Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manganese Nodule Sample
2.2. Smelter Slag
2.3. Reagent and Leaching Test
2.4. Experimental Design
2.5. MnO2/Fe Ratio Effect
2.6. The Effect of Particle Size
3. Results and Discussion
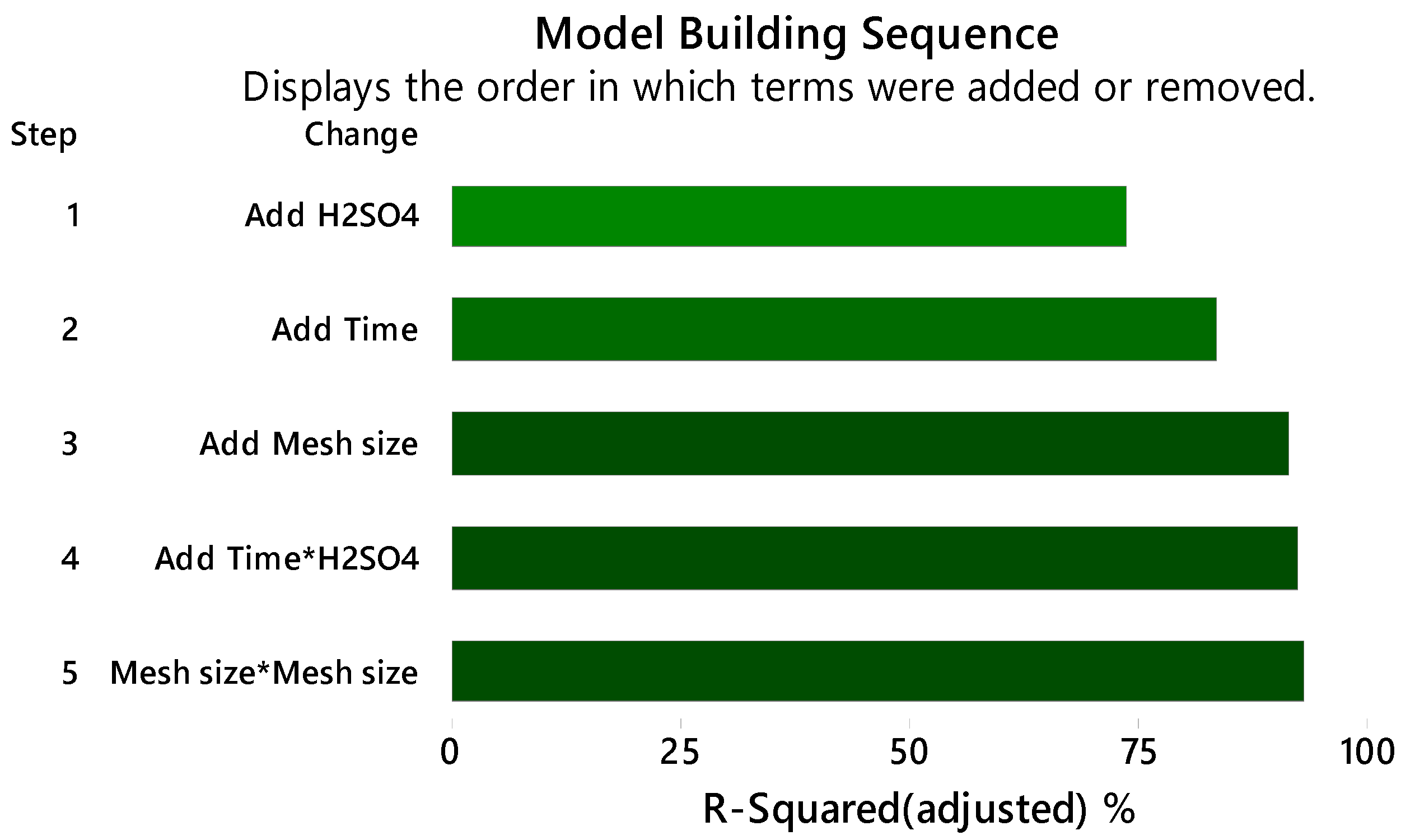
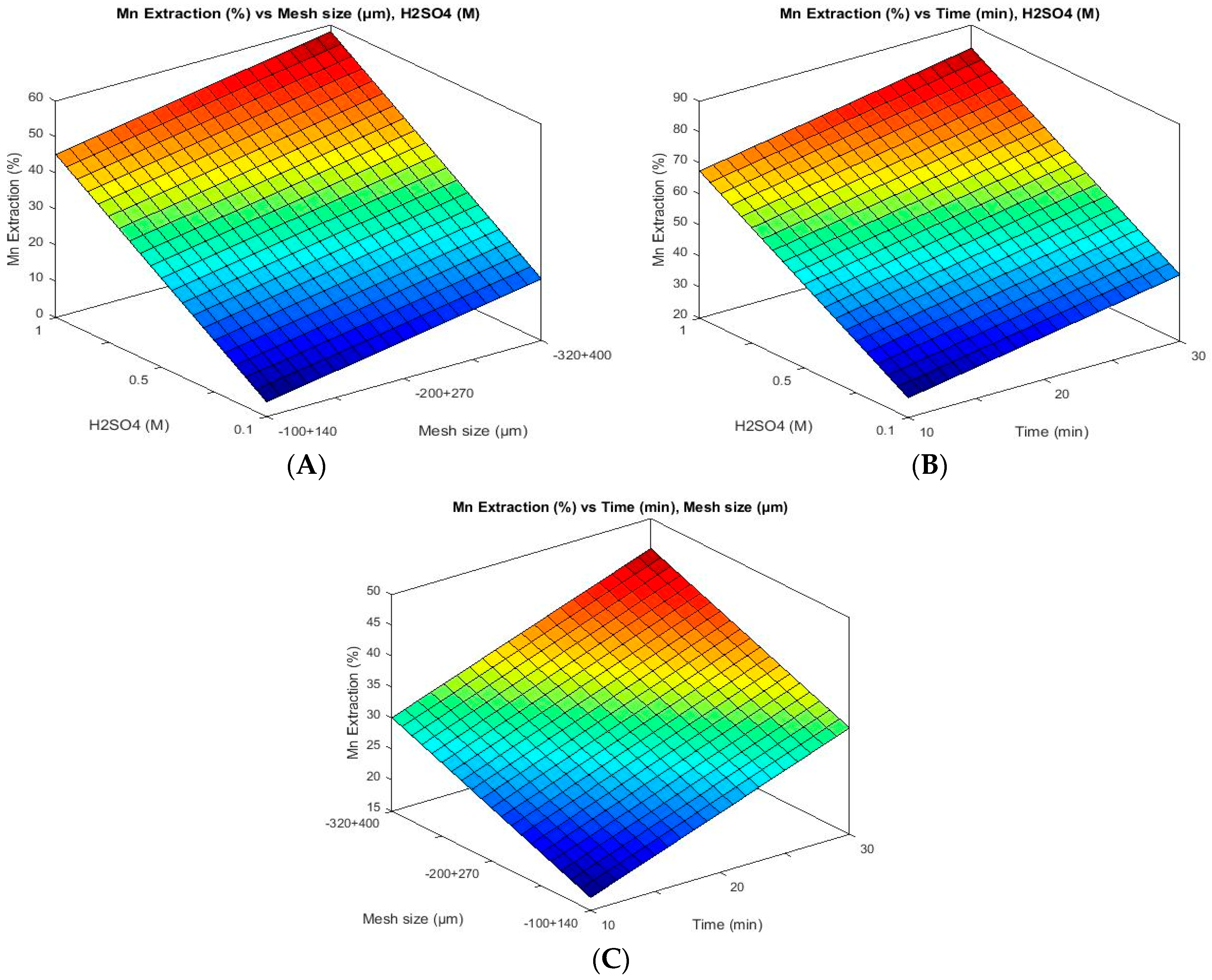
3.1. Methodology
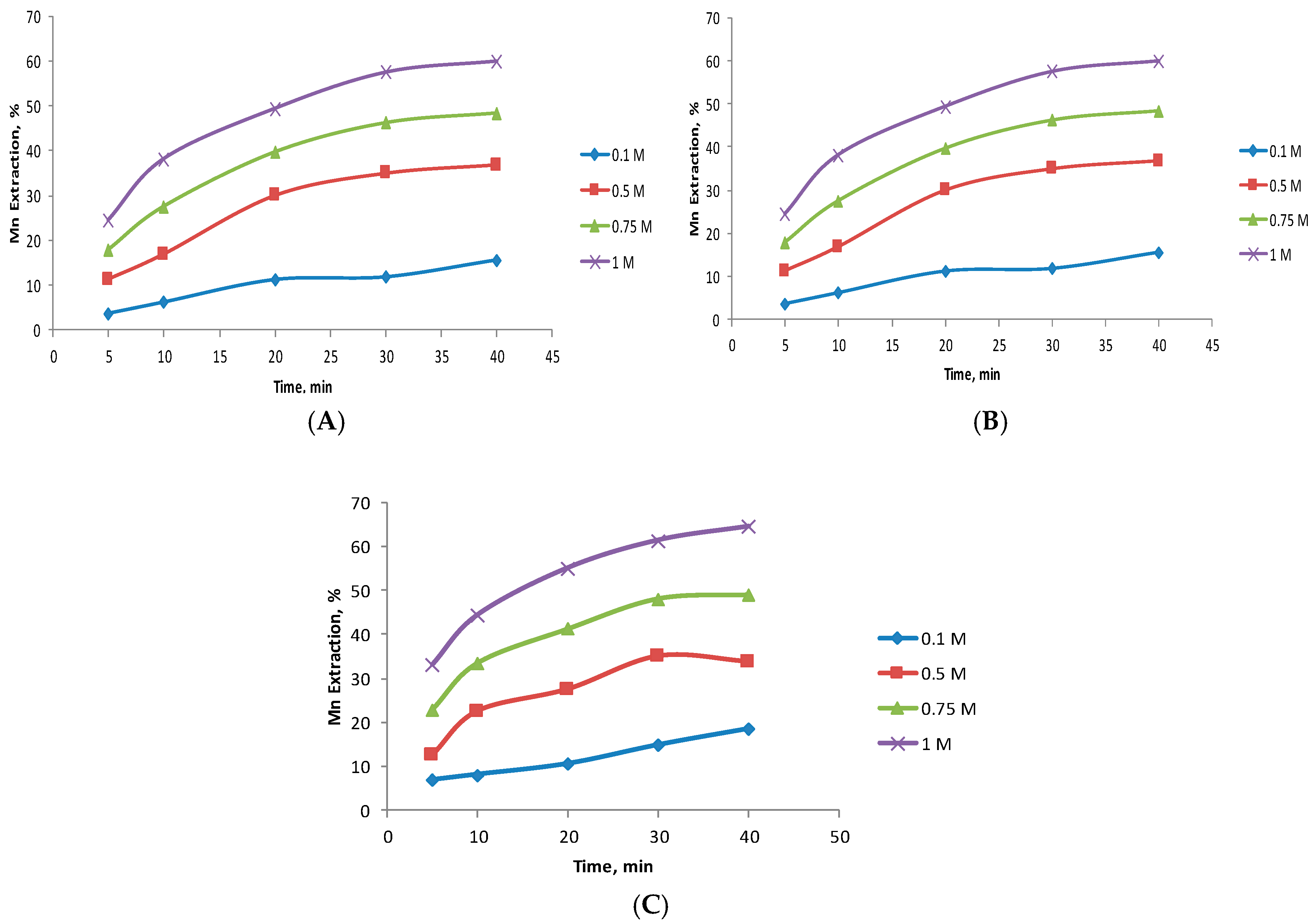
3.2. Effect of the MnO2/Fe Ratio
3.3. Effect of Particle Size
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somoza, L.; González, F.; León, R.; Medialdea, T.; De Torres, T.; Ortiz, J.; Lunar, R.; Martínez-Frías, J.; Merinero, R. Ferromanganese nodules and micro-hardgrounds associated with the Cadiz Contourite Channel (NE Atlantic): Palaeoenvironmental records of fluid venting and bottom currents. Chem. Geol. 2012, 310–311, 56–78. [Google Scholar]
- Senanayake, G. Acid leaching of metals from deep-sea manganese nodules—A critical review of fundamentals and applications. Miner. Eng. 2011, 24, 1379–1396. [Google Scholar] [CrossRef]
- Lenoble, J.P. Polymetallic Nodules; International Seabed Authority: Kingston, Jamaica, 2000; p. 8. [Google Scholar]
- Kanungo, S.B. Rate process of the reduction leaching of manganese nodules in dilute HCl in presence of pyrite. Part I. Dissolution behavior of iron and sulphur species during leaching. Hydrometallurgy 1999, 52, 313–330. [Google Scholar] [CrossRef]
- Kanungo, S.B. Rate process of the reduction leaching of manganese nodules in dilute HCl in presence of pyrite: Part II. Leaching behavior of manganese. Hydrometallurgy 1999, 52, 331–347. [Google Scholar] [CrossRef]
- Zakeri, A.; Bafghi, M.S.; Shahriari, S. Dissolution of manganese dioxide ore in sulfuric acid in the presence of ferrous ion. Iran. J. Mater. Sci. Engi. 2007, 4, 22–27. [Google Scholar]
- Su, H.; Liu, H.; Wang, F.; Lü, X.; Wen, Y. Kinetics of Reductive Leaching of Low-grade Pyrolusite with Molasses Alcohol Wastewater in H2SO4. Chin. J. Chem. Eng. 2010, 18, 730–735. [Google Scholar] [CrossRef]
- Bafghi, M.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Kanungo, S.B.; Das, R.P. Extraction of metals from manganese nodules of the Indian Ocean by leaching in aqueous solution of sulphur dioxide. Hydrometallurgy 1988, 20, 135–146. [Google Scholar] [CrossRef]
- Charewicz, W.A.; Chaoyin, Z.; Chmielewski, T. The leaching behavior of ocean polymetallic nodules in chloride solutions. Physicochem. Probl. Miner. Process. 2001, 35, 55–56. [Google Scholar]
- Kanungo, S.B.; Jena, P.K. Reduction leaching of manganese nodules of Indian Ocean origin in dilute hydrochloric acid. Hydrometallurgy 1998, 2, 41–58. [Google Scholar] [CrossRef]
- Han, K.N.; Fuerstenau, D.W. Acid leaching of ocean floor manganese nodules at elevated temperature. Int. J. Miner. Process. 1975, 2, 163–171. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, Y.; Huang, Z.; Zhang, B.; Qiu, G. Leaching kinetics of pyrolusite from manganese–silver ores in the presence of hydrogen peroxide. Hydrometallurgy 2004, 72, 129–138. [Google Scholar] [CrossRef]
- Petrie, L.M. Molecular interpretation for SO2 dissolution kinetics of pyrolusite, manganite and hematite. Appl. Geochem. 1995, 10, 253–267. [Google Scholar] [CrossRef]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4∙H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Process. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Hariprasad, D.; Mohapatra, M.; Anand, S. Non-isothermal self-sustained one pot dissolution of metal values from manganese nodules using NH3OHCl as a novel reductant in sulfuric acid medium. J. Chem. Technol. Biotechnol. 2013, 88, 1114–1120. [Google Scholar] [CrossRef]
- White, F.; Peterson, M.L.; Hochella, M.F. Electrochemistry and dissolution kinetics of magnetite and ilmenite. Geochim. Cosmochim. Acta 1994, 58, 1859–1875. [Google Scholar] [CrossRef]
- Nijjer, S.; Thonstad, J.; Haarberg, G.M. Oxidation of manganese(II) and reduction of manganese dioxide in sulphuric acid. Electrochim. Acta 2000, 46, 395–399. [Google Scholar] [CrossRef]
- Godunov, E.B.; Izotov, A.D.; Gorichev, I.G. Reactions of manganese oxides with sulfuric acid solutions studied by kinetic and electrochemical methods. Inorg. Mater. 2017, 53, 831–837. [Google Scholar] [CrossRef]
- Anacleto, N.; Ostrovski, O.; Ganguly, S. Reduction of Manganese Oxides by Methane-containing Gas. ISIJ Int. 2004, 44, 1480–1487. [Google Scholar] [CrossRef] [Green Version]
- Sesen, F.E. Practical reduction of manganese oxide. J. Chem. Technol. Appl. 2017, 1, 1–2. [Google Scholar]
- COCHILCO. Análisis del Catastro de Depósitos de Relaves en Chile y guía de estructura de datos. Servicio Nacional de Geología y Minería. 2018. Available online: http://www.sernageomin.cl/wp-content/uploads/2018/05/An%C3%A1lisis-de-los-Dep%C3%B3sitos-de-Relaves-en-Chile_VF.pdf (accessed on 1 December 2018).
- DGA 1998, Universidad de Chile. Informe País: Estado del Medio Ambiente en Chile 2012. 2013. Available online: http://www.repositorio.uchile.cl/handle/2250/123564 (accessed on 1 December 2018).
- Dean, A.; Voss, D.; Draguljic, D. Response Surface Methodology. Des. Anal. Exp. 2017, 565–614. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.D.; Maurer, R.E.; Celli, G.B. Multiple Linear Regression. In Experimental Design; Springer International Publishing: Cham, Switzerland, 2018; pp. 505–532. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; Wiley: Hoboken, NJ, USA, 2012; Volume 8, pp. 3–10. [Google Scholar]
- Paramguru, R.K.; Kanungu, S.B. Electrochemical phenomena in MnO2–FeS2 leaching in dilute HCl. Part 3. Manganese dissolution from indian ocean nodules. Can. Metall. Q. 1998, 37, 405–417. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bazdanis, G.; Bartzas, G.; Sahinkaya, E.; Zaharaki, D. Removal of heavy metals from leachates using organic/ inorganic permeable reactive barriers. Desalin. Water Treat. 2013, 51, 3052–3059. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef] [PubMed]
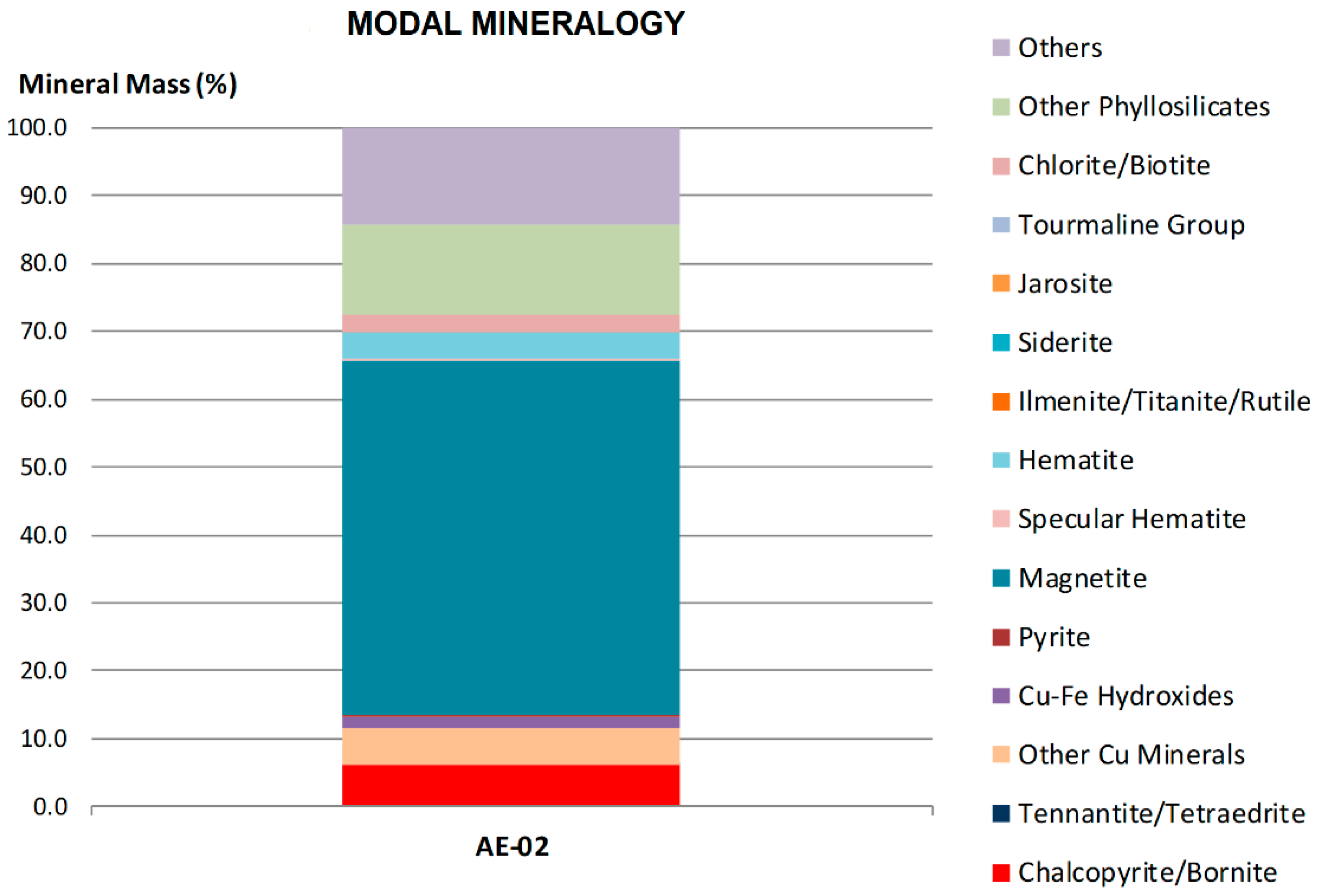



| Component | Mn | Fe | Cu | Co |
|---|---|---|---|---|
| Mass (%) | 15.96 | 0.45 | 0.12 | 0.29 |
| Component | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | MnO2 | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass (%) | 3.54 | 3.69 | 2.97 | 7.20 | 1.17 | 0.33 | 22.48 | 1.07 | 29.85 | 26.02 |
| Mineral | Amount % w/w |
|---|---|
| Chalcopyrite/bornite | 6.05 |
| Tennantite/tetraedrite | 0.24 |
| Other Cu minerals | 5.22 |
| Cu-Fe hydroxides | 1.80 |
| Pyrite | 0.18 |
| Magnetite | 52.11 |
| Specular hematite | 0.47 |
| Hematite | 3.79 |
| Ilminite/titanite/rutile | 0.03 |
| Siderite | 0.07 |
| Chlorite/biotite | 2.55 |
| Other phyllosilicates | 13.14 |
| Others | 14.35 |
| Total | 100.00 |
| Exp. No. | Time (min) | Sieve Fraction (Tyler Mesh) | Particle Size (µm) | Sulphuric Acid (M) | Mn Extraction (%) |
|---|---|---|---|---|---|
| 1 | 10 | −200 + 270 | −75 + 53 | 0.1 | 8.77 |
| 2 | 20 | −100 + 140 | −150 + 106 | 0.5 | 30.08 |
| 3 | 20 | −200 + 270 | −75 + 53 | 1.0 | 58.27 |
| 4 | 30 | −200 + 270 | −75 + 53 | 1.0 | 69.55 |
| 5 | 10 | −320 + 400 | −47 + 38 | 0.5 | 22.56 |
| 6 | 20 | −100 + 140 | −150 + 106 | 0.1 | 11.28 |
| 7 | 30 | −100 + 140 | −150 + 106 | 1.0 | 57.64 |
| 8 | 30 | −320 + 400 | −47 + 38 | 0.1 | 15.04 |
| 9 | 10 | −100 + 140 | −150 + 106 | 0.5 | 16.92 |
| 10 | 10 | −100 + 140 | −150 + 106 | 1.0 | 38.22 |
| 11 | 20 | −200 + 270 | −75 + 53 | 0.5 | 53.88 |
| 12 | 30 | −100 + 140 | −150 + 106 | 0.1 | 11.90 |
| 13 | 20 | −200 + 270 | −75 + 53 | 0.1 | 17.54 |
| 14 | 10 | −100 + 140 | −150 + 106 | 0.1 | 6.27 |
| 15 | 10 | −200 + 270 | −75 + 53 | 1.0 | 45.74 |
| 16 | 10 | −320 + 400 | −47 + 38 | 0.1 | 8.15 |
| 17 | 20 | −320 + 400 | −47 + 38 | 0.1 | 10.65 |
| 18 | 20 | −320 + 400 | −47 + 38 | 0.5 | 27.57 |
| 19 | 30 | −200 + 270 | −75 + 53 | 0.1 | 20.05 |
| 20 | 30 | −200 + 270 | −75 + 53 | 0.5 | 60.78 |
| 21 | 10 | −320 + 400 | −47 + 38 | 1.0 | 44.49 |
| 22 | 20 | −320 + 400 | −47 + 38 | 1.0 | 55.14 |
| 23 | 20 | −100 + 140 | −150 + 106 | 1.0 | 49.50 |
| 24 | 30 | −320 + 400 | −47 + 38 | 0.5 | 35.09 |
| 25 | 10 | −200 + 270 | −75 + 53 | 0.5 | 39.47 |
| 26 | 30 | −320 + 400 | −47 + 38 | 1.0 | 61.40 |
| 27 | 30 | −100 + 140 | −150 + 106 | 0.5 | 35.09 |
| Parameters | Values |
|---|---|
| Sieve fraction (Tyler mesh) | −100 + 140, −200 + 270, −320 + 400 |
| Particle size (µm) | −150 + 106, −75 + 53, −47 + 38 |
| Time (in min) | 5, 10, 20, 30, 40 |
| H2SO4 (M) | 0.1, 0.5, 0.75, 1 |
| MnO2/Fe (slag) | 1/1 |
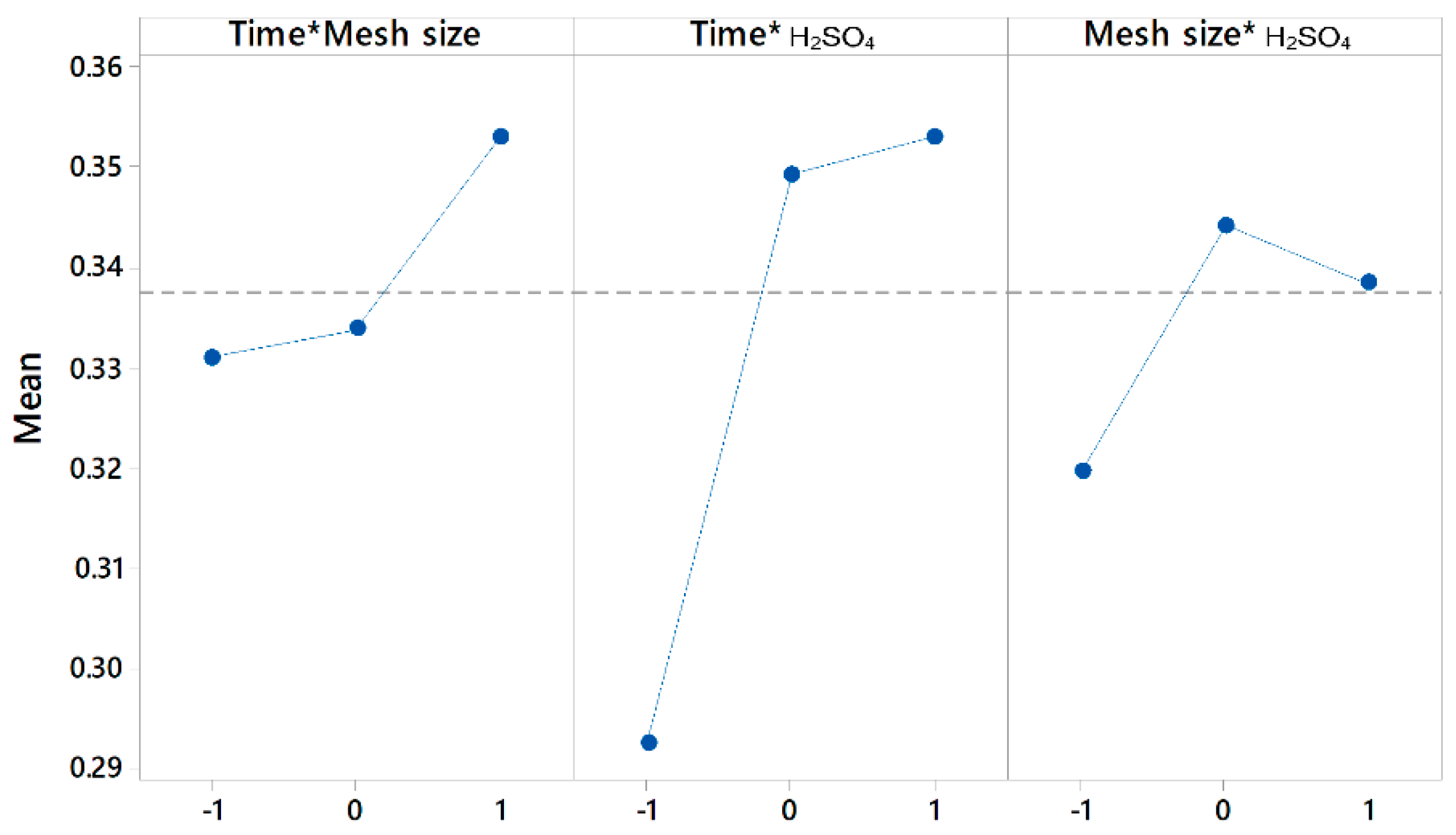
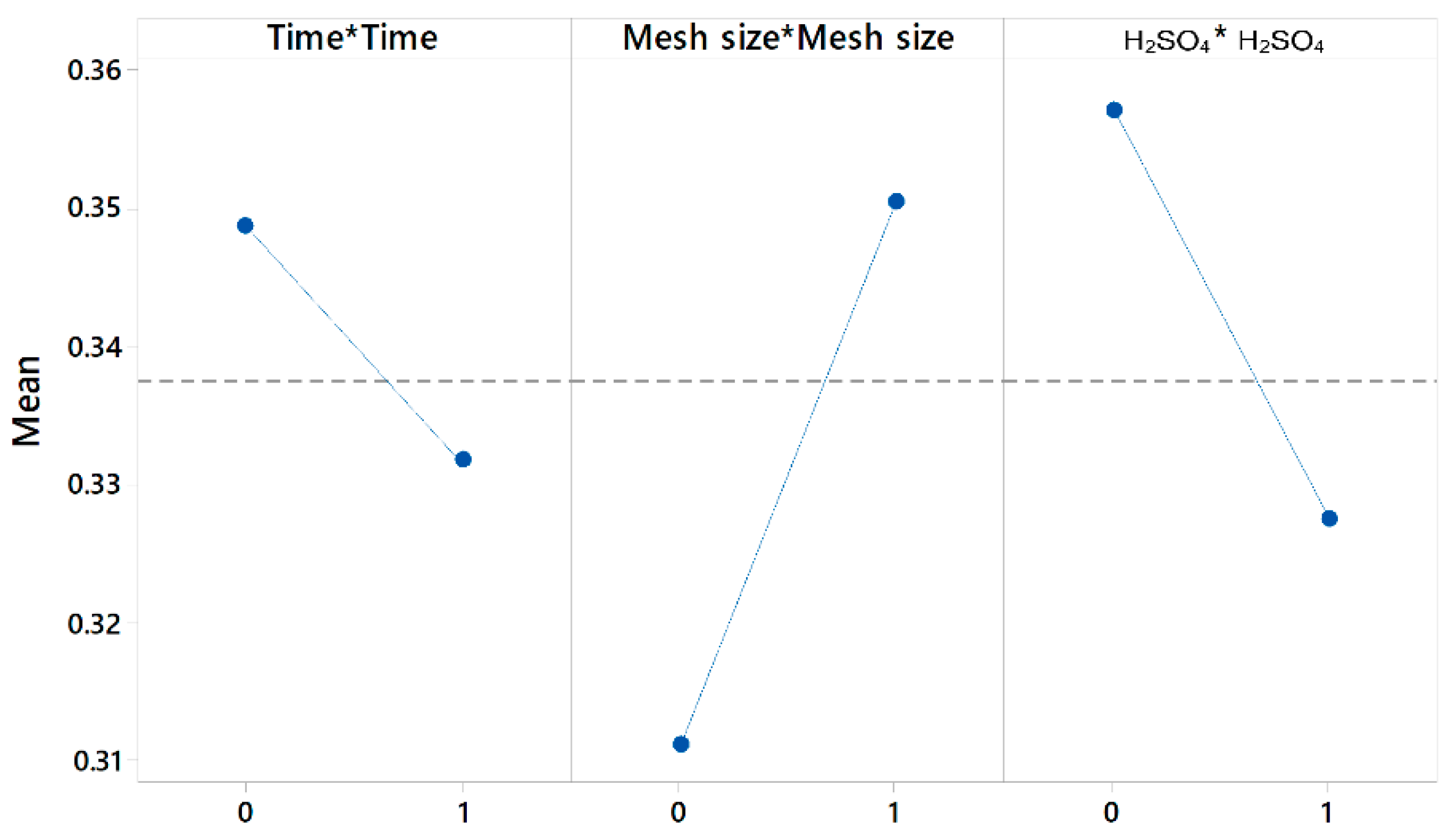
| Source | F-Value | p-Value |
|---|---|---|
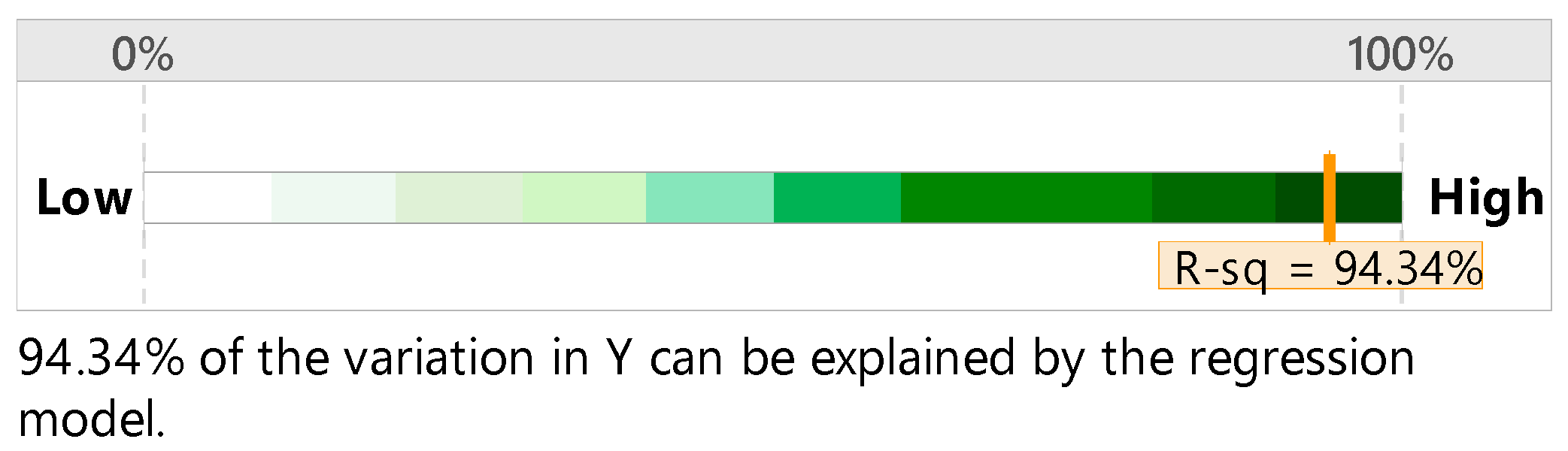
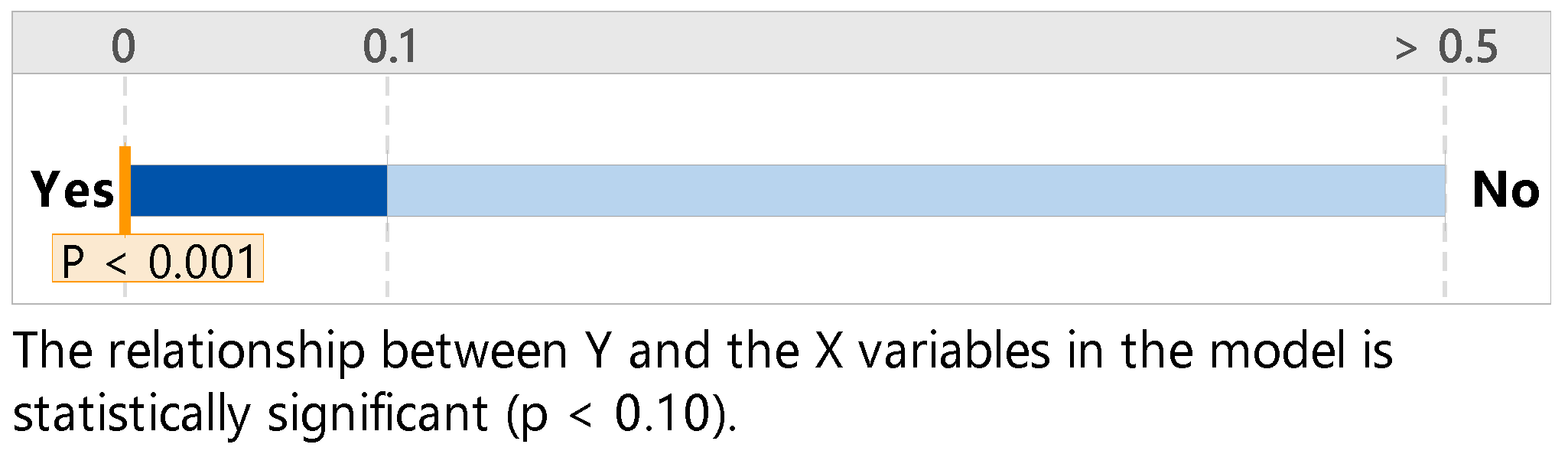
| Regression | 38.11 | 0.000 |
| Time | 36.29 | 0.000 |
| Mesh size | 26.95 | 0.000 |
| H2SO4 | 269.22 | 0.000 |
| Time × Mesh size | 0.51 | 0.485 |
| Time × H2SO4 | 3.89 | 0.065 |
| Mesh size × H2SO4 | 0.37 | 0.549 |
| Time × Time | 0.62 | 0.443 |
| Mesh size × Mesh size | 3.28 | 0.088 |
| H2SO4 × H2SO4 | 1.86 | 0.191 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, N.; Herrera, N.; Castillo, J.; Torres, C.M.; Sepúlveda, R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. https://doi.org/10.3390/min8120565
Toro N, Herrera N, Castillo J, Torres CM, Sepúlveda R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals. 2018; 8(12):565. https://doi.org/10.3390/min8120565
Chicago/Turabian StyleToro, Norman, Nelson Herrera, Jonathan Castillo, Cynthia M. Torres, and Rossana Sepúlveda. 2018. "Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I" Minerals 8, no. 12: 565. https://doi.org/10.3390/min8120565







