minerals 2018, 8, 521. https://doi.org/10.3390/min8110521
Liu B, Sun H, Peng T, He Q. One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures. Minerals. 2018; 8(11):521. https://doi.org/10.3390/min8110521
Chicago/Turabian StyleLiu, Bo, Hongjuan Sun, Tongjiang Peng, and Qian He. 2018. "One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures" Minerals 8, no. 11: 521. https://doi.org/10.3390/min8110521





