An Isotope Study of the Dzhida Mo–W Ore Field (Western Transbaikalia, Russia)
Abstract
:1. Introduction
2. Geological Background
2.1. The Pervomaika Deposit
2.2. The Inkur Deposit
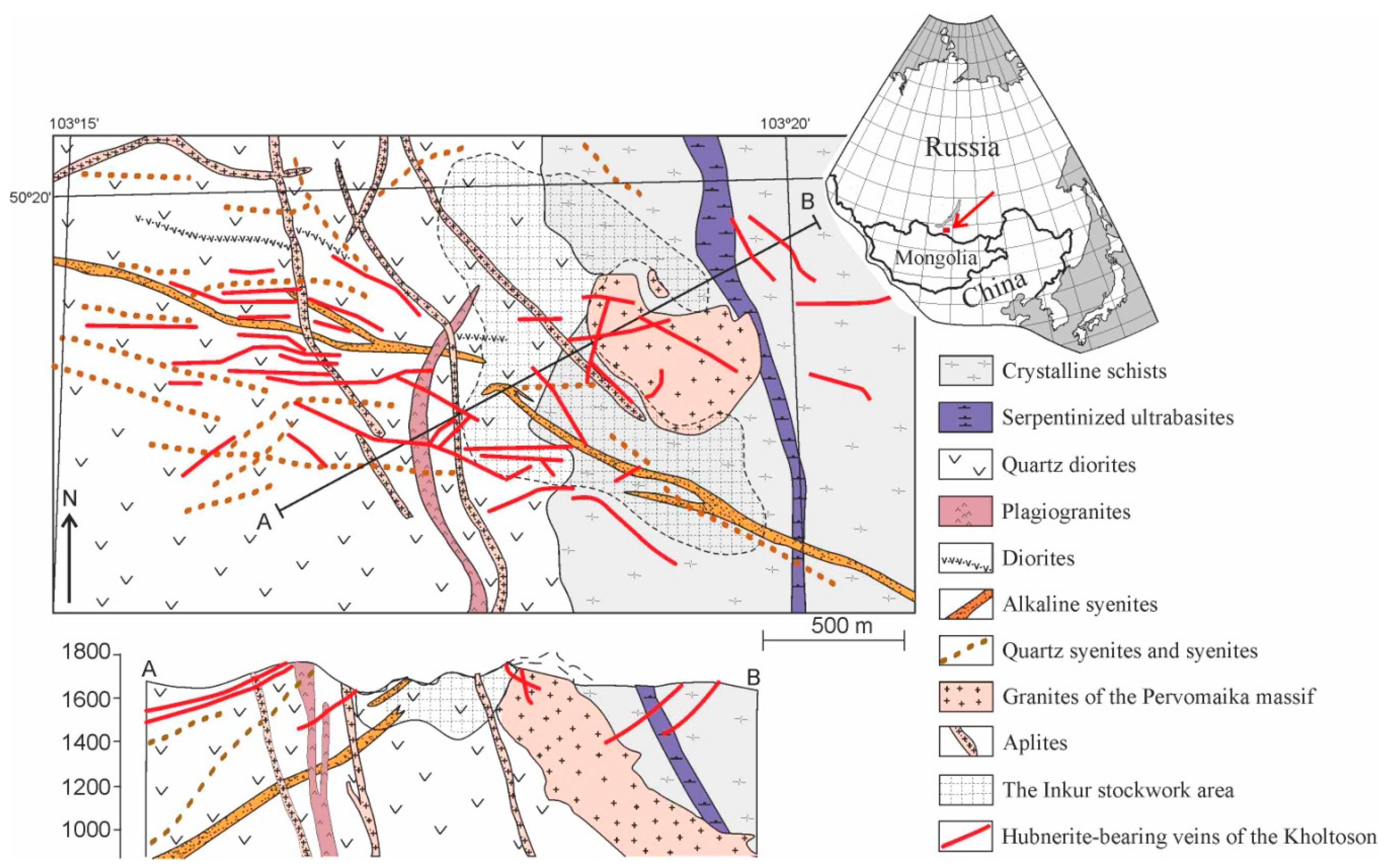
2.3. The Kholtoson Deposit
3. Analytical Methods
4. Results
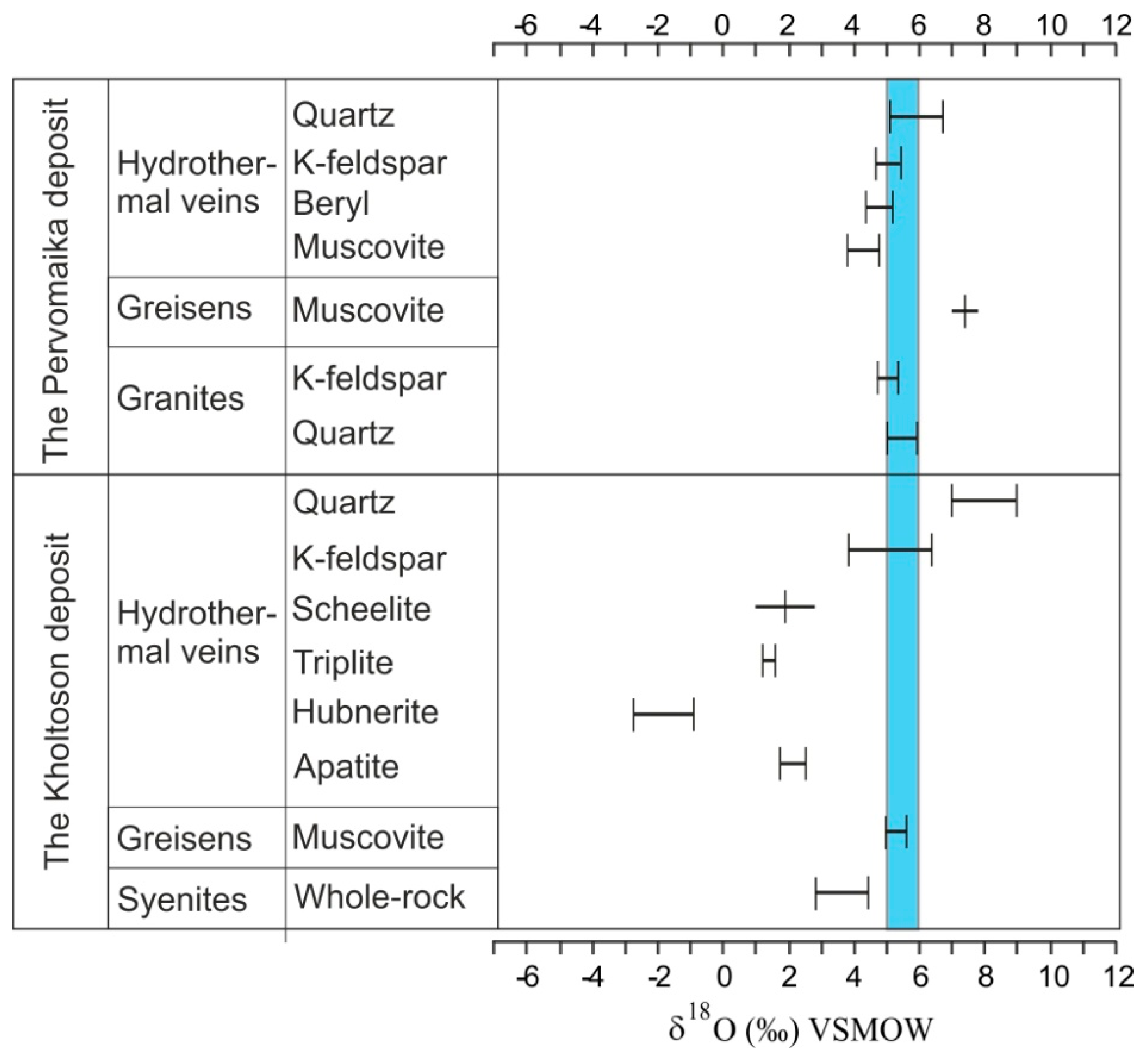
4.1. O, C, H Isotope Data
4.1.1. The Pervomaika Deposit
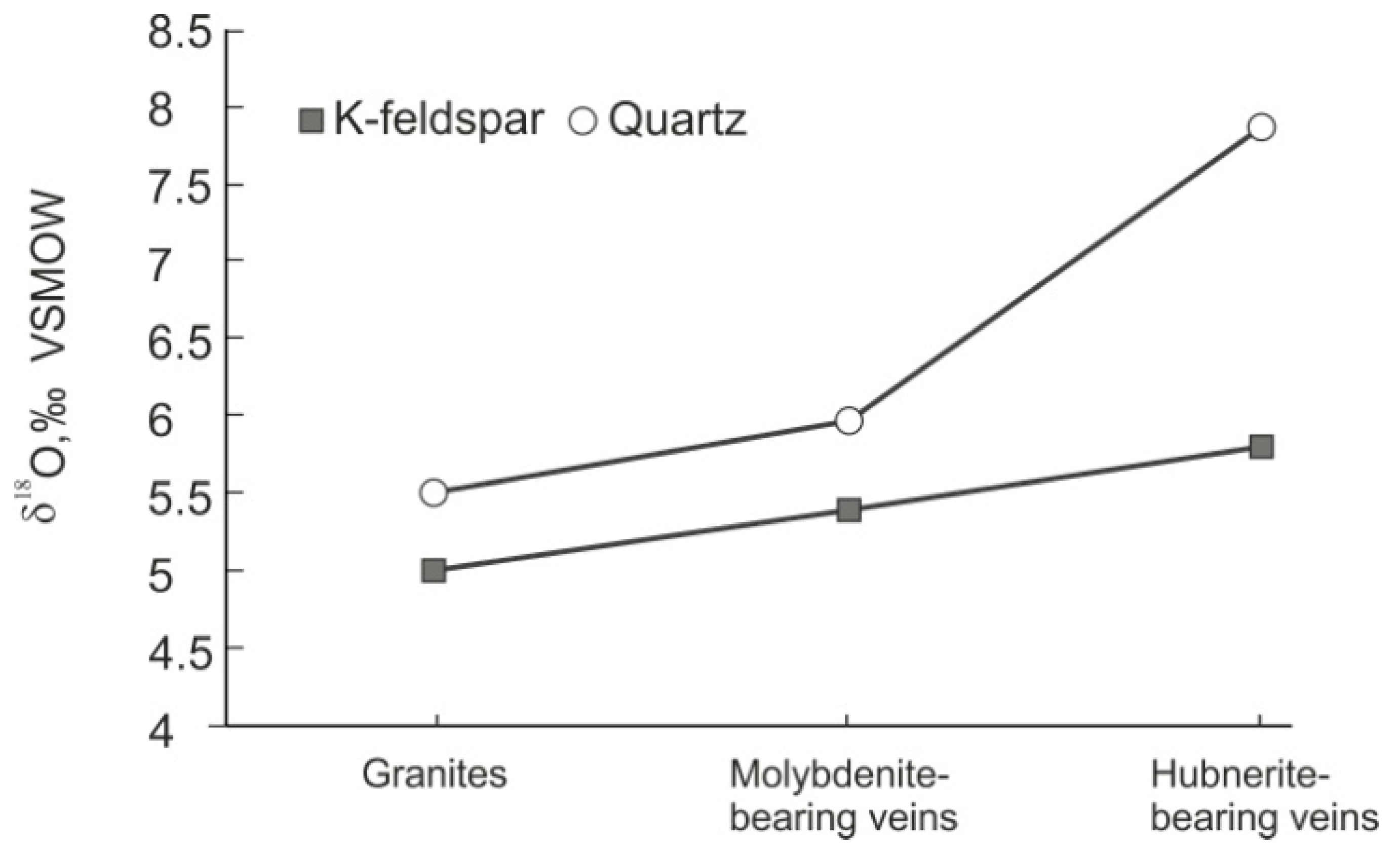
4.1.2. The Kholtoson Deposit
4.2. S Isotope Data
4.3. Sr, Nd Isotope Data
5. Discussion
6. Conclusions
- Granites in the Pervomaika massif were formed as a result of the melting of the crustal substrate following exposure to high-temperature mantle fluid. The low δ18O values and initial ratios of 87Sr/86Sr and the positive values of εNd (T) provide evidence of this formation.
- Components of deep (mantle) source such as F, S, and CO2 were involved in the formation of the Dzhida ore field.
- The ore-forming fluids included waters from the meteoric source at the later stages of deposit formation.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.-J.; Pirajno, F.; Li, N.; Deng, X.-H. Molybdenum deposits in China. Ore Geol. Rev. 2017, 81, 401–404. [Google Scholar] [CrossRef]
- Zeng, Q.-D.; Sun, Y.; Chu, S.-X.; Duan, X.-X.; Liu, J. Geochemistry and geochronology of the Dongshanwan porphyry Mo–W. deposit, Northeast China: Implications for the Late Jurassic tectonic setting. J. Asian Earth Sci. 2015, 97, 472–485. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, X.; Ding, Z.; Cawood, P.A.; Yue, S. Geology, geochronology and isotopic geochemistry of the Xiaoliugou W-Mo ore field in the Qilian Orogen, NW China: Case study of a skarn system formed during continental collision. Ore Geol. Rev. 2017, 81, 575–586. [Google Scholar] [CrossRef]
- Yang, Y.F.; Li, N.; Chen, Y.J. Fluid inclusion study of the Nannihu giant porphyry Mo-W deposit, Henan Province, China: Implications for the nature of porphyry ore-fluid systems formed in continental collision regime. Ore Geol. Rev. 2012, 46, 83–94. [Google Scholar] [CrossRef]
- Deng, X.H.; Chen, Y.J.; Santosh, M.; Wang, J.B.; Li, C.; Yue, S.W.; Zheng, Z.; Chen, H.J.; Tang, H.S.; Dong, L.H.; et al. U–Pb zircon, Re–Os molybdenite geochronology and Rb–Sr geochemistry from the Xiaobaishitou W(–Mo) deposit: Implications for Triassic tectonic setting in eastern Tianshan, NW China. Ore Geol. Rev. 2017, 80, 332–351. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.-J.; Deng, X.-H. Mineralization mechanisms in the Shangfanggou giant porphyry-skarn Mo–Fe deposit of the east Qinling, China: Constraints from H–O–C–S–Pb isotopes. Ore Geol. Rev. 2017, 81, 535–547. [Google Scholar] [CrossRef]
- Li, G.M.; Cao, M.; Qin, K.; Evans, N.J.; Hollings, P.; Seitmuratova, E.Y. Geochronology, petrogenesis and tectonic settings of pre- and syn-ore granites from the W-Mo deposits (East Kounrad, Zhanet and Akshatau), Central Kazakhstan. Lithos 2016, 252–253, 16–31. [Google Scholar] [CrossRef]
- Chernyshev, I.V.; Gol’tsman, Y.V.; Bairova, E.D.; Ivanova, G.F. Rb-Sr geochronometry of sequential granite formation, greisenization, and hydrothermal mineralization: Evidence from the Dzhida W-Mo deposit, Western Transbaikal region. Dokl. Earth Sci. 1998, 360, 613–616. [Google Scholar]
- Chernyshev, I.V.; Agapova, A.A.; Troitsky, V.A. Pb-Pb isotope characteristics and problem of the source of large-scale W-Mo mineralization in the Dzhida ore field (Western Transbaikalia). Dokl. Earth Sci. 1999, 366, 819–822. [Google Scholar]
- Matveeva, S.S.; Spasennikh, M.Y.; Sushevskaya, T.M. Geochemical model of formation of the Spokininsky deposit (East Transbaikalia). Geol. Ore Depos. 2002, 44, 125–132. (In Russian) [Google Scholar]
- Breiter, K. Nearly contemporaneous evolution of the A-and S-type fractionated granites in the Krušné hory/Erzgebirge Mts.; Central Europe. Lithos 2012, 151, 105–121. [Google Scholar] [CrossRef]
- Gomes, M.; Neiva, A. Petrogenesis of tin-bearing granites from Ervedosa, northern Portugal: The importance of magmatic processes. Chem. der Erde-Geochem. 2002, 62, 47–72. [Google Scholar] [CrossRef]
- Raimbault, L.; Cuney, M.; Azencott, C.; Duthou, J.; Joron, J.L. Geochemical evidence for a multistage magmatic genesis of Ta-Sn-Li mineralization in the granite at Beauvoir, French Massif Central. Econ. Geol. 1995, 90, 548–576. [Google Scholar] [CrossRef]
- Kelly, W.C.; Rye, R.O. Geologic, fluid inclusion, and stable isotope studies of the tin-tungsten deposits of Panasqueira, Portugal. Econ. Geol. 1979, 74, 1721–1822. [Google Scholar] [CrossRef]
- Siegel, K.; Williams-Jones, A.E.; Van Hinsberg, V.J. The amphiboles of the REE-rich A-type peralkaline Strange Lake pluton—Fingerprints of magma evolution. Lithos 2017, 288, 156–174. [Google Scholar] [CrossRef]
- Khoshnoodi, K.; Behzadi, M.; Gannadi-Maragheh, M.; Yazdi, M. Alkali Metasomatism and Th-REE Mineralization in the Choghart deposit, Bafq district, Central Iran. Geol. Croat. 2017, 70, 53–69. [Google Scholar] [CrossRef]
- Beus, A.A.; Severov, A.S.; Sitin, A.A.; Subbotin, K.D. Albitized and Greisenized Granites (Apogranites); The USSR Academy of Sciences: Moscow, Russia, 1962; 193p. (In Russian) [Google Scholar]
- Neiva, A. Portuguese granites associated with Sn-W and Au mineralizations. Bull.-Geol. Soc. Finl. 2002, 74, 79–101. [Google Scholar] [CrossRef]
- Taylor, B.E.; O’Neil, J.R. Stable isotope studies of metasomatic Ca-Fe-Al-Si-skarns and associated metamorphic and igneous rocks Osgood mountains, Nevada. Contrib. Mineral. Petrol. 1977, 63, 1–50. [Google Scholar] [CrossRef]
- Burtseva, M.V.; Ripp, G.S.; Posokhov, V.F.; Murzintseva, A.E. Nephrites of East Siberia: Geochemical features and problems of genesis. Russ. Geol. Geophys. 2015, 56, 516–527. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Heaton, T.; Wall, F.; Gunn, G. Tracing the fluid source of heavy REE mineralisation in carbonatites using a novel method of oxygen-isotope analysis in apatite: The example of Songwe Hill, Malawi. Chem. Geol. 2016, 440, 275–287. [Google Scholar] [CrossRef]
- Johnson, T.M.; Ripley, E.M. Hydrogen and oxygen isotopic systematic of berillium mineralisation Spor Mauntain, Utah. Geol. Soc. Am. Abstr. Progr. 1998, 30, 127. [Google Scholar]
- Christiansen, E.H.; Bikun, J.V.; Sheridan, M.F.; Burt, D.M. Geochemical evolution of topaz rhyolites from the Thomas Range and Spor Mountain, Utah. Am. Mineral. 1984, 69, 223–236. [Google Scholar]
- Sheglov, A.D. Fundamentals of Metallogenic Analysis; Nedra: Moscow, Russia, 1976; 295p. (In Russian) [Google Scholar]
- Baturina, E.E.; Ripp, G.S. Mo-W Deposits of the Western Transbaikalia; Nauka: Moscow, Russia, 1984; 152p. (In Russian) [Google Scholar]
- Ripp, G.S. Geochemistry of Endogenous Mineralization and Forecasting Criteria in Folded Regions; Nauka: Moscow, Russia, 1984; 192p. (In Russian) [Google Scholar]
- Kushnarev, M.P. The Structure of the Dzhida Deposit; Academy of Sciences of the USSR: Moscow, Russia, 1954; 140p. (In Russian) [Google Scholar]
- Ignatovich, V.I. Mineral and raw materials base of molybdenum. Razvedka I ohrana nedr 2007, 12, 37–43. (In Russian) [Google Scholar]
- Povilaitis, M.M. Endogenous Deposits of Tungsten and the Conditions for Their Formation; Nedra: Moscow, Russia, 1979; 152p. (In Russian) [Google Scholar]
- Ontoev, D.O. Stages of Mineralization and Zoning of the Deposits of Transbaikalia; Nauka: Moscow, Russia, 1974; 244p. (In Russian) [Google Scholar]
- Pokalov, V.T. Age and geotectonics, the position of W-Mo mineralization in the Dzhida region of the Western Transbaikalia. Dokl. Earth Sci. 1979, 247, 678–681. [Google Scholar]
- Ivanova, G.F.; Smirnova, O.K.; Ignatenko, K.I. Chemical composition of the wolframite-mineralization of the Dzhida ore field. Zap. Russ. Mineral. Soc. 1991, 4, 77–88. (In Russian) [Google Scholar]
- Borshchevsky, Y.A.; Apeltsin, F.R.; Borisova, S.L. Isotope composition of oxygen of wolframite from tungsten deposits of various formation and genetic types. Zap. Russ. Mineral. Soc. 1980, 4, 633–643. (In Russian) [Google Scholar]
- Platov, V.S.; Savchenko, A.A.; Ignatov, A.M.; Gorokhovsky, D.V. State Geological Map of the Russian Federation; Scale 1: 1000000 (Third Generation) Aldan-Transbaikalian Series; M-48. Ulan-Ude; VSEGEI: St. Petersburg, Russia, 2009; 271p. (In Russian) [Google Scholar]
- Shapenko, V.V. Genetic features of tungsten mineralization of the Dzhida ore field (South-Western Transbaikalia). Geol. Ore Depos. 1982, 5, 18–29. [Google Scholar]
- Reyf, F.G. Ore-Forming Potential of Granites and Conditions for Its Realization; Nauka: Moscow, Russia, 1990; p. 180. (In Russian) [Google Scholar]
- Coplen, T.B. Normalization of oxygen and hydrogen data. Chem. Geol. 1988, 72, 293–297. [Google Scholar]
- Friedman, I.; O’Neil, J.; Cebula, G. Two new carbonate stable isotope standards. Geostand. Newsl. 1982, 6, 11–12. [Google Scholar] [CrossRef]
- Beaudoin, G.; Therrien, P. The updated web stable isotope fractionation calculator. In Handbook of Stable Isotope Analytical Techniques; Groot, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1120–1122. [Google Scholar]
- Zhang, X.P.; Yao, T.D. World spatial characteristics of oxygen isotope ratio in precipitation. J. Glaciol. Geocryol. 1994, 16, 202–210. [Google Scholar]
- Zheng, Y.-F. Oxygen isotope fractionation in wolframite. Eur. J. Mineral. 1992, 4, 1331–1335. [Google Scholar] [CrossRef]
- Zheng, Y.-F. Calculation of oxygen isotope fractionation in anhydrous silicate minerals. Geochim. Cosmochim. Acta 1993, 57, 1079–1091. [Google Scholar] [CrossRef]
- Zheng, Y.-F. Calculation of oxygen isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Vennemann, T.W.; O’Neil, J.R. A simple and inexpensive method of hydrogen isotope and water analyses of minerals and rocks based on zinc reagent. Chem. Geol. (Isot. Geosci. Sect.) 1993, 103, 227–234. [Google Scholar] [CrossRef]
- Grinenko, V.A. Preparation of sulfur dioxide for isotopic analysis. Inorg. Chem. 1962, 7, 2478–2482. (In Russian) [Google Scholar]
- Savatenkov, V.M.; Morozova, I.M.; Levsky, L.K. Behavior of the Sm-Nd, Rb-Sr, K-Ar, and U-Pb isotopic systems during alkaline metasomatism: Fenites in the outer-contact zone of an ultramafic-alkaline intrusion. Geochem. Int. 2004, 10, 899–920. [Google Scholar]
- Wickham, S.M.; Alberts, A.D.; Zanvilevich, A.N.; Litvinovsky, B.A.; Bindeman, J.N.; Schanble, E.A. A stable Isotope Study of Anorogenic Magmatism in East Central Asia. Petrology 1996, 37, 1063–1095. [Google Scholar] [CrossRef]
- Litvinovsky, B.A.; Tsygankov, A.A.; Jahn, B.M.; Katzir, Y.; Be’eri-Shlevin, Y. The Late Paleozoic post-collisional igneous province of Transbaikalia (Russia). Lithos 2011, 125, 845–874. [Google Scholar] [CrossRef]
- Tsygankov, A.A. Late paleozoic granitoids in Western Transbaikalia: Sequence of formation, sources of magmas, and geodynamics. Russ. Geol. Geophys. 2014, 2, 153–176. [Google Scholar] [CrossRef]
- Taylor, H.P.; Frechen, J.; Degens, E.T. Oxygen and carbon isotope studies of carbonatites from the Laacher See district, West Germany and the Alno district, Sweden. Geochim. Cosmochim. Acta 1967, 31, 407–430. [Google Scholar] [CrossRef]
- Yang, J.; Barling, J.; Siebert, C.; Fietzke, J.; Stephens, E.; Halliday, A.N. The molybdenium isotopic compositions of I-S and A-type granitic suites. Geochim. Cosmochim. Acta 2017, 205, 168–186. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 2009; 286p. [Google Scholar]
- Letnikov, F.A. Fluid regime of endogenic proceces and ore-formation processes. Russ. Geol. Geophys. 2006, 47, 1296–1308. [Google Scholar]
- Chukhrov, F.V.; Ermilova, L.P.; Vinogradov, V.I. Isotopic Composition and Origin of Sulfur of Some Tungsten Deposits in Central Kazakhstan. Isotopes of Sulfur and Ore Formation; Nauka: Moscow, Russia, 1987; pp. 46–58. (In Russian) [Google Scholar]
- Borovikov, A.A.; Borisenko, A.S.; Shabalin, S.I.; Goverdovskiy, V.A.; Bryanskiy, N.V. Composition and metal contents of ore-forming fluids of the Kalguty Mo-W(Be) deposit (Gorny Altai). Russ. Geol. Geophys. 2016, 4, 507–518. [Google Scholar] [CrossRef]


| ID No. | Sample No. | Description | Mineral | δ18O‰ | δ18O‰ | Proportion of Meteoric Water (%) |
|---|---|---|---|---|---|---|
| VSMOW | Fluid | |||||
| Granite (750 °C) | ||||||
| 1 | P-15-1a | Euhedral grains | Quartz | 5.9 | 4.6 | 13.7 |
| 2 | P-15-1б | Interstitial grains | K-feldspar | 5.3 | 5.3 | 11.2 |
| 3 | P-15-2a | Euhedral grains | Quartz | 5.5 | 4.2 | 15.1 |
| 4 | P-15-2б | K-feldspar | 4.7 | 4.7 | 13.3 | |
| Pre-ore stage (Greisen, 350 °C) | ||||||
| 5 | P-15-9 | Aggregates of dense bladed crystals | Muscovite | 7.3 | 6.7 | 6.3 |
| Hydrothermal stage (350 °C) | ||||||
| 6 | P-15-7 | Lamellar aggregate from quartz–molybdenite veins | Muscovite | 3.9 | 3.3 | 18.3 |
| 7 | P-17-1 | Muscovite | 4.8 | 4.2 | 15.1 | |
| 8 | P-15-7б | Euhedral grains from quartz–K-feldspar–beryl veinlets | K-feldspar | 3.8 | 0.7 | 27.4 |
| 9 | P-15-6 | K-feldspar | 5.4 | 2.3 | 21.8 | |
| 10 | P-15-7a | Beryl | 4.5 | 5.0 | 12.3 | |
| 11 | P-17-2 | Beryl | 5.3 | 5.8 | 9.5 | |
| 12 | P-15-1 | Quartz | 6.5 | 0.9 | 26.7 | |
| 13 | P-15-2/1 | Quartz | 5.3 | −0.3 | 30.9 | |
| 14 | P-15-3 | Quartz–molybdenite veinlets | Quartz | 5.7 | 0.1 | 29.5 |
| 15 | P-15-4 | Quartz | 5.7 | 0.1 | 29.5 | |
| 16 | P-15-5 | Quartz | 6.6 | 1.0 | 26.3 | |
| ID No. | Sample No. | Description | Mineral | δ18O‰ | δ18O‰ | Proportion of Meteoric Water (%) |
|---|---|---|---|---|---|---|
| VSMOW | Fluid | |||||
| Syenite | ||||||
| 1 | 113-46 | Whole-rock | 2.9 | |||
| 2 | KH-17 | Whole-rock | 4.4 | |||
| Pre-ore stage (Greisen 350 °C) | ||||||
| 3 | Dzh-1C | Lamellar aggregate | Muscovite | 4.9 | 4.6 | 13.7 |
| 4 | KH-15-6 | Muscovite | 5.3 | 5.0 | 12.3 | |
| 5 | KH-15-1 | Muscovite | 5.0 | 4.7 | 13.3 | |
| 6 | KH-15-2 | Muscovite | 5.5 | 5.2 | 11.6 | |
| 7 | KH-15-5a | Muscovite | 5.3 | 5.0 | 12.28 | |
| Ore stage (300 °C) | ||||||
| 8 | Dzh-1к | Quartz–hubnerite veins | Quartz | 7.3 | 0.3 | 28.8 |
| 9 | Dzh-13/87 | Quartz | 8.8 | 1.8 | 23.5 | |
| 10 | KH-15-9a | Quartz | 7.2 | 0.2 | 29.1 | |
| 11 | KH-15-6a | Quartz | 9.7 | 2.7 | 20.4 | |
| 12 | KH-15-13 | Quartz | 8.5 | 1.5 | 24.6 | |
| 13 | KH-15-12/1 | Quartz | 7.4 | 0.4 | 28.4 | |
| 14 | KH-12/2 | Quartz | 7.8 | 0.8 | 27.0 | |
| 15 | KH-15-18 | Quartz | 9.1 | 2.1 | 22.5 | |
| 16 | KH-15-5 | Quartz | 8.7 | 1.7 | 23.9 | |
| 17 | KH-15-11 | Quartz–carbonate–hubnerite veins | Quartz | 7.3 | 0.3 | 28.8 |
| 18 | KH-15-10a | Quartz | 7.0 | 0 | 29.8 | |
| 19 | KH-15-8 | Quartz | 7.7 | 0.7 | 27.4 | |
| 20 | GM-1210 | Quartz | 8.9 | 1.9 | 23.2 | |
| 21 | KH-15-9б | Euhedral grains from quartz–hubnerite veins | K-feldspar | 5.6 | 1.4 | 24.9 |
| 22 | Dzh-13/87 | K-feldspar | 6.4 | 2.2 | 22.1 | |
| 23 | KH-15-8a | K-feldspar | 5.7 | −0.4 | 31.2 | |
| 24 | KH-15-14a | K-feldspar | 5.8 | 1.6 | 24.2 | |
| 25 | KH-15-11a | K-feldspar | 4.8 | 0.6 | 27.7 | |
| 26 | KH-15-10 | K-feldspar | 5.9 | 1.7 | 23.9 | |
| 27 | KH-15-7б | Euhedral grains from quartz–carbonate–hubnerite veins | K-feldspar | 3.8 | 1.5 | 24.6 |
| 28 | KH-15-14 | Euhedral grains from quartz–hubnerite veins | Hubnerite | −1.1 | 0.7 | 27.4 |
| 29 | Dzh-1в | Hubnerite | −0.7 | 1.1 | 26.0 | |
| 30 | KH-15-13 | Hubnerite | −1.1 | 0.7 | 27.4 | |
| 31 | KH-15-15 | Hubnerite | −2.7 | −0.9 | 33.0 | |
| 32 | KH-15-10 | Hubnerite | −2.5 | −0.7 | 32.3 | |
| 33 | GM-12-10 | Euhedral grains from quartz–carbonate–hubnerite veins | Hubnerite | −0.9 | 0.9 | 26.7 |
| 34 | P-15-9a | Hubnerite | −1.4 | 0.4 | 28.4 | |
| 35 | P-18-4 | Hubnerite | −1.4 | 0.4 | 28.4 | |
| 36 | Dzh 13/87 | Triplite | 1.5 | |||
| 37 | Dzh-2 | Triplite | 1.1 | |||
| 38 | GM-1210 | Scheelite | 1.9 | −0.8 | 32.6 | |
| 39 | P-18-2 | Apatite | 2.6 | |||
| 40 | P-18-4 | Apatite | 1.9 | |||
| Sample | GM-1210 | KH-15-10a | KH-15-13 | KH-15-9a | KH-15-5 | Sample | KH-15-8 | Dzh-13/87 | KH-15-11 |
|---|---|---|---|---|---|---|---|---|---|
| Quartz | 8.9 | 7.0 | 8.5 | 7.2 | 8.7 | Quartz | 7.7 | 8.8 | 7.3 |
| Hubnerite | −0.9 | −2.5 | −1.1 | −1.4 | −1.1 | K-feldspar | 5.7 | 6.4 | 4.8 |
| ∆ | 9.8 | 9.5 | 9.6 | 8.6 | 9.8 | ∆ | 2.0 | 2.4 | 2.5 |
| T (Q-Hb) | 277 | 290 | 286 | 333 | 281 | T(Q-Kfs) | 391 | 313 | 297 |
| Sample No. | Mineral | δ18O, ‰ VSMOW | δ13C, ‰ VPDB |
|---|---|---|---|
| Dzh-1 | Rhodochrosite | 9.3 | −7.0 |
| 23/4 | Rhodochrosite | 6.8 | −5.7 |
| P-1 | Rhodochrosite | 7.8 | −7.5 |
| P-2 | Ankerite | 9.2 | −4.4 |
| P-16 | Ankerite | 9.8 | −4.2 |
| KH-15-18 | Ankerite | 8.14 | −3.8 |
| 1300 | Calcite | 5.8 | −8.9 |
| Sample No. | Description | Mineral | δ34S‰ VCTD |
|---|---|---|---|
| The Pervomaika deposit | |||
| 201/1 | Blade crystals from quartz–molybdenite veinlets | Molybdenite | 2.1 |
| 201/2 | Molybdenite | 0.3 | |
| 201/3 | Molybdenite | 1.5 | |
| C-160 | Blade crystals from molybdenite veinlets | Molybdenite | −0.2 |
| P-17 | Molybdenite | −0.2 | |
| P-C-223 | Euhedral crystals from quartz–molybdenite veinlets | Pyrite | −1.3 |
| P-C-223-1 | Pyrite | −1.8 | |
| P-C-228 | Pyrite | −0.9 | |
| P-C-123 | Pyrite | 0.9 | |
| P-C-123-1a | Sphalerite | −1.9 | |
| P-C-123-2 | Sphalerite | 1.1 | |
| The Inkur deposit | |||
| 105-78 | Euhedral crystals from quartz–K-feldspar–hubnerite veinlets | Pyrite | −0.7 |
| 105-78-1 | Sphalerite | −2.2 | |
| 105-78-2 | Chalcopyrite | 1.2 | |
| The Kholtoson deposit | |||
| 1615-4 | Euhedral crystals from quartz–sulfide–hubnerite veins | Pyrite | 0.3 |
| 1370-6 | Pyrite | 2.4 | |
| 182-383 | Pyrite | 2.3 | |
| C-33-182 | Pyrite | 1.3 | |
| 247-1 | Sphalerite | 2.2 | |
| 1370 | Chalcopyrite | −0.4 | |
| 1370-1 | Euhedral grains from quartz–carbonate–hubnerite veins | Galena | −5.3 |
| C-105-15 | Galena | 1.5 | |
| 1700 | Galena | −0.9 | |
| 247 | Galena | −1.8 | |
| 247-1a | Galena | −1.8 | |
| 1789 | Galena | −6.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripp, G.S.; Smirnova, O.K.; Izbrodin, I.A.; Lastochkin, E.I.; Rampilov, M.O.; Posokhov, V.F. An Isotope Study of the Dzhida Mo–W Ore Field (Western Transbaikalia, Russia). Minerals 2018, 8, 546. https://doi.org/10.3390/min8120546
Ripp GS, Smirnova OK, Izbrodin IA, Lastochkin EI, Rampilov MO, Posokhov VF. An Isotope Study of the Dzhida Mo–W Ore Field (Western Transbaikalia, Russia). Minerals. 2018; 8(12):546. https://doi.org/10.3390/min8120546
Chicago/Turabian StyleRipp, German S., Olga K. Smirnova, Ivan A. Izbrodin, Eugeny I. Lastochkin, Mikhail O. Rampilov, and Viktor F. Posokhov. 2018. "An Isotope Study of the Dzhida Mo–W Ore Field (Western Transbaikalia, Russia)" Minerals 8, no. 12: 546. https://doi.org/10.3390/min8120546




